Peer reviewed
Performance
m
etrics
in
c
linical
trials
Intertwining Quality
Management Systems
with Metrics to Improve
Trial Quality
Liz Wool, RN, BSN, CCRA, CMT
M
anaging quality in clinical trials is the focus of daily activities that,
per the Clinical Trials Transformation Initiative (CTTI), substantiate
the clinical research community’s ability “to effectively and efficiently
an-swer the intended question about the benefits and risks of a medical
prod-uct (therapeutic or diagnostic) or procedure while assuring protection of
human subjects.”
1At the Drug Information Association’s 2011 Annual Meeting, a presenter
from the European Medicines Agency (EMA) provided a description of
“quality” as “sufficient to support the decision-making process on medicines
throughout the clinical development and postmarketing authorization.”
2With the advent of increasing protocol complexities, technological
capabili-ties, and globalization of research, a prospective, systematic, and methodical
approach to ensuring quality in clinical trials is needed and is being advocated
by regulators. Additionally, both the Food and Drug Administration (FDA) and
EMA are collaborating with all stakeholders in clinical research to define and
describe the elements of quality by design (QbD) in the context of clinical
trial conduct. QbD incorporates the elements of a quality management system
with benchmarks to the International Conference on Harmonization (ICH) in
the ICH Q7 through Q10 documents and the International Organization for
Standardization’s ISO 9000 standards for quality management systems.
In May 2012, the EMA hosted a workshop that focused on a reflection
paper by the agency’s Good Clinical Practice (GCP) International Working
Group about risk-based quality management in clinical trials. The group
requested input from various stakeholders (including ACRP) on what the
QbD elements, context, and framework are for clinical trials. Similarly, the
FDA and CTTI are launching the QbD workstream in 2012.
As clinical researchers in the 21st century, stating that we have standard
operating procedures (SOPs) and training is not defining a quality management
system. This article describes a targeted review of a quality management
sys-tem that, when adhered to in its entirety, provides the organization with steps
for defining, planning, monitoring, measuring, and continuously improving
the quality of its work, leading to the inherent ability to identify, analyze, and
address possible performance issues through the appropriate use of metrics.
Quality Management System
Rather than focus solely on GCP compliance, SOPs, processes, forms, and
training, there needs to be a renewed focus on “building quality within an
organization” that inherently possesses the culture of quality.
3A quality
This article describes
a targeted review of a
quality management
system that provides
the organization
with the inherent
ability to identify,
analyze, and address
possible performance
issues through the
appropriate use of
metrics.
A quality management system
provides the prospective, systematic,
methodical, scientifically based
frame-work to plan, manage, monitor, and
measure the quality of the organization
and its performance throughout the
clinical trial and product development
lifecycles. Specifically, the quality
man-agement system establishes the
stan-dards under which work will be
per-formed and how the organization and
personnel perform and document their
assigned clinical trial activities, duties,
and functions. An additional
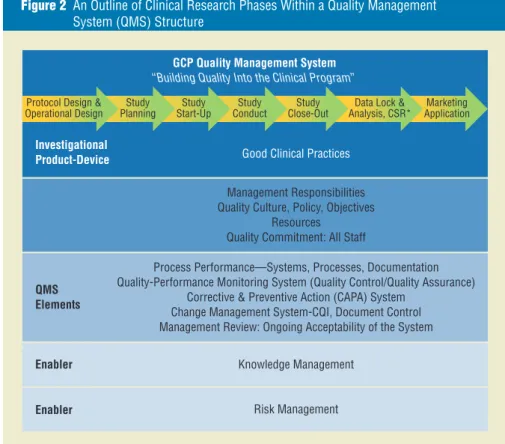
illustra-tion is noted in Figure 2, which outlines
the phases of product development for
clinical research benchmarking to
simi-lar illustrations for the ICH Q10
Phar-maceutical Quality System Guideline.
7This network system of interrelated
processes provides uniformity and
con-sistency for people and actions,
describ-ing the work performed and how the
work is documented, as outlined in
Fig-ure 3. In the clinical trials context, this
quality data obtained without
compro-mising the protection of human
sub-jects’ rights and welfare.
After implementing a quality
man-agement system, assessing, monitoring,
and measuring how well the
organiza-tion is performing to the established
standards and methods is required.
Using visual inspection and
confirma-tion, document review, data analytics
and review, and metrics, the
organiza-tion implements the foundaorganiza-tional
cor-nerstones for assessing its performance.
Elements of a Quality
Management System
The overarching framework for a
qual-ity management system is illustrated
in Figure 1, which visualizes the
criti-cal “plan, do, check, act” approach of
a committed organization to quality
through the development,
implemen-tation, and maintenance of a quality
management system.
management system sets out the
stan-dards to be achieved and the method to
meet them. The system should define
what people, actions, and documents
should be employed to conduct the
work in a consistent manner, leaving
evidence of what has happened. It may
include manuals, handbooks,
proce-dures, policies, records, and templates.
4In 1946, the International
Organi-zation for StandardiOrgani-zation (ISO) was
founded, and in 1987, it published
the first ISO 9000 standard for quality
management systems.
5Many people
believe that ISO 9000 focuses only
on manufacturing of products;
how-ever, ISO 9001:2008 was written such
that small businesses (e.g., consulting
firms) can implement ISO 9000 for
their organizations.
The ISO definition for quality states
that “the
quality
of something can be
determined by comparing a set of
inher-ent characteristics with a set of
require-ments.” With this in mind, this article will
discuss the quality management system
principles espoused in ISO 9000-9001,
extrapolating its use in the global
clini-cal research arena. Additional terms that
organizations may use to describe their
quality management system include
clinical quality system, integrated
qual-ity management, qualqual-ity management,
or total quality management.
From a practical standpoint, it is
important to understand how the
ele-ments and components of an
organiza-tion’s quality management system relate
to this article’s description of a quality
management system in order to perform
a comprehensive gap analysis. Due to
space limitations, alternative theories
and methods will not be discussed here.
Kleppinger and Ball, in their article
“Building Quality into Clinical Trials with
the Use of a Quality Systems Approach,”
describe the utility and application of
ISO 9000-9001 quality management
systems principles for clinical trial
plan-ning, execution, ongoing monitoring,
and continuous improvement during
the clinical trial lifecycle.
6The authors
assert that, even though a quality
sys-tem does not impose something totally
new on clinical research, a systematic
approach will produce a more reliable
and useful end product
—
that is,
high-Figure 1 Framework for a Quality Management System
Training Performance Dashboards Process Monitoring and Analyses Change Control Deviation Management Corrective and Preventive Action Program Process Improvement GCP Quality Assurance Unit, Annual Audit Plan Issue Escalation Management Responsibility, Management Review of QMS Metrics System Quality Management System (QMS) Framework Risk
Management Quality Policy, Quality Manual
Procedural Documents, Document
correctly per the standards outlined
in the monitoring visit report
com-pletion guidelines, monitoring visit
report template, monitoring plan, and
monitoring SOP? Did the CRA/monitor
capture critical issues, protocol
devia-tions, or violations in the monitoring
report as identified by either database
review of deviation listings or during
an onsite quality assessment visit that
was performed by his or her supervisor
or the sponsor? Additional examples
are described in Table 2.
Upon identification of a metric that
has either met or is out of range of the
predetermined tolerance limits, the next
execution of the clinical trial to the
established quality standards.
Specifi-cally, quality metrics provide the
abil-ity to measure progress to the standards
and goals defined by the organization.
Quality metrics are reported as the
“error rate,” and require predefined
tolerance limits for effective
moni-toring, measurement, and reporting
of quality and compliance signals to
the organization. Note that what most
organizations refer to as “key quality
indicators” are a subset of performance
indicators. For example, a site
moni-toring report may be completed on
time; however, is the report completed
framework establishes a reliable network
of commitments throughout the
organi-zation and business enterprise, which
each employee of and contributor to the
research site knows and understands and
to which everyone performs. Thereby,
the organization or business establishes,
maintains, and manages this network of
commitments, which supports building
quality into the clinical trial practices.
8A quality management
systems approach in
clinical research is a
further extension of
delivering quality care
for those patients who
volunteer to participate
in clinical research.
Inherent in a quality management
system is documenting the work
per-formed, evaluating deviations from the
established quality standards and
con-trols, and taking the necessary actions
for immediate and continuous process
improvement. This framework for
qual-ity is not so different from that used in
hospitals and medical institutions, which
must obtain and maintain accreditation
of their facility, per country/state
require-ments. Therefore, a quality management
systems approach in clinical research is
a further extension of delivering quality
care for those patients who volunteer to
participate in clinical research.
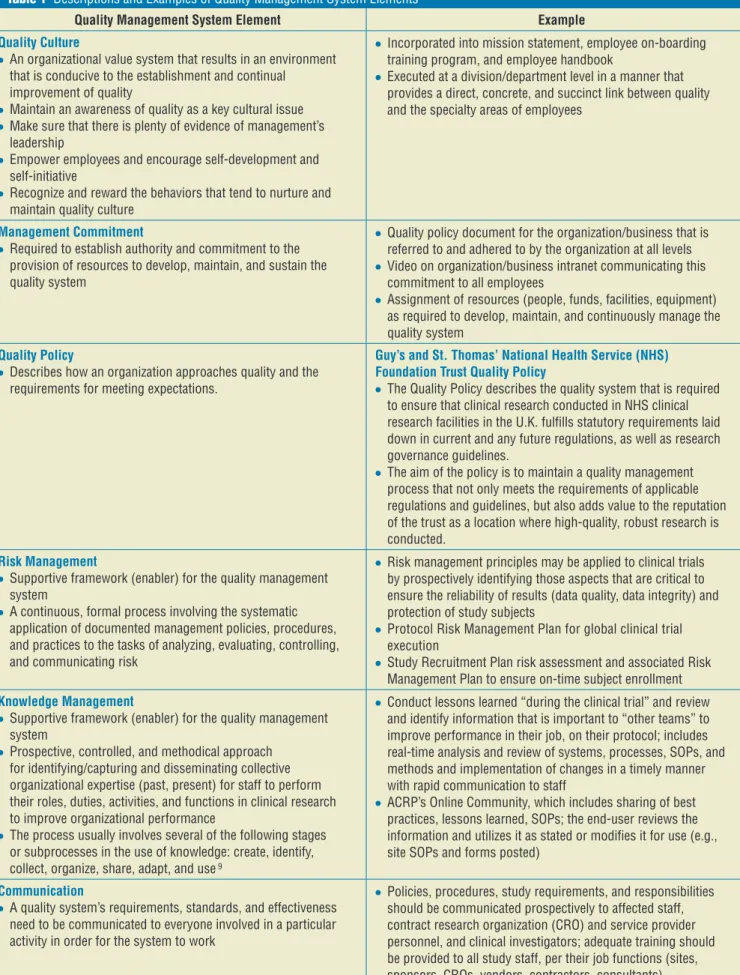
Table 1 presents a targeted
descrip-tion and applicadescrip-tion (examples) of
crit-ical aspects of both the quality
man-agement system framework and the
plan, do, check, act principles.
Metrics: Reporting
Performance of the Quality
Management System
When defined and used appropriately,
metrics are an effective tool for
moni-toring and measuring the performance
of an organization’s quality system and
Figure 3 The Network of Interrelated Processes
Figure 2 An Outline of Clinical Research Phases Within a Quality Management System (QMS) Structure
Marketing Application Data Lock &
Analysis, CSR*
GCP Quality Management System
“Building Quality Into the Clinical Program” Protocol Design &
Operational Design
Enabler Enabler
Good Clinical Practices
Management Responsibilities Quality Culture, Policy, Objectives
Resources Quality Commitment: All Staff
Process Performance—Systems, Processes, Documentation Quality-Performance Monitoring System (Quality Control/Quality Assurance)
Corrective & Preventive Action (CAPA) System Change Management System-CQI, Document Control Management Review: Ongoing Acceptability of the System
Knowledge Management Risk Management
Study
Planning Start-UpStudy ConductStudy Close-OutStudy
Investigational Product-Device
QMS Elements
People Work
Network of interrelated processes with each process made up of:
Supplies, Tools, Equipment Reports, Materials Resources, Rules, Regulations Records, Documents, Forms Activities, Tasks
Table 1 Descriptions and Examples of Quality Management System Elements
Quality Management System Element Example
Quality Culture
●
● An organizational value system that results in an environment
that is conducive to the establishment and continual improvement of quality
●
● Maintain an awareness of quality as a key cultural issue ●
● Make sure that there is plenty of evidence of management’s
leadership
●
● Empower employees and encourage self-development and
self-initiative
●
● Recognize and reward the behaviors that tend to nurture and
maintain quality culture
●
● Incorporated into mission statement, employee on-boarding
training program, and employee handbook
●
● Executed at a division/department level in a manner that
provides a direct, concrete, and succinct link between quality and the specialty areas of employees
Management Commitment
●
● Required to establish authority and commitment to the
provision of resources to develop, maintain, and sustain the quality system
●
● Quality policy document for the organization/business that is
referred to and adhered to by the organization at all levels
●
● Video on organization/business intranet communicating this
commitment to all employees
●
● Assignment of resources (people, funds, facilities, equipment)
as required to develop, maintain, and continuously manage the quality system
Quality Policy
●
● Describes how an organization approaches quality and the
requirements for meeting expectations.
Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust Quality Policy
●
● The Quality Policy describes the quality system that is required
to ensure that clinical research conducted in NHS clinical research facilities in the U.K. fulfills statutory requirements laid down in current and any future regulations, as well as research governance guidelines.
●
● The aim of the policy is to maintain a quality management
process that not only meets the requirements of applicable regulations and guidelines, but also adds value to the reputation of the trust as a location where high-quality, robust research is conducted.
Risk Management
●
● Supportive framework (enabler) for the quality management
system
●
● A continuous, formal process involving the systematic
application of documented management policies, procedures, and practices to the tasks of analyzing, evaluating, controlling, and communicating risk
●
● Risk management principles may be applied to clinical trials
by prospectively identifying those aspects that are critical to ensure the reliability of results (data quality, data integrity) and protection of study subjects
●
● Protocol Risk Management Plan for global clinical trial
execution
●
● Study Recruitment Plan risk assessment and associated Risk
Management Plan to ensure on-time subject enrollment Knowledge Management
●
● Supportive framework (enabler) for the quality management
system
●
● Prospective, controlled, and methodical approach
for identifying/capturing and disseminating collective organizational expertise (past, present) for staff to perform their roles, duties, activities, and functions in clinical research to improve organizational performance
●
● The process usually involves several of the following stages
or subprocesses in the use of knowledge: create, identify, collect, organize, share, adapt, and use 9
●
● Conduct lessons learned “during the clinical trial” and review
and identify information that is important to “other teams” to improve performance in their job, on their protocol; includes real-time analysis and review of systems, processes, SOPs, and methods and implementation of changes in a timely manner with rapid communication to staff
●
● ACRP’s Online Community, which includes sharing of best
practices, lessons learned, SOPs; the end-user reviews the information and utilizes it as stated or modifies it for use (e.g., site SOPs and forms posted)
Communication
●
● A quality system’s requirements, standards, and effectiveness
need to be communicated to everyone involved in a particular activity in order for the system to work
●
● Policies, procedures, study requirements, and responsibilities
should be communicated prospectively to affected staff, contract research organization (CRO) and service provider personnel, and clinical investigators; adequate training should be provided to all study staff, per their job functions (sites, sponsors, CROs, vendors, contractors, consultants)
Table 1 Descriptions and Examples of Quality Management System Elements (continued)
Quality Management System Element Example
Job Responsibilities and Assessment of Personnel Competencies
●
● Job descriptions are present and current, and there is a
methodology for continuously reviewing and updating them according to changes in the regulatory landscape and other job responsibilities
●
● Do personnel possess competencies, knowledge, experience,
skills, and training to execute their assigned responsibilities, duties, functions, and activities?
●
● Each position has a current and documented job description,
employee/contractor training plan, and training file
Document Control
●
● Document control is a consistent method of controlling
documents that includes version numbering, dating, issuing, and withdrawing as controlled procedures
●
● Ensures that only the current version of the procedural
document is used by all personnel
●
● Procedural documents include SOPs, informed consent
templates, and investigational product accountability logs, etc.
Quality Plans
●
● Documents specifying which procedures and associated
resources shall be applied, by whom, and when to a specific project, product, process, or contract; describes how the quality system is applied to a specific deliverable
●
● Protocol-Specific Quality Management Plan ●
● Monitoring Plan, Data Management Plan, Project Plan,
Recruitment Plan, Quality Oversight Plan of Third Parties Plan, Data Monitoring Committee Charter, Data-Safety Monitoring Plan, Annual Audit Plan
Quality Standards
●
● Organizational standards/requirements for conducting
business
●
● Regulations, guidances, guidelines, regulatory authority
inspection manuals
●
● Protocol-specific requirements for endpoint assessments (i.e., by
an MD or certified assessor), and other study-related procedures/ activities
●
● Each informed consent is obtained prior to any study-specific
procedures performed on the subject
●
● Protocol (e.g., subject enrollment criteria) ●
● Trial-specific procedures and expectations (e.g., correct data
entry per the source documentation into electronic data capture)
●
● Predefined requirements (e.g., temperature at which
investigational product must be stored) Quality Control
●
● A set of activities intended to ensure that quality requirements
are actually being met
●
● Organizational controlled, procedural documents (e.g., SOPs
[unblinding, randomization], templates, forms, job aids [flow charts, reference cards, business operations manuals])
●
● Procedural documents include: SOPs, informed consent
templates, investigational product accountability logs
●
● Sponsor’s clinical research associate (CRA)/monitor perform
onsite monitoring visits to a clinical investigator
●
● Refrigerator and freezer temperature is routinely checked/monitored
to ensure the temperature is within specified limits (requirements); this routine check/monitoring is documented in the temperature log Monitoring and Measurement of the Quality System
●
● Organization monitors customers’ perceptions of whether it
has met their requirements
●
● Suitable methods are utilized to monitor and measure the
organization’s performance
●
● Data analyses of performance (predefined/predetermined
metrics and tolerance limits)
●
● Customer report of product quality complaints ●
● Internal audits of the quality system (see quality assurance
section) Facilities and Equipment
●
● Controlled environments required to execute the protocol
●
● Clinical site’s refrigerators/freezers possess the
protocol-required temperature range with documented evidence of ongoing monitoring, calibration, and maintenance per manufacturer specifications
Quality Assurance
●
● The aspect of quality management that focuses on the
confidence that quality requirements are fulfilled
●
● Self-inspection audits of the quality system, processes,
activities, and documents to independently assure that the defined requirements/standards for the protocol/system/ process/procedure have been adhered to
●
● Conducted by defined, qualified personnel independent of the
activity; performed in a systematic manner
●
● Organization’s routine audit of the quality system ●
● Clinical study report audit ●
● Clinical investigator site audit ●
● Quality systems audit of vendor/third party ●
● Trial Master File audit
3. Which aspects of the system are
performing adequately?
4. What is ideal performance?
5. Are the improvements having
the desired effect?
Figure 4 presents some useful
guide-lines for successfully using and
evalu-ating quality systems metrics.
10Summary
Effective execution of clinical trials
featuring “quality built within” requires
assumptions reference in the
develop-ment of the risk managedevelop-ment plan and
revise that plan accordingly? Is this an
issue we did not expect, thereby
neces-sitating the need for the development
of a new risk management plan?
Quality systems metrics
analy-sis focuses on the following critical
questions:
101. How is the system performing?
2. Which aspects of the system
are performing poorly or need
improvement?
step is the evaluation of the metric and
any relevant companion metrics. This
analysis supports a robust and
compre-hensive root cause analysis as to what
the issue is, the issue’s impact, and what
corresponding directed and focused
CAPA steps and continuous
improve-ment measures should be taken.
Further, metrics need to be
evalu-ated with the associevalu-ated risk
manage-ment plan and actions analyzed: Did
our mitigation plans work? Should we
implement our predefined contingency
plans? Do we need to reevaluate the
Quality Management System Element Example
Deviation Management
●
● Supports learning in the organization through the identification,
recording, and investigation of activities that are not performed correctly or as planned, and provides the framework on how to investigate, plan, and change the way activities are performed
●
● Protocol deviation log maintained by the site and by CRAs/
monitors
Corrective and Preventive Action (CAPA) Program
●
● Corrective action aims to address and manage identified areas
of noncompliance and nonconformity (an issue or problem) by investigating the “root cause” in order to accurately eliminate it
●
● Preventive actions aim to establish proactive methods/steps/
actions to foresee any issues and to prevent them from occurring
●
● Incorrect version of the informed consent used for a study
subject (corrective action: current version of consent in the file)
●
● Subject’s investigational product dose not adjusted per the
results of their liver or renal values, as required by the protocol (preventive action: checklist, per patient visit, outlining requirements)
Continuous Improvement
●
● The organization shall continually improve the effectiveness
of the quality system through the use of the quality policy, quality objectives, audit results, analysis of data, CAPA program, and management review
●
● Build on the knowledge “known” and “learned” to make
proactive improvements in individual trials and across all trials, and in the business enterprise (close correlation to knowledge management for the organization)
●
● Use of audit findings, audit conclusions, analysis of data,
management reviews, and deviation management to improve the quality system/organization
●
● CAPA plans implemented as a result of audit conclusions, audit
findings, internal monitoring of systems/processes and quality system by staff, CRA/site monitor feedback
Issue Escalation
●
● The issue escalation process describes how the project
identifies, tracks, and manages issues and action items that are generated throughout the project life cycle; it also defines how to escalate an issue to a higher level of management for resolution and how resolutions are documented
●
● Unanticipated issues and action items are assigned to a
specific person for action and are tracked to resolution
●
● Cases of suspected scientific/ethical misconduct and/or
fraud are escalated within 24 hours to the compliance/quality assurance department and senior management for investigation
Management Review of the Quality System’s Performance
●
● Senior management’s overarching responsibility for review
and analysis, at predetermined intervals, of the functioning/ adequacy of the quality system utilizing key indicators of performance, quality, and revenue
●
● Has the quality system provided management with
the information to reassure them of compliance to the organization’s quality system?
●
● Through the assessment of metrics (performance, quality
indicators), does the organization need to add anything or change anything in the quality system to meet organizational quality objectives?
●
● Biannual meeting and review of the quality system utilizing
metric reports and performance dashboards Table 1 Descriptions and Examples of Quality Management System Elements (continued)
References
1. Clinical Trials Transformation Initiative. Available at https://www.trialstransforma tion.org/scope.
2. Sweeney F. 2011. Defining Quality in Clinical Trials. DIA Annual Meeting, 2011.
3. Cameron K, Since W. 1999. A framework for organizational quality culture. Quality
Man-agement Journal, 1999, pp. 7–25.
4. BARQA. 2010. Quality Systems Workbook.
Available at www.barqa.org (free download
available).
5. International Organization for
Standardiza-tion; www.iso.org.
6. Kleppinger C, Ball L. 2010. Building quality into clinical trials with use of a quality sys-tems approach. Clinical Infectious Disease
Journal 51(Supp. 1): S111-S116.
7. ICH Q10 Pharmaceutical Quality System
pre-sentation, www.ich.org/products/guidelines
/quality/quality-single/article/pharmaceutical -quality-system.html.
8. Burrow D. CDER BIMO Warning Letters as Case Studies-Building Quality in Clinical Tri-als. Presentation, ACRP Global Conference, 2012.
9. American Productivity and Quality Center Publication. 2000. Stages of
Implementa-tion: A Guide for Your Journey to Knowledge Management Best Practices. Houston, Texas.
10. Zuckerman D. 2006. Pharmaceutical R & D Metrics. Gower Publishing Ltd., England.
Additional Sources
Cianfrani C, Tsikais J, West J. 2009. ISO 9001:
2008, Explained. Milwaukee, Wis.: ASQ
Quality Press.
Clinical Trials Transformation Initiative.
Develop-ing Effective Quality Systems in Clinical
Tri-als: An Enlightened Approach; www.ctti.org.
ISO 9001:2008. Quality Management Systems—
Requirements; www.iso.org.
Ribière V, Khorramshahgol R. 2004. Integrating total quality management and knowledge management. Journal of Management Sys-tems 16(1).
Toth-Allen J. 2012. Building Quality into Clinical Trials: An FDA Perspective. FDA Webinar, 04 May 2012.
Tricker R. 2010. ISO 9001:2008 for Small
Busi-nesses. Burlington, Mass.: Elsevier.
Liz Wool, RN, BSN, CCRA, CMT, has 22 years of experience in the clinical research industry. She is presi-dent and CEO of QD-Quality and Training Solutions, Inc. (QD-QTS), a clinical quality systems, training, auditing, and CRO-vendor oversight consulting firm providing ser-vices to institutions, investigators, sponsors, and CROs. QD-QTS has offices in San Bruno, Calif., and Franklin, Tenn. A certified Master Trainer and instructional designer, she is also a member of ACRP’s Association Board of Trustees and Editorial Advisory Board. She can be reached at lizwool@qd-qts.com.
ity management system principles
described in this article represent the
framework and components of
progres-sive 21st century practices for use by
all stakeholders in the clinical research
enterprise.
a prospective, systematic approach to
quality through the implementation
of a robust and comprehensive
qual-ity management system whereby
met-rics provide the ability to measure an
organization’s performance. The
qual-Figure 4 Guidelines for Using and Evaluating Quality Systems Metrics Successfully Using Quality Systems Metrics
●
● Identify the stakeholders and the metrics for their activities ●
● Determine the metrics required for periodic management review of the quality
system
●
● Determine how the metrics will change the way you perform your business ●
● Select the right metrics and rationalize the calculation rules (do you have this
requisite expertise inhouse?)
●
● Determine how you will use the results ●
● Define the reporting mechanisms (scorecard, dashboard, real-time reports) and
issue escalation pathways both internally and externally with other parties
●
● Determine the right source of the data ●
● Collect the data and validate the results ●
● Continuously evaluate the metrics (obtain feedback and study the utility of your
measurement)
●
● Continuously improve the process ●
● Communicate the results
Successfully Evaluating Quality Systems Metrics
●
● What does this metric measurement mean? ●
● What will I do with this information? ●
● How will this communicate a “quality performance” threshold or tolerance limit
requiring investigation or evaluation?
●
● Do I need another metric to get the “whole picture”? ●
● Identify any “companion metrics” to assist with the evaluation
Table 2 Examples of How Quality Indicators are Subsets of Performance Indicators
Performance Indicator Quality Indicator
Case report forms (CRFs) completed and submitted on time > 90%
CRF query rate < 5% Imaging study completed on time >95% Image readability > 98% Blood samples collected on time > 95% Quantity not sufficient < 1% Staff trained on SOPs prior to performing
responsibilities/tasks in SOP > 95% SOP deviation rate < 3% Staff trained to the protocol-investigational
plan prior to performing delegated study tasks > 95%
Protocol deviations-violations < 2%
Site staff delegated tasks prior to study-start > 95%
Staff delegated responsibilities correctly per licensure and/or certification requirements per state/country 100%
Subjects enrolled on time > 85% Subjects enrolled meet enrollment criteria 100%
Informed consent obtained 100% Subjects consented with the correct ICF
version 100%
Informed consent obtained 100% Subjects consented prior to study-specific