Excessive daytime sleepiness in patients suering from dierent
levels of obstructive sleep apnoea syndrome
C . S A U T E R
1, S . A S E N B A U M
1, R . P O P O V I C
2, H . B A U E R
3, C . L A M M
3,
G . K L OÈ S C H
1and J . Z E I T L H O F E R
11University Clinic of Neurology, Vienna,2University Clinic of Pulmology, Vienna,3University of Psychology, Brain Research Lab, Vienna, Austria
Accepted in revised form 30 March 2000; received 25 August 1999
INTRODUCTION
Excessive daytime sleepiness (EDS) is a serious eect of obstructive sleep apnoea (OSA). Its clinical features are a strong feeling of abnormal daytime tiredness, reduced wake-fulness and vigilance. In addition to this higher risk of internal and neurological disease (Zeitlhofer et al. 1995), patients suering from untreated OSA are more frequently involved in industrial and automobile accidents (Findley et al. 1988, 1995; Casselet al. 1991). EDS may also lead to problems at work and to a deterioration in psychosocial and cognitive
function (Rothet al. 1988). In general, EDS can have a major impact on quality of life.
EDS in OSA is caused by altered quantity and quality of night sleep. Dierent aspects of disturbed sleep, such as changes in breathing like apnoea/hypopnea or hypoxia (Greenberg et al. 1987), have been postulated as the main generator of EDS in OSA. The restorative nature of sleep might also be reduced by arousals (Stepanski et al. 1984; Zucconiet al. 1994; Kingshottet al. 1998), fragmentation of sleep (Roehrset al. 1989; Bennettet al. 1999), a lack of slow-wave sleep (SWS; Martinet al. 1997b) or a reduction in total sleep time (TST; Chughet al. 1996), likewise resulting in EDS. This study was designed to evaluate objective and subjective aspects of EDS in untreated patients with dierent levels of OSA. Another purpose was to elucidate the possible
connec-Correspondence: Prof. Dr J. Zeitlhofer, UniversitaÈtsklinik fuÈr Neurologie, WaÈhringer GuÈrtel 18-20, A-1090 Wien, Austria. Tel.: 431 40 400 3100, Fax: 431 40 400 3144, e-mail: josef.zeitlhofer@ univie.ac.at
SUMMARY Excessive daytime sleepiness (EDS) is a frequent symptom of patients with obstructive sleep apnoea (OSA). EDS is a high-risk factor for accidents at work and on the road. Thirty untreated patients with dierent levels of severity of OSA were studied concerning night sleep and EDS. The criterion for severity was the respiratory disturbance index (RDI): 15 patients were classi®ed as `moderately' apnoeic (RDI < 40), 15 as `severely' apnoeic (RDI > 40). Following night-time polysomnog-raphy, objective and subjective aspects of EDS were studied. To assess objective EDS the Maintenance of Wakefulness Test (MWT) and a computer-based vigilance performance test were used. Subjective EDS was determined using the Stanford Sleepiness Scale (SSS), the Epworth Sleepiness Scale (ESS) and the Visual Analogue Scales for Performance (VAS-P) and Tiredness (VAS-T). Well-being was assessed using the Scale of Well-Being by von Zerssen (Bf-S/Bf-S¢). Severe apnoea patients spent more time in stage 1 and less in slow-wave sleep. MWT latencies tended to be shorter in the severe apnoea group. Vigilance testing revealed no group dierences. Patients with moderate apnoea described themselves as more impaired in all subjective scales, but only SSS scores reached statistical signi®cance. Our results suggest that there is no simple correlation between polysomnographic and respiratory sleep variables at night on the one hand, and the extent of EDS on the other hand. Furthermore, subjective and objective evaluation of EDS does not yield the same results. New approaches which allow a more detailed analysis of night sleep and daytime function are required to identify high-risked patients.
KEYWORDS obstructive sleep apnoea, Maintenance of Wakefulness Test, excessive
tions between the severity of OSA at night and the extent of EDS.
METHODS Patients
Thirty (six female and 24 male) untreated OSA-patients underwent polysomnographic procedures and evaluation of EDS at the Departments of Neurology and Pulmology at the University Hospital of Vienna. Patients had a mean age of 50.3 (SD) 10.3 years and a mean body mass index (BMI) of 33.65 7.75. The mean respiratory disturbance index (RDI, i.e. the number of apnoeas and hypopneas per hour of sleep) was 48.5 28.14.
Patients were divided into two subgroups representing moderate and severe apnoea in OSA. Using the classi®cation of NaeÈgele et al. (1995); patients with an RDI < 40 were considered moderately apnoeic (n15), those with an RDI > 40 were classi®ed as severely apnoeic (n15).
Nocturnal sleep studies
Polysomnography preceding the day of the evaluation of EDS was performed in accordance with the standard methods of Rechtschaen and Kales (1968). Air¯ow was recorded using thermistors at the nose and mouth, thoracic and abdominal respiratory movements by a strain gauge, arterial oxygen saturation was measured continuously with a ®nger oxymeter. Sleep was scored automatically (Sleep Analyzing Computer 847, Oxford Instruments, UK) and veri®ed visually on the basis of Rechtschaen and Kales (1968).
Variables in relation to sleep were TST, sleep eciency (SE; TST/total bed time´100), sleep latency (SL), percentage of light sleep (stages 1 and 2), SWS (stages 3 and 4), rapid eye movement (REM) and time awake (W).
Respiratory variables were obstructive apnoeas and hypop-neas according to standard criteria. From these variables indices were computed, i.e. the number of respiratory events per hour of sleep (AI, apnoea index; HI, hypopnea index; RDI, total of AI and HI), and decreases in arterial oxygen saturation (SaO2) by 4% or more were considered.
Maintenance of Wakefulness Test
The Maintenance of Wakefulness Test (MWT; Mitler et al. 1982) was performed at 09.00, 11.00, 13.00 and 15.00. The patient was seated in an armchair and was told to keep awake for as long as possible. In accordance with the recommenda-tions of Doghramjis et al. (1997), sleep latencies were calcu-lated from the time that light was dimmed to the appearance of the ®rst 30-s epoch of any stage of sleep. Trials lasted for 30 min, or were terminated after 10 min of continuous sleep. This duration of the test was chosen to make the results more comparable with those in the vigilance test.
Vigilance test
Taking into account the circadian variation of performance (Mitler et al. 1988), vigilance was tested at the expected maximum in the late morning and at the nadir in the early afternoon, i.e. at 10.00 and 14.00. At these two times, the patients completed the vigilance test `Quatember-Maly' (Wiener TestsystemTM, Schufried, Austria), a computerized
version of the Mackworth clock test (Mackworth 1957), taking 30 min (training inclusive). The important variables were `hits' (correct response to target stimulus), `false' (response when not requested) and `reaction time' to a stimulus.
Epworth Sleepiness Scale
To evaluate the level of general sleepiness with regard to the past few weeks, patients completed the Epworth Sleepiness Scale (ESS, Johns 1991) both in the morning and at the end of testing in the afternoon. The test was performed twice to see if patients estimated their general level of sleepiness dierently after dealing with the topic for a whole day. Higher scores in the ESS (range: 0±24) imply a greater average sleep propensity. Normal values range between 2 and 10, scores >14 are indicative of a high level of daytime sleepiness (Johns 1991, 1994).
Stanford Sleepiness Scale
Beginning at 09.00, the current level of subjective sleepiness was evaluated every 30 min using the Stanford Sleepiness Scale (SSS, Hoddeset al. 1972). The SSS contains seven statements describing dierent levels of current sleepiness ranging from 1 `feeling active and vital; alert; wide awake' to 7 `almost in reverie; sleep onset soon; lost struggle to remain awake'. The patient has to choose the most appropriate description of his subjective level of sleepiness.
Visual analogue scales
Two visual analogue scales (VAS; Folstein and Luria 1973; Herbertet al. 1976; Leeet al. 1991), one for tiredness (VAS-T) and one for performance (VAS-P), were presented to the patients every 30 min. High scores in VAS-T indicate pro-nounced subjective tiredness, high values in VAS-P correspond to high subjective performance.
Scale of Well-Being
To evaluate current well-being and condition of the patient, the self-rating Scale of Well-Being (`Be®ndlichkeitsskala', Bf-S/Bf-S¢) by von Zerssen (1976) was administered. Patients ®lled in this scale four times, immediately before each MWT, alternating between Bf-S and the parallel form Bf-S¢. The higher the score, the worse the patient's condition at the moment of evaluation.
Procedure
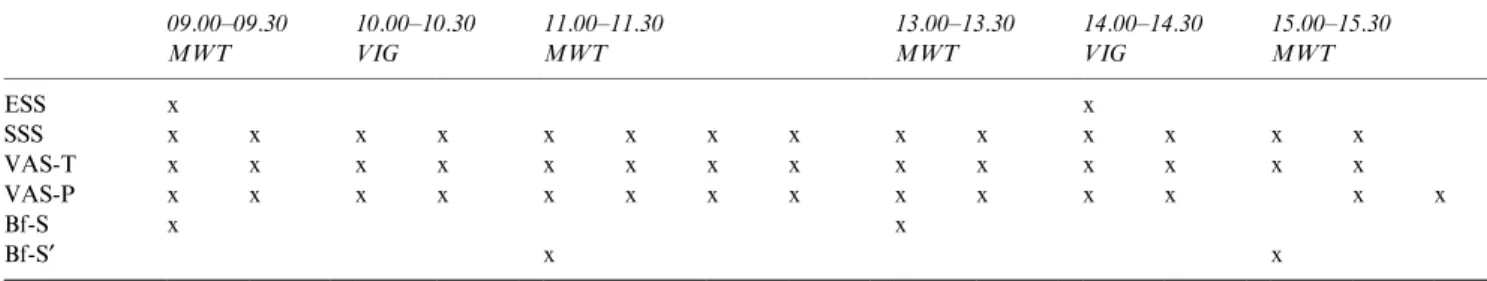
Patients' sleep was recorded in the sleep laboratory. Details concerning EDS testing performed on the following day are given in Table 1. The use of alcohol, caeine and nicotine was not permitted for the duration of the study.
Data analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences for Windows 6.0 (SPSS). All normally distributed physiological variables (age, RDI, BMI,SaO2) and
polysomnographic variables obtained at night were analysed by means of t-tests for independent samples. In non-normally distributed variables dierences between groups were assessed by using Mann±Whitney tests. As all tests and scales of EDS were administered several times, repeated two-way analysis of variances (ANOVA) was computed with `group' (`moderate' or
`severe') as the between factor and `time' (dierent times of testing) as the within factor for normally distributed vari-ables. For non-normally distributed variables, Wilcoxon's rank sum test was used. To take expected dierences in circadian in¯uences into account means of the subjective scales, ®lled out every 30 min were computed for each patient at 10.00 and 14.00. When appropriate, the Pearson correla-tion coecientror Spearman rank correlations were used to detect associations between dierent variables. As sleep and respiration during sleep, as well as daytime performance, are in¯uenced by age (Spiegel 1990) all these correlations were partial, controlling for age. A probability value of < 0.05
was accepted as statistically signi®cant for all procedures. In total, 126 correlations were calculated, 12 of which proved to be signi®cant. Owing to that large number of correlations, some of the 12 found to be signi®cant could yield signi®cance merely by chance. Therefore, the binomial theorem (Cross and Chan 1982) was used to interpret results. The likeli-hood of obtaining this result of 12 signi®cant correlations for P(12 : 126,0.05)0.0953, implying that they are not signif-icant by chance alone.
RESULTS
Characteristics of subgroups
Moderately apnoeic OSA patients were aged 51.3 12.0 years, had a RDI of 26.9 10.5 and a BMI of 31.1 5.7 kg/m2. In contrast, severely apnoeic OSA patients
were aged 49.3 8.54 years, had a RDI of 70.2 22.9 and a BMI of 36.2 8.9 kg/m2. Both groups were comparable with
regard to age (t0.51, NS) and BMI (t)1.87, NS). Severely apnoeic patients showed signi®cantly lower mean (90.33 3.04 vs. 93.60 2.67; t3.13, P< 0.01) and lowest arterial oxygen saturations (60.00 8.94 vs. 93.60 2.67;t5.61,P< 0.0001).
Polysomnographic ®ndings
The two groups diered signi®cantly with regard to the percentage of time spent awake after sleep onset (stage W), stage 1 and SWS. There was no dierence regarding SL, TST,
Table 1 Schedule of EDS evaluation
09.00±09.30 10.00±10.30 11.00±11.30 13.00±13.30 14.00±14.30 15.00±15.30 MWT VIG MWT MWT VIG MWT ESS x x SSS x x x x x x x x x x x x x x VAS-T x x x x x x x x x x x x x x VAS-P x x x x x x x x x x x x x x Bf-S x x Bf-S¢ x x
Bf-S, Scale of Well-Being; Bf-S¢, Scale of Well-Being (parallel-form); EDS, Excessive Daytime Sleepiness; ESS, Epworth Sleepiness Scale; MWT, Maintenance of Wakefulness Test; SSS, Stanford Sleepiness Scale; VAS-P, Visual Analogue Scale of Performance; VAS-T, Visual Analogue Scale of Tiredness; VIG, Vigilance Test.
Table 2 Comparison of sleep parameters between moderately (RDI < 40) and severely (RDI > 40) apnoeic OSA patients (t-test)
RDI < 40 (mean SD) RDI > 40 (mean SD) t P
SL 9.83 7.67 11.27 13.99 )0.35 0.730 TST 391.17 47.42 423.23 45.64 )1.89 0.070 SE 80.53 9.88 86.80 8.83 )1.83 0.078 Stage W 17.47 10.00 10.26 6.16 2.38 0.012 Stage 1 20.81 9.20 39.15 13.63 )4.32 0.000 Stage 2 37.69 6.55 38.71 13.93 )0.26 0.400 Stage 3 8.27 4.18 3.07 4.62 3.23 0.002 Stage 4 3.62 5.46 0.07 0.18 2.51 0.013 REM 11.95 6.56 8.64 3.75 1.7 0.100
RDI, Respiratory Disturbance Index; SE, Sleep Eciency; SL, Sleep Latency; Stage W, Stage Awake; TST, Total Sleep Time.
SE, and the percentage of stage 2 sleep. Polysomnographic ®ndings are shown in Table 2.
Maintenance of Wakefulness Test
Mean latencies for each group and times of testing are shown in Fig. 1. Analysis of variance did not reveal any dierences between the two groups (F1.14, NS) or between the four times of testing (F0.25, NS). There were no signi®cant interactions between groups and times of testing (F1.86, NS). Although no statistically signi®cant results were obtained, severely apnoeic OSA patients were less able to keep awake in three of four tests. Other studies used longer (40 min) or shorter versions (20 min) of the MWT (for a review see Doghramjiet al. 1997). Owing to dierent `ceiling eects' in those versions, the results shown here can not be compared with those data.
Vigilance test
None of the three variables measured with the `Quatember-Maly' vigilance test distinguished signi®cantly between the two groups (`hits':Z)0.272, NS; `false':Z)0.477, NS; `mean reaction-time': Z)0.667, NS). In both groups of patients results did not dier between the two times of testing (RDI < 40: `hits': Z)0.359, NS; `false':Z)1.610, NS; `mean reaction time': Z)1.297, NS; RDI > 40: `hits': Z)0.985, NS; `false':Z)1.065, NS; `mean reaction time': Z)0.826, NS). In general, one third of all patients were promptly able to detect all 100 signals given. The mean scores for both groups were similar to the mean of a normal population. One third of the subjects in each group showed extremely low values, i.e. a percentile of <25 with `hits'. The number of false reactions was negatively correlated with the percentage of REM during night sleep (q)0.466;P< 0.01) and the number of `hits' was correlated with the mean of subjective performance in the VAS-P (q0.379;P< 0.039). Epworth Sleepiness Scale
OSA patients with a RDI < 40 tended to score higher at both times, i.e. they rated their general level of sleepiness in dierent sopori®c situations of daily life higher. Mean values for
moderately apnoeic OSA patients were 13.4 4.71 in the morning and 13.73 5.13 in the afternoon, whereas severely apnoeic OSA patients had means of 11.47 5.64 and 12.33 5.41. ESS scores did not dier between RDI groups (F0.73, NS), or between the times of testing (F1.61, NS). There were no signi®cant interactions between groups and times of testing (F0.09, NS). Mean ESS scores were weakly correlated with mean subjective tiredness (r)0.4579, P< 0.05) and performance (r0.4762; P< 0.01) in the VAS, but not with any other polygraphic or EDS measure. Stanford Sleepiness Scale
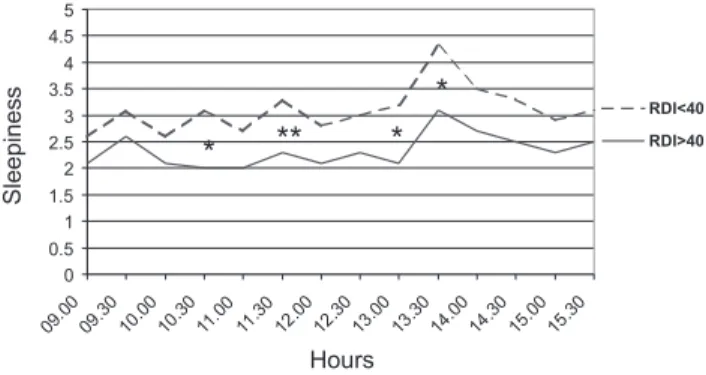
ANOVArevealed signi®cant RDI intergroup dierences (high
and low RDI levels) in subjective sleepiness (F4.6, P< 0.05), and signi®cant dierences between the dierent times of testing (F5.30,P< 0.001). Patients with mod-erate OSA described themselves as sleepier throughout the whole day, with signi®cantly higher SSS scores (Student's t-test) at fur of the 14 times of testing (Fig. 2). The means computed between 10.00 and 14.00 did not dier between groups (RDI < 40: 3.1 vs. RDI > 40: 2.4). There were no signi®cant interactions between RDI groups and times of testing. Both groups reached highest sleepiness scores in the early afternoon and these values altered almost in parallel. Values correlated signi®cantly with SL (r0.5003, P< 0.01), SE (r)0.5651; P< 0.01), TST (r)0.5623; P< 0.01), Stage W (r0.4577; P< 0.05). SSS means and means of the VAS-T (r0.6622;P< 0.000) and VAS-P (r)0.7185; P< 0.000) were correlated highly signi®-cantly.
Visual analogue scales
Diurnal ¯uctuations of VAS-T and VAS-P scores for both groups are illustrated in Fig. 3. No signi®cant dierences were found between the RDI groups for VAS-T (F1.98, NS) or VAS-P (F2.75, NS). There were signi®cant dierences between the various times of testing for VAS-T (F4.24, P< 0.01) and VAS-P (F2.79, P< 0.05). Interactions
20 15 10 5 0
Sleep latencies (minutes)
Total 15.00 13.00 11.00 09.00 Hours 18.6 RDI < 40 RDI > 40 13.415.4 17 16.9 12.9 19.2 17.5 14.1 13
Figure 1. Mean MWT sleep latencies for moderately and severely apnoeic OSA patients and total mean score for each group. Dierences between groups did not reach statistical signi®cance.
Figure 2. Mean scores of subjective sleepiness in the Stanford Sleep-iness Scale for moderately and severely apnoeic patients. Scores were evaluated every 30 min, high values indicate increased sleepiness. Moderately apnoeic patients described themselves as sleepier at four time points (*P< 0.05, **P< 0.01).
between RDI groups and times of testing for VAS-T (F0.85, NS) or VAS-P (F0.82, NS) were not signi®cant. Tiredness was increased in the afternoon and reached a peak at 13.30, whereas subjective performance declined in parallel in both groups, i.e. VAS-P and VAS-T correlated highly signi®cantly (r)0.8590,P< 0.000).
Scale of Well-Being
Groups did not dier concerning well-being in the Bf-S/Bf-S¢
(F2.37, NS) and there were no signi®cant interactions between times of testing and group membership (F0.57, NS). Well-being altered between the four times of testing (F3.85, P< 0.05), but patients rated their condition as most impaired in the morning. Until 13.00 well-being was ameliorated in both groups. Only in patients with moderate apnoea in OSA, did well-being worsen in the last evaluation. The mean Bf-S/Bf-S¢score for severely apnoeic OSA patients was comparable with normative data. Patients with a RDI <40 were less well-balanced but still comparable with healthy controls. Results are shown in Fig. 4. The mean of well-being was weakly correlated with the mean of the VAS-P (q0.379; P< 0.05).
DISCUSSION
Patients with OSA show excessive daytime sleepiness as a result of altered quantity and quality of night sleep. This study tried to evaluate dierent aspects of EDS using objective (ability to maintain awake, vigilance performance in monot-onous situations) and subjective (general and current level of sleepiness, tiredness, performance and well-being) measures. Furthermore, we tried to determine what kind of relationship exists between these parameters with respect to the dierent levels of OSA.
Polygraphic ®ndings showed clear dierences between the two groups. In severely apnoeic OSA patients, the percentage
of stage 1 sleep was increased and that of SWS decreased, whereas moderately apnoeic OSA patients spent more time in stage 4 and stage W, which led to a decreased sleep eciency and total sleep time. Cassel et al. (1994) also observed that patients with a moderate degree of OSA are more likely to suer from insomnia.
In the severely aected group, mean sleep latencies in the MWT sessions tended to be shorter in three of the four times, without reaching statistical signi®cance. There were no time-of-day eects. MWT results did not correlate with polygraphic ®ndings or with any other objective or subject-ive variables. OSA studies using the MWT showed a weak negative correlation of MWT sleep latency with the respir-atory disturbance index (Sangal et al. 1992; Sangal and Sangal 1997
1 ), the number of arousals caused by respiratory events (Poceta et al. 1992), the lowest oxygen saturation (Kingshott et al. 1998) and percentage of stage 1 (Sangal et al. 1997). A study conducted by Doghramjiet al. 1997 in normal subjects showed that sleep eciency was the only nocturnal sleep variable that was correlated with MWT sleep latency.
Other studies in healthy adults have shown SWS duration (Martinet al. 1996) and TST (HaÈrmaÈet al. 1998) to be the best predictors of MWT sleep latency. Although ®ndings are inconsistent, the MWT is successfully used to assess improve-ments in alertness following therapeutic interventions in narcoleptics (Mitleret al. 1986; Mitleret al. 1990; Mitler and Hajdukovich 1991; Mitler 1994) and OSA patients (Sangal et al. 1992; Meuriceet al. 1996; Tihonen and Partinen 1998), but not in patients with mild OSA, treated with CPAP (Englemanet al. 1999).
The `Quatember-Maly' vigilance test did not reveal any dierences between the two groups. One-third of patients in each group showed extremely impaired vigilance. No time-of-day eects could be discerned. Our computer-based test might have been too short to distinguish between groups. Weeû et al. (1998) suggested that vigilance tasks in the diagnosis of sleep and waking disorders should last at least 30 min to reveal de®cits. But even tests of longer duration were in some cases unable to distinguish between dierent levels of
Figure 3. Mean scores of the visual analogue scales of tiredness (VAS-T) and performance (VAS-P) in moderately and severely apnoeic patients. High scores in VAS-T indicate strong subjective tiredness, high values in VAS-P correspond to high subjective performance. Dierences between groups did not reach statistical signi®cance.
Figure 4. Mean scores for the Scale of Well-Being, evaluated every 2 h and global score in moderately and severely apnoeic patients. High values indicate reduced subjective well-being. Dierences between groups did not reach statistical signi®cance.
OSA-severity (Weeû et al. 1994). Nevertheless most vigilance tests are sensitive enough to evaluate therapeutic eects (e.g. CPAP) in OSA (Schwarzenberger-Kesperet al. 1987; Rande-rathet al. 1997; Conradtet al. 1998).
The number of `hits' showed a weak correlation with the mean VAS-P score of the total sample; the better a patient's feeling about his subjective performance, the more hits he made. Furthermore, the number of false reactions showed a weak negative correlation with the percentage of REM sleep, which cannot be interpreted satisfactorily. One must consider that in spite of the use of the binomial theorem some of the correlations could still be a product of chance alone. No other objective or subjective variables were signi®cantly correlated with the vigilance testing. In a study of 117 OSA patients, Hofmann and Klein (1993) found no correlation between the number of false reactions in the `Quatember-Maly' test and polygraphic and respiratory parameters. Kotterbaet al. (1997) examined vigilance in 40 OSA patients with a version similar to our test. They found no correlation between the RDI and subjective EDS. Compared with the MWT, the vigilance test was less capable of distinguishing between the two groups. It could be assumed that the MWT session is to a lesser extent in¯uenced by a masking motivational eect, because no response (to a signal) is demanded and patients are not allowed to activate themselves.
The level of general daytime sleepiness in the ESS did not dier between RDI groups. In both groups, the mean score of general sleepiness was elevated (>10) compared with healthy controls (Johns 1994). Five of the moderately aected patients and six of the severely aected patients showed pathological levels of sleepiness (>14). Johns (1991) found that OSA patients with severe apnoea (RDI49.5 9.6) showed signi®cantly higher levels of sleepiness than moderately apnoeic OSA patients (RDI21.1 4.0), whereas Smolley et al. (1993) and Rohmfeldet al. (1995) found no dierences when comparing groups with a RDI < 30 and >30. It seems that the ESS is not able to dierentiate well between dierent levels of severity of obstructive sleep apnoea, or alternatively, scores are dependent on the kind of grouping variable(s) used to dierentiate between dierent levels of severity of OSA. In this study we only found a weak correlation of the ESS with the VAS scales of Performance and Tiredness (negative direction), but with any polysomnographic variables. As the ESS intends to measure the general level of sleepiness with respect to the last few weeks, data from one night of sleep polysomnography might not be representative. Johns (1993) found the strongest correlation between the RDI, minimal oxygen saturation and the ESS in OSA patients. Other studies did not ®nd any signi®cant correlations between the ESS and polysomnographic parameters (Smolleyet al. 1993; Kingshott et al. 1995), even when taking into account measures of sleep fragmentation (Kingshottet al. 1998) and dierent de®nitions of microarousals (Martinet al. 1997a).
Moderately apnoeic OSA patients tended to score higher in all subjective measures of sleepiness, tiredness, performance and well-being, but statistical signi®cance was reached only in
the SSS. Subjective ratings in VAS-P, VAS-T and ESS showed moderate correlations, indicating that they measure similar, but not identical, attributes (Johnson et al. 1990). Another point is that the stronger correlations between subjective ratings than between subjective and objective ones could be related to other factors (Brioneset al. 1996). The MWT and the vigilance test are dependent more on the ability to cope with sleepiness, whereas the subjective scales are aected to a lesser extent by motivation concerning the duration of the test. The latter are in¯uenced more by the patient's ability to re¯ect upon himself. The lack of a closer relationship with other objective measures was also observed in other studies (Harnish et al. 1996; Redlineet al. 1997; Sangalet al. 1997; Kingshott et al. 1998).
In contrast, Bennettet al. (1998) found stronger correlations between EDS (ESS and modi®ed MWT) and sleep fragmen-tation variables using EEG as well as non-EEG sleep fragmentation indices in untreated patients. But not more than»25% of variance of EDS could be explained using this approach.
Subjective EDS, as measured by the SSS, VAS-P and VAS-T and Bf-S/Bf-S¢, showed varying scores during the day, with the highest subjective levels of sleepiness, tiredness and low performance in the mid-afternoon. This could be due to the bimodal pattern of sleepiness (Mitleret al. 1988).
Considering all patients, there were only signi®cant corre-lations of the SSS with sleep latency, sleep eciency, percent-age of wake-time and total sleep time. These four polysomnographic variables are highly interdependent by de®nition and relationships with SSS means were of only a moderate nature. SSS means did not correlate signi®cantly with any other sleep or respiratory variable. Other studies also detected only a weak or even no correlation between the SSS and polysomnography (Johnson et al. 1990; Sforza and Lugaresi 1995; Harnish et al. 1996; Lugaresi and Plazzi 1997). Mean SSS scores were not related to any other objective measures of EDS. Previous studies have shown that SSS scores and objective sleepiness do not always move in parallel (Roth et al. 1980; Harnish et al. 1996; Huterer et al. 1996; Martin et al. 1997b). Lugaresi and Plazzi (1997) stated that neither the number of apnoeas nor the degree of oxygen saturation during sleep is a good predictor of EDS. Our results verify their ®ndings to some extent. Stradlinget al. (1996) and Stradling and Davies (1996)
2 tried to ®nd the missing link between EDS and measures of OSA severity. They implemented neural network processing on EEG data to detect variations in the degree of arousal accompanying obstructive respiratory events and leading to dierent degrees of sleep disturbance. The dierent arousal magnitudes found between patients could explain the diverse severity levels in EDS, but further research in this ®eld is needed.
The obviously lower level of subjective daytime sleepiness in severely apnoeic OSA patients compared with moderately apnoeic ones could be due to the fact that sleepy patients no longer have a frame of reference for judging their own sleepiness (Hartse et al. 1982). They may be more used to
their sleepiness and thus may no longer be aware of it. Moderately apnoeic patients tended to have less TST, which was related to higher subjective sleepiness in the SSS. Maybe these patients were more aware of a reduced quality of night sleep, which made them feel less recovered during the day. This might explain the reduced well-being in the morning in this group. Subjective judgement of prior night sleep, which we did not evaluate suciently, may help to answer these questions.
In contrast to subjective ®ndings, MWT latencies showed that in three of four sessions severely apnoeic OSA patients tended to doze o earlier than the moderate apnoea group.
In addition to a more detailed analysis of polysomnography of night sleep (e.g. Stradling et al. 1999
3 ), a more elaborated
signal analysis (spectral analysis) of the ongoing EEG during MWT sessions might clarify the relationship between subject-ive and objectsubject-ive measures of daytime tiredness. In a vigilance EEG study in sleep apnoea patients and healthy controls utilizing EEG mapping techniques, Saletu et al. (1996) and Saletu et al. (in press) found a high correlation between nocturnal respiratory distress and deteriorated daytime brain functioning; mid-morning V-EEG mapping exhibited less total power, more delta and theta and less alpha, as well as a slower dominant frequency and centroid of the total activity in apnoea patients.
In conclusion, EDS in OSA seems almost independent of prior night sleep. Furthermore, objective and subjective variables are not clearly related in our study. These results could be due to: (i) an arbitrarily de®ned criterion of RDI as an indicator of severity; (ii) a nonlinear development of subjective and objective EDS in OSA; (iii) intruding factors of motivation, ability of self-re¯ection, self-rating and illness-judgement; and (iv) a de®cit of adequate measurements for night sleep and daytime function.
Therefore, in order to understand these underlying mecha-nisms of excessive sleepiness in OSA, further research is needed to detect high-risk patients. This implies new instruments for measuring night sleep (e.g. neural net processing) and for EDS (e.g. V-EEG) on the one hand and ®eld studies in the patient's everyday life (e.g. ambulant vigilance monitoring) on the other hand.
ACKNOWLEDGEMENTS
The authors thank the sta of the sleep laboratory, Peter Anderer for his advices in statistics and Elisabeth GraÈtzhofer and Richard Hallinan for editing the manuscript.
REFERENCES
Bennett, L. S., Barbour, C., Langford, B., Stradling, J. R. and Davies, R. J. O. Health status in obstructive sleep apnea. Relationship with sleep fragmentation and daytime sleepiness, and eects of continu-ous positive airway pressure treatment. Am. J. Respir. Crit. Care Med., 1999, 159: 1884±1890.
Bennett, L. S., Langford, B. A., Stradling, J. R. and Davies, R. J. O. Sleep fragmentation indices as predictors of daytime sleepiness and
nCPAP response in obstructive sleep apnea.Am. J. Respir. Crit. Care Med., 1998, 158: 778±786.
Briones, B., Adams, N., Strauss, M., Rosenberg, C., Whalen, C., Carskadon, M., Roebuck, T., Winters, M. and Redlins, S. Relationship between sleepiness and general health status.Sleep, 1996, 19: 583±588.
Cassel, W., Ploch, T., Peter, J. H. and von Wichert, P. Unfallgefahr von Patienten mit naÈchtlichen AtmungsstoÈrungen. Pneumologie, 1991, 45: 271±275.
Cassel, W., Stammnitz, A., Schneider, H., Becker, C. and Conradt, R. Insomniebeschwerden im Rahmen Schlafbezogener AtmungsstoÈr-ungen. In: C. Becker-Carus (Ed) Fortschritte der Schlafmedizin. Aktuelle BeitraÈge Zur Insomnieforschung (Forum Streb- und Sch-laorschung). Lit.,
4 MuÈnster, 1994: 220±229.
Chugh, D. K., Weaver, T. E. and Dinges, D. F. Neurobehavioral consequences of arousals.Sleep, 1996, 19 (Suppl. 10): 198±201. Conradt, R., Hochban, W., Heitmann, J., Brandenburg, U., Casssel,
W., Penzel, T. and Peter, J. H. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandib-ular advancement compared to CPAP therapy.J. Sleep Res., 1998, 7: 217±223.
Cross, E. M. and Chan, W. W. Use of the binomial theorem in interpreting results of multiple tests of signi®cance.Educat. Psychol. Measure., 1982, 42: 25±34.
Doghramji, K., Mitler, M. M., Sangal, R. B., Shapiro, C., Taylor, S., Walsleben, J., Belisle, C., Erman, M. K., Hayduk, R., Hosn, R., O'Malley, E. B., Sangal, J. M., Schutte, S. L. and Youakim, J. M. A normative study of the maintenance of wakefulness test (MWT).
Electroenceph. Clin. Neurophysiol., 1997, 103: 554±562.
Engleman, H. M., Kingshott, R. N., Wraith, P. K., Mackay, T. W., Deary, I. J. and Douglas, N. J. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome.Am. J. Respir. Crit. Care Med., 1999, 159: 461±467.
Findley, L., Unverzagt, M., Guchu, R., Fabrizio, M., Buckner, J. and Suratt, P. Vigilance and automobile accidents in patients with sleep apnea or narcolepsy.Chest, 1995, 108: 619±624.
Findley, L. J., Unverzagt, M. E. and Suratt, P. M. Automobile accidents involving patients with obstructive sleep apnea.Am. Rev. Resp. Dis., 1988, 138: 337±340.
Folstein, F. M. and Luria, R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychol. Med., 1973, 3: 479±486.
Greenberg, G. D., Watson, R. K. and Deptula, D. Neuropsychological dysfunction in sleep apnea.Sleep, 1987, 10: 254±262.
HaÈrmaÈ, M., Suvanto, S., Popkin, S., Pulli, K., Mulder, M. and Hirvonen, K. A dose±response study of total sleep time and the ability to maintain wakefulness.J. Sleep Res., 1998, 7: 167±174. Harnish, M. J., Chard, S. R. and Orr, W. C. Relationship between measures of objective and subjective sleepiness.Sleep Res., 1996, 25: 492.
Hartse, K. M., Roth, T. and Zorick, F. J. Daytime sleepiness and daytime wakefulness: the eect of instruction.Sleep, 1982, 5 (Suppl. 2): 107±118.
Herbert, M., Johns, M. W. and DoreÂ, C. Factor analysis of analogue scales measuring subjective feelings before and after sleep.Br. J. Med. Psychol., 1976, 49: 373±379.
Hoddes, E., Dement, W. C. and Zarcone, V. The development and use of the Stanford Sleepiness Scale (SSS).Psychophysiology, 1972, 9: 150.
Hofmann, G. and Klein, H. E. Der Vigilanz-Test nach Quatember-Maly als diagnostische Hilfsuntersuchung bei Schlafapnoe. In: K. Meier-Ewert and E. RuÈther (Eds)Schlafmedizin.Gustav Fischer, Stuttgart, 1993: 272±273.
Huterer, N., Salahovic, D. and Shapiro, C. M. Does night sleep predict subjective or objective daytime sleepiness? Sleep Res., 1996, 25: 497.
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale.Sleep, 1991, 14: 540±545.
Johns, M. W. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale.Chest, 1993, 103: 30±36. Johns, M. W. Sleepiness in dierent situations measured by the
Epworth Sleepiness Scale.Sleep, 1994, 17: 703±710.
Johnson, L. C., Spinweber, C. L., Gomez, S. A. and Matteson, L. C. Daytime sleepiness, performance, mood, nocturnal sleep: the eect of benzodiazepine and caeine on their relationship.Sleep, 1990, 13: 121±135.
Kingshott, R. N., Engleman, H. M., Deary, I. J. and Douglas, N. J. Does arousal frequency predict daytime function?Eur. Respir. J., 1998, 12: 1264±1270.
Kingshott, R. N., Sime, P. J., Engleman, H. M. and Douglas, N. J. Self assessment of daytime sleepiness: patient versus partner.Thorax, 1995, 50: 994±995.
Kotterba, S., Widdig, W., Duscha, C. H. and Rasche, K. Ereignis-korrelierte Potentiale und neuropsychologische Untersuchungen bei Schlafapnoepatienten.Pneumologie, 1997, 51: 712±715.
Lee, K. A., Hicks, G. and Nino-Murcia, G. Validity and reliability of a scale to assess fatigue.Psychiatry Res., 1991, 36: 291±298. Lugaresi, E. and Plazzi, G. Heavy snorer disease: from snoring to the
sleep apnea syndrome ± an overview.Respiration, 1997, 64 (Suppl. 1): 11±14.
Mackworth, N. H. Vigilance.Advancement Sci., 1957, 53: 389±393. Martin, S., Engleman, H. M., Deary, I. J. and Douglas, N. J. The eect
of sleep fragmentation on daytime function.Am. J. Respir. Crit. Care. Med., 1996, 153: 1328±1332.
Martin, S. E., Engleman, H. M., Kingshott, R. N. and Douglas, N. J. Microarousals in patients with sleep apnoea/hypopnea syndrome.
J. Sleep Res., 1997a, 6: 276±280.
Martin, S. E., Wraith, P. K., Deary, I. J. and Douglas, N. J. The eect of nonvisible sleep fragmentation on daytime function. Am. J. Respir. Crit. Care Med., 1997b, 155: 1596±1601.
Meurice, J.-C., Marc, I. and SeÂrieÁs, F. Ecacy of Auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome.Am. J. Respir. Crit. Care Med., 1996, 153: 794±798.
Mitler, M. M. Evaluation of treatment with stimulations in narcolepsy.
Sleep, 1994, 17 (Suppl. 8): 103±106.
Mitler, M. M., Carskadon, M. A., Czeisler, C. A., Dement, W. C., Dinges, D. F. and Graeber, R. C. Catastrophes, sleep, and public policy: consensus report.Sleep, 1988, 11: 100±109.
Mitler, M. M., Gujavarty, K. S. and Browman, C. P. Maintenance of Wakefulness Test: a polysomnographic technique for evaluating treatment ecacy in patients with excessive somnolence. Electroen-ceph. Clin. Neurophys., 1982, 53: 658±661.
Mitler, M. M. and Hajdukovich, R. Relative ecacy of drugs for treatment of sleepiness in narcolepsy.Sleep, 1991, 14: 218±220. Mitler, M. M., Hajdukovich, R., Erman, M. and Koziol, J. A.
Narcolepsy.J. Clin. Neurophysiol., 1990, 7: 93±118.
Mitler, M. M., Shafor, R., Hajdukovich, R., Timms, R. and Browman, C. Treatment of narcolepsy: objective studies on methylphenidate, pemoline, and protryptyline.Sleep, 1986, 9: 260±264.
NaeÈgeleÂ, B., Thouvard, V., PeÂpin, J.-L., LeÂvy, P., Bonnet, C., Perret, J. E., Pellat, J. and Feuerstein, C. De®cits of cognitive executive functions in patients with sleep apnea syndrome.Sleep, 1995, 18: 43±52.
Poceta, J. S., Timms, R. M., Jeong, D.-U., Ho, S., Erman, M. K. and Mitler, M. M. Maintenance of Wakefulness Test in obstructive sleep apnea syndrome.Chest, 1992, 101: 893±897.
Randerath, W., Gerdesmeyer, C., StroÈhlein, G. and RuÈhle, K.-H. Messung der Vigilanz mittels Fahrsimulator vor und nach nCPAP ± Vergleich zweier Simulationsprogramme mit unterschiedlicher Er-eignishaÈu®gkeit.Somnologie, 1997, 1: 110±114.
Rechtschaen, A. and Kales, A., eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of
Human Subjects.Brain Information Service/Brain Research Insti-tute, UCLA, Los Angeles, 1968.
Redline, S., Strauss, M. E., Adams, N., Winters, M., Roebuck, T., Spry, K., Rosenberg, C. and Adams, K. Neuropsychological function in mild sleep-disordered breathing.Sleep, 1997, 20: 160± 167.
Roehrs, T., Zorick, F., Wittig, R., Conway, W. and Roth, T. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders.Chest, 1989, 95: 1202±1206. Rohmfeld, R., Lund, R., Weeû, H.-G., Steinberg, R., Pritzel, M. and
SchroÈder, A. Der moderierende Eekt kognitiver Stille auf die Symptombelastung bei obstruktivem Schlaf-Apnoe-Syndrom (OSAS).3. Deutscher Kongreû fuÈr Schlaorschung und Schlafmed-izin. Leistung und Schlaf. Jahrestagung.der Deutschen Gesellschaft fuÈr Schlaorschung und Schlafmedizin, Ruhr-UniversitaÈt Bochum 19±21 Oktober 1995: 112.
Roth, T., Hartse, K. M., Zorick, F. and Conway, W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airway sleep apnea.Sleep, 1980, 3: 425±439.
Roth, T., Roehrs, T. A. and Conway, W. A. Behavioural morbidity of apnea.Semin. Res. Med., 1988, 9: 554±559.
Saletu, B., Anderer, P., Kircheis, L., KloÈsch, G., Gruber, G., Mandl, M., Parapatics, S., Stanger, E., Tschida, U. and Winkler, A. Sleep apnea and daytime vigilance objectivated by EEG-mapping.J. Sleep Res., 1996, 5 (Suppl. 1): 201.
Saletu, M., Oberndorfer, S., Anderer, P., Gruber, G., Hauer, C., Mandl, M., Popovic, R. and Saletu, B. Daytime vigilance correlates with nocturnal respiratory and arousal variables in sleep apnea patients: polysomnography and EEG-mapping investigations. Wie-ner Klin. Wochenschr., 2000, 112, 6: 281±289.
6
Sangal, R. B. and Sangal, J. M. Measurement of P300 and sleep characteristics in patients with hypersomnia: Do P300 latencies, P300 amplitudes, and Multiple Sleep Latency and Maintenance of Wakefulness Test measure dierent factors?Clin. EEG, 1997, 28: 179±184.
Sangal, R. B., Thomas, L. and Mitler, M. M. Disorders of excessive sleepiness. Treatment improves ability to stay awake but does not reduce sleepiness.Chest, 1992, 102: 699±703.
Schwarzenberger-Kesper, F., Becker, H., Penzel, T., Peter, J. H., Weber, P. and von Wichert, P. Die exzessive Einschlafneigung am. Tage (EDS) beim Apnoe-Patienten ± Diagnostische Bedeutung und Objektivierung mittels Vigilanztest und synchroner EEG-Registrie-rung am Tage.Praxis Klin. Pneumol., 1987, 41: 401±405.
Sforza, E. and Lugaresi, E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syn-drome patients: eects of chronic treatment and 1-night therapy withdrawal.Sleep, 1995, 18: 195±201.
Smolley, L. A., Ivey, M., Farkas, E., Faucette, E. and Murphy, S. Epworth sleepiness scale is useful for monitoring daytime sleepiness.
Sleep Res., 1993, 22: 389.
Spiegel, R. Sleep, sleep disorders and the regulation of vigilance in physiological and pathological aging. In: M. Bergener and S. I. Finkel (Eds)Clinical and Scienti®c Psychogeriatrics, Vol. 1. Sprin-ger, New York, 1990: 216±249.
Stepanski, J., Lamphere, J., Badia, P., Zorick, F. and Roth, T. Sleep fragmentation and daytime sleepiness.Sleep, 1984, 7: 18±26. Stradling, J. R. and Davies, R. J. O. Is it necessary to record sleep?
Sleep, 1996, 19: S251±S254.
Stradling, J. R., Pitson, D. J., Bennett, L., Barbour, C. and Davies, R. J. O. Variation in the arousal pattern after obstructive events in obstructive sleep apnea.Am. J. Respir. Crit. Care Med., 1999, 159: 130±136.
Tihonen, M. and Partinen, M. Polysomnography and Maintenance of Wakefulness Test as predictors of CPAP eectiveness in obstructive sleep apnea. Electroenceph. Clin. Neurophys., 1998, 107: 383±386.
Weeû, H.-G., Lund, R., Gresele, C., BoÈhning, W., Sauter, C. and Steinberg, R. und Arbeitsgruppe Vigilanz der Deutschen Gesellschaft fuÈr Schlaorschung und Schlafmedizin (DGSM). Vigilanz, Einschlafneigung, Daueraufmerksamkeit, MuÈdigkeit, SchlaÈfrigkeit. Die Messung muÈdigkeitsbezogener Prozesse bei Hypersomnien. Theoretische Grundlagen. Somnologie, 1998, 2: 32±41.
Weeû, H.-G., Rohmfeld, R. and Steinberg, R. Aufmerksamkeits- und Vigilanzleistung bei Hypersomnien am Beispiel des Schlaf-Apnoe Syndroms. In: C. Becker-Carus (Ed)Fortschritte der Schlafmedizin.
Aktuelle BeitraÈge Zur Insomnieforschung. Lit., MuÈnchen, 1994: 230±245.
Zeitlhofer, J., Rieder, A., Kapfhammer, G., Aull, S., Bolitschek, J., Kunze, M., Saletu, B. and Lechner, H. Die Schlafapnoe als Risikofaktor.Acta Med. Austriaca, 1995, 22: 64±68.
von Zerssen, D.Die Be®ndlichkeits-Skala.Beltz, Weinheim, 1976. Zucconi, M., Oldani, A., Ferini-Strambi, L., Calori, G., Castranovo,
C. and Smirne, S. EEG arousal pattern in habitual snorers with and without obstructive sleep apnoea (OSA). J. Sleep Res., 1994, 4: 107±112.