Indian J.Plant Physioi., Vol. XXX, No.4, pp. 321-331, 1987 , ,
/
PHYSIOLOGICAL SIGNIFICANCE'OF
ZINC
AND IRON-RETROSPECT AND PROSPECTO.K.OARO·
Department of Plant Physiology, Institute of Agricultural Scienc~s", Banaras Hindu University, Varanasi-al 005
The constraint arising out of iron and zinc deficiency in the Indiall' agricultural field is recurringly expanding owing to a general negligence in the
use:
of such vital elements. The level of mineral nutrients in soil, or of an externally applied fertilizer, is an important factor that seems to influence the growth and yield potentiality of a crop. If the level of an element is lower than the optimum, the growth will not be up to the mark, and the plants may show symptoms of deficiency. On the other hand, ifthe soil nutrient is above the optimum requirement of crop, it may develop signs of toxicity. So it is important to find out a suitable level of nutrient elements for different crops. Coupled with these, the processes. involved in senescence are important because they occur during grain fill, and evidences suggest that earl} senescence may be yield limiting. Iron is involved ill energy producing and utilizing processes in plants and is important in many redox. reactions. In higher plants the essentiality of Fe for development and maintenance of photosynthetically active tissues has been recognized because Fe is involved in the synthesis of chrolophyll (Miller et al., 1984). Presently, Zn is also recognised as an essential .compotent in several dehydrogenases, proteinases etc. A large number of growth and metabolic effects of Fe and Zn remain unexplained relative to their roles as biocatalysts. Furthermore, iron is an integral part of the nitrogen fixing complex, i.e., nitrogenase, leghaemoglobin and ferredoxins (Evans and Russel, 1971). While presenting the work done at Banaras Hindu University with my reseerch colleague Dr. A, Hemantaranjan, I would cover the physiological significance of zinc fertilization in wheat and the effect(s) of iron and zinc fertiliza tion on senescence in french bean (PhaseolWl vulgaris L.) with reference to nodula tion and nitrogen fixation.EtI'eet of various levels of zinc on the growth and yield of wheat Triticum aestivum (1..)
Fertilization of zinc in the form of zinc sulphate produces distinct improve ment in the growth and yield of wheat plants. As shown in Table 1, there are
I
~:':~~:~~~F'
J
322
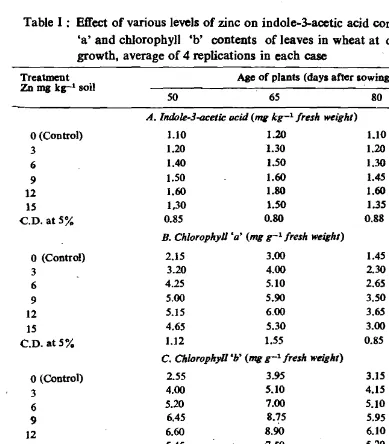
O.Jt.·GARGTable 1: Effect of various levels of zinc on indole-3-acetic acid content, chlorophyll 'a' and chlorophyll 'b' contents of leaves in wheat at different stages of growth, average of 4 replications in each case
Treatment Age: of plants (daya after aowiq)
Zn IIII kg-I soil
SO 65 80 95
A. Indole-3-ocetic ucid ("" kg-I fresh weight)
o
(Control) 1.10 1.20 1.10 0.003 1.20 1.30 1.20 1.10
6 1.40 1.50 1.30 1.20
9 1.50 1.60 1.45 1.25
12 1.60 1.80 1.60 1.45
15 1,30 1.50 1.35 1.20
C.D. at5% 0.85 0.80 0.88 0.65
B. ChlorophyU 'a' (mg g-l fresh weight)
o
(Control) 2.15 3.00 1.45 0.003 3.20 4.00 2.30 1.00
6 4.25 5.10 2.65 1.15
9 5.00 5.90 3.50 1.40
12 5.15 6.00 3.65 1.45
IS 4.65 5.30 3.00 1.20
C.D.at5% 1.12 l.S5 0.85 078
C. Chlorophyll 'b' (mg g-1 fresh weight)
o
(Control) 2.55 3.95 3.15 0.853 4.00 5.10 4.15 2.00
6 5.20 7.00 5.10 2.60
~ 6.45 8.?5 5.95 3.50
12 6.60 8.90 6.10 3.55
IS 5.45 7.50 5.20 3.00
C.D. at 5% 1.00 1.22 1.18 1.00
r
a
PHYSIOLOGICAL SIGNIFICANCE OF ZINC AND IRON 323
Table II : Effect of various levels of zinc on the area of leaves and total dry matter content (g) of wheat at different stages of growth; average of 4 replications in each case
Treatment Age of plants (days after sowing)
Zn mg kg-l soil
50 65 80 95
A. Areao/lea/(cm)
o(Control)
33.20 60.62 69.75 73.063 42.07 72.41 84.60 89.46
6 49.64 76.85 95.87 103.33
9 59.95 111.65 115.63 117.00
12 61.27 113.25 121.86 124.10
15 44.49 87.75 92.11 94.00
C.D. at 5% 0.08 0.13 0.81 0.99
B. Total dry Weight (g)
o(Control) 5.30 11.55 16.40 18.91
3 6.16 12.47 16.83 20.05
6 7.65 14.78 19.63 23.54
9 9.46 17.90 24.38 27.40
12 10.20 18.58 25.60 27.97
15 7.84 15.32 18.86 21.25
C.D.at5% 2.51 2.27 4.76 2.39
Table III : Effect of various levels of zinc on certain yield characters of wheat at the time of harvest; average of 4 replications in each Case
Treatment Murober of ear Length of ear Yield of seeds Bulk weight
Zn rog kg-l soil heads heads per plant of 1000
(em) (g) seeds (g)
o(Control) 10.00 9.74 13.58 38.34
3 11.00 11.25 18.65 44.10
6 12.00 11.50 19.95 47.05
9 15.00 12.35 22.78 52.90
12 16.00 12.45 22.95 53.25
15 13.00 11.50 20.00 45.38
C.D. at 5% 1.28 1.70 0.06 0.16
324
O.L'GAllGthe 'enhanCed levers of zinc which might have favoured chlorophyll content in leaves even after the post-flowering stage (Voica. 1969). Indole-3-acetic acid is
also responsible for the inhibition ot' the destruction of chlorophyll 'a' and 'b' {Mishra and Biswal, 1980; Hemantaranjan and Garg, 1984). Obviously, these phYSiological chmrges could have led the plants tOr better photosynthesis for a longer period and resulted into the greater dry matter production and improved ,grain yield. Yield decreased at higher levels of zinc application, as it was observed at IS rng kg-I soil of zinc, which may be due to the toxic effect of zinc at this level.
In the light of vital role played by zinc in plant metabolism and of results ()f this investigation, if there is greater availability of zinc at cellular level within an optimum range (9 and 12 mg kg-I soil), the growth and yield potentiality of .of plants will stand improved.
hoo and zioc fertilizatioD 011 leaf seoeseeaee iD freoeb beea (Phaseolus .ulgaris L.)
The results indicate that chlorophyll 'a' and 'b' IAA and nitrate reductase activity in leaves increased with increased concentrations of Fe. However, a .decline in the above physiological changes were noted at 20 mg kg-I soil of Fe (Table 4 and S). These were found lObe increased at the levels of zinc application too. Increases in the amount of chlorophyll 'a' and 'b', indole-3-acetic acid concentrations and nitrate reductase activity were recorded in leaves treated with the combination of Fe and Zn. The combinations of 5 Fe
+
5 Zn Jp.g kg-I soil .howed maximum increases in all the parameters noted above. Iron @ 10 mg kg-1PHYSIOLOGICAL SIGNIFICANCE OF ZINC AND UlON 325
Table IV : Effect(s) of Fe and Zn fertilization on chlorophyU 'a' and 'b' of french bean leilves at three dates of growth
Treatment Chlorophyll 'a' Chlorophyll 'b,
Fe' Zn
20 days 40 days 60 days 20 days 40 days 60 days
mg kg-1 soil mg g-1 fresh weight mg ar1 fresh weight
0
o
(Control) 1.30 2.40 1.80 2.25 3.85 3.055 0 2.00 3.30 3.00 2.95 4.95 4.50
10 0 2.90 4.00 3.65 3.85 5.15 5.90
20 0 1.90 3.10 2.90 2,80 2.25 3.95
0 5 J.85 3.00 2.75 2.70 4.15 3.80
0 10 1.90 3.10 2.80 2.85 4.20 3.85
0 20 2.00 3.25 2.96 2.95 4.40 4.00
5 5 3.50 5.90 5.60 4.45 7.05 6.65
]0 10 3.15 5.00 4.75 4.15 6.15 5.80
20 20 3.00 4.50 4.20 3.95 5.65 5.lS
C.D. at 5% 0.90 1.20 1.00 0.90 1.30 ],05
Table V : EffcctsCs) of Fe and Zn fertilization on indole-3-acetic acid and nitrate reductase activity of french bean leaves at different stages of growth
Treatment Indole-3-acetic acid Nitrate reductase activity
Fe Zn
20 days 40 days 60 days 20 days 40 days 60 days
mgkg-l soH mg g-l fresh weight p. mol h-l ar1 fresh wt
0
o(Control)
0.96 1.21 0.91 0.30 1.32 1.225 0 1.10 1.42 1.31 1.21 2.33 2.21
10 0 1.13 1.44 1.38 1.41 2.66 2.57
20 0 1.08 1.40 1.33 1.07 2.26 2.16
0 5 1.20 1.56 I.4S 1.00 2.10 2.02
0 10 1.28 1.63 1.51 1.04 2.20 2.11
0 20 1.33 1.67 1.58 1.06 2.23 2.14
5 5 1.51 1.88 1.81 2.12 3.48 3.39
10 10 1.58 1.94 1.80 2.10 3.44 3.33
20 20 1.44 1.71 1.60 2.06 3.40 3.21
C.D.atS% 0.90 0.36 0.22 0.31 0.39 0.41
activity and the formation of ferredoxin. Ferredoxin may be necessary to activate the a-aminolevulinic acid (ALA) synthesizing enzyme. With Fe-deficient plants, ferredoxin would be limiting and could directly affect chlorophyll biosynthesis
(Miner et aI" 1984). The increases in the level of IAA might have delayed the senescence of leaves in Zn- and Fe-treated plants since IAA is known to inhibit
~hlorophyll· loss during senescence in wheat (Mishra and Biswal, 1980;
q ".
''F-;;;.:'~c~~'':;::;;c':~~ptb
1
I
!
326 O.K. GARG
plants than Fe-treated plants may have been related with Zn-involvemcnt in auxin metabolism (Takaki and Kushizaki, 1970). Further, maximum nitrate reductase activity at the 4O-day stage of growth may have been because of rapid growth and requirement fer higher energy to reduce N. Nitrate reductase in an Fe-containing enzyme and Fe may play a key role in changing the oxidation state through electron transfer (Hall, 1977; Smith, 1984). A marked reduction in nitrate reductase activity of control plants indicated the importance of Fe and Zn individually or in combination for the maintenance of nitrate reductase activity in plants. This led to the conclusion that iron alone or in combination with zinc applied contri buted to the inhibition of senescence in french bean plants.
boa and zinc fertilization in relation to nodulation aDd nitrogen fixation in french bean
Iron alone (at a concentration of 5 or 10 mg kg-1 soil) as well as in combination with the idential concentration(s) of zinc, initiated significant nodule formation (Table 6). The nudule processing under the laboratory conditions have shown the appearance of N:~-fixing bacteroids Rhizobia sp.) inside the newly formed nodules. As a matter of fact, french bean plants grown in Varanasi soils. lack nitrogen-fixing nodules. The availability of iron and zinc in the soil is not more than 0.60 and 2-3 mg kg-1 soil respectively, which is near the deficiency
level in the soil. During the course of investigations no exogenous supplementation of Rhizobium was made in the experimental soil. At a threshold level of 5 mg kg-I soil the two salts (ferrous sulphate and zinc sulphate) exhibited maximum nodula tion and an increase in the leghaemoglobin content. Fe at 5 and 10 mg kg-I soil
Table VI: Effects(s) of Fe and Zn on nodule number, dry matter yield and their leghaemoglobin content in french been at two dates of growth
Treatment Mumber of nodules Dary matter yield Lcghaemoglobin
Fe Zn
40 days 80 days 40 days 80 days 40 days 80 days
mg kg-1 soil 4 plants-1 g pOt-1 mg 4 plants-1
0
o
(Control) 0.0 0.0 0.0 0.0 0.0 0.05 0 30.0 46.0 0.10 0.95 0.06 0.61
10 0 47.0 59.0 0.19 1.10 0.12 0.32
20 0 21.0 30.0 0.07 0.77 0.03 0.49
20 5 14.0 22.0 0.05 0.41 0.02 0.33
0 10 19.0 27.0 0.06 0.60 0.03 0.52
0 20 21.0 34.0 0.07 0.79 0.03 0.52
5 5 59.0 76.0 0.26 1.30 0.18 1.04
10 to 54.0 70.0 0.22 1.18 0.16 0.89
20 20 51.0 66.0 0.21 1.13 0.15 0.84
r
PHYSIOLOGICAL SIGNIFICANCBOF ZINC AND IRON 327
.and Zn at 5, 10 and 20 mg kg~1 soil showed a trend of increase in the number of nodules, dry matter yield and leghaemo81obin content. In contrast, Fe (@ 20 mg kg~1 soil) alone or in combination with Zn (@ 20 mg kg-I soil) revealed a consider
able decrease in the above at both the stages.
In the present investigation a significant increase in total dry matter and
in l'il'O nitrogen fixation were also found on the addition of either of the two salts
(alone or in combination) at a concentration of 5 or 10 mg kg-I soil (Table 7). at all the three stages of growth. Finally. at harvest, the data recorded for the yield attributes were also better over the control (Tab1e 8) and specially at a concentra 1ion of 5 or 10 mg kg-1 soil of the two salts (alone or in combination.
The increases in nodulation and leghaemoglobin content at different levels of Fe and Zn are probably associated with their specific physiolopical roles in -plant and Rhizobium growth up to a certain level; the decrease in activities with the higher Fe and/or Zn levels might be due to a possible inter-molecular inter ference with the plant/microbe nutrition. Rai et al. 0984) reported that iron and
its inter relationship with other micronutrients is an important factor controlling 1he nitrogenase-make up and activity inside the nodules. Extrinsic factors, which limit the synthesis and functions of leghaemoglobin, ferredoxin and active iron (Fe++) of nodules, are of great importance. The in vivo nitrogen fixation might be due to the fact that at the levels of initial fixation of dinitrogen, the energetic -electrons required for the reduction are carried by ferredoxin, a low molecular weight protein containing several per cent iron (Dart, 1979). It is all the more important to mention at this point that nitrogenase activity is due primarily to an iron-molybdenum (Fe-Mo) co-factor (Pienkos et al., 1977 and Shah el al., 1977) which is an active site of component-I of nitrogenase and the site to which molecular nitrogen binds and is subsequently reduced during Nz-fixation {Shah el al., 1978}. Naturally then, an Fe-deficiency below the critical level is expected to block the formation of nitrogenase enzyme precursor. Therefore, on supplementation with a readily assimilable Fe-salt. the Fe
deficiency was made up leading to possible activation of the nitrogenase complex • .and hence the significant nitrogen fixation. Such roles of exogenous Fe has already been examined in a Nz-fixing photosynthetic microbial system (Vaishampayan,
1984)•.
The fore going discussion stimulate the idea that iron may affect the produc 1ion of nitrogen by french bean plants in three ways. It may act directly on the
initiation, development and enzymatic functions of nodules, it may influence the .efficiency of host~Rhizobium symbiosis, or it may play an essential role in plant'
j ; J-.
328 O.K. GAllO
Table VII : Effectll(s) of Fe and Zn fertilization on the total dry
matter
yield and total nitrogen content in french bean at different stages of growthTreatment Total dry matter Total oitropn
Fe Zn
40 days 80 days 106 days 40 days 80 days 106 days
(Maturity) (Maturity)
Il1I q-l soil
0 ·0 9.72
g 3 plants-1
23.94 36.00 50.00
m, 3 plants-1
191.20 372.50
5 0 12.96 33.31 51.13 60.60 300.10 603.60
10 0 18.92 37.11 54.00 67.20 326.00 655.00
20 0 11.92 32.14 45.41 58.50 275.50 556.80
0 5 12.80 33.00 47.44 54.39 251.SO 507.30
0 10 ·.5.92 36.13 51.60 57.00 265.00 538.60
0 20 13.90 36.00 51.43 58.10 270.50 543.80
5 5 27.20 ·48.35 63.00 74.80 354.00 724.90
.0 10 24.88 45.66 60.61 72.00 348.00 702.60
20 20 22.72 42.24 57.13 69.00 335.00 682.30
CD. at 5% 2.20 1.90 1.98 3.10 4.10 11.10
Table VIII: Effect(s) of Fe and Zn fertiliZJltion on certain yield characters of french bean
Treatmcnt Number of pods Number ofseeds Weight ofseeds Wci8btof
Fe Zn per plant per pod per plants (g) 1000 seeds (g)
mg q-l soil
I
o(Control)
4.00 4.00 6.15' 410.205 0 6.00 5.00 14.00 463.00
10 0 7.00 5.SO 18.40 479.00
20 0 6.00 4.50 12.15 442.50
0 5 6.00 5.00 14.98 440.00
0 10 6,00 5.00 16.00 446.00
0 20 7.00 5.50 18.00 451.00
5 5 10.00 6.00 32.20 538.80
10 10. 9.00 6.00 28.24 529.20
20 20 6.50 5.00 16.71 508.00
C.D. at5% 1.80 2.00 3.10 49.00
PHYSIOLOGICAL SIGNIFICANCE OF ZINC AND IRON
element to fulfil this need. Its saturated d orbitals and small size favour tetra hedral complexes which give importance of zinc by taking part in various processes of plant metabolism. This is very clear from the present experimental findings, especially when zine was combined with iron. This lead to the conclusion that the french bean plants which grow in the soils of Varanasi lack the N2-fixing nodules. This may be in response to the proper nutritional deficiency apparent from the fore going experiments. The addition of various levels of Fe and/or Zn to Varanasi soils created a condition conducive to the formation of nodules morphologically similar to those present in the legume plants of other areas. Our microbial culture experiments have confirmed the bssociation of some Ni1ixing Rhizobium strain in these nodule$. and the phenomena clearly indicate the existence of Rhizobia in and/or around the Varanasi soil. However under specific nutritional state of tbe soil. as stated above, these Rhizobia can undergo a symbiotic relation with bean plants for a significant yield of nitrogen and can finally increase the yield of french bean plants (Table 8) by the efficient nitrosen f>upply to the photosynthetic parts and delaying senescence of leaves.
CONCLUSIONS
In spite of the specific and significant role(s) of iron and zinc in plant metabolism zinc and/or iron deficiency in crop plants is prevelent in Indian agricultural fields. During the last few years some critical experiments were performed with the cereal crop wheat and pulse crop french bean with the applica tion of zinc (in wheat) and zinc and iron (in french bean) in the deficient soils of Varanasi (Zn-availability-O.60 mg kg-1 soil and Fe-availability-2.0 to 3.0
mg kg-1 soil). The effect of various levels of zinc on the growth and yield of
wheat proved that increasing levels of zinc upto 12 mg kg-1 soil provided required
availability of this element to wheat plants and thereby improved their growth and yield.
330 O.K. GAllG
On the basis of the conclusions, discussed, it may be suggested that zinc and iron command a great significance in maintaining a 'physiological balance' in crop plants. The growers should, therefore, take care that these nutrients are not <lecificient in the soil.
It is optimistically expected that major advances in combatting zinc and iron problems will continue to be made in the coming years by utilizing genotypic variation. The door that has been opened by such investigations will permit the researchers to examine the physiological significance and practicability of zinc and iron nutrition in other economic crops.
REFERENCES
OarkSOIl, T. and Hanson. B.J. (1980). 'I'b8 mineral nutrition of higher plants. Ann. Rev. Plant Physiol., 31 : 239·298.
Dart, P. 1979.10 : A Treatise on Dinitropn FIXation, Section UI (R.W.H. Hardy and W.S. Silver Eds.). lohn Wiley and Sons, Inc., New York, p. 419.
Evans. H.J. and Russel, S.A. (1971). lh : The Chemistry and Biochemistry of Nitrogen fixation, l.R. Postgate (Ed.), Plenum Press, London, pp. 191-215.
(larg, O.K., Hemantaranjan A. and Ramesh. C. 1986. Effect of iron and zinc fertilization on senescence in french bean (Piroseolus vulgaris L.). I. Plant Nutr., 9: 251-266.
Hall, D.G. 1977. Iron-Sulphur proteins and energy conversion systems. p. 67-80. In R. Buvet, J.J. Allen and J.R. Massue (ed). Living systems as energy con'ferters. North Holland Publ., Amsterdam.
Han, N.P., Keys. J.A. and Merrett, J.M. (1978). Ribulose di-phosphate corboxylase protein during flag leaf senescence. I. Exp. Bot., 29 : 31-37.
Hemantaranjan, A. and Garg. O.K. (1984), Effect of zinc fertilization on the senescence of wheat varieties. Indian I. Plant Physiol., Z8 : 239-246 •
.Jacobson, B.S., Pong, P. and Health, R.L. (1975). Carbonic anhydrase of spinach. Studies on its location, inhibition and physiological fonction. Plan t Physiol., SS : 468-474.
Miller, G.W., Pushnik, J.C. and Welkie G.W. (1984). Iron chlorosis, a world wide problem, the relation of chlorophyll biosynthesis to iron, I. Plant Nutr., 7 : 1-22.
Misbra, A.N. and Biswal, U.C. (1980). Effect of phytohormones on chlorophyll degradation during agini of chloroplasts in vivo and in vitro. Proto plasma. lOS: 1-8.
Patterson, T.G. and Moss, D.N. (1979). Senescens in fiield grown wheat. Crop Sci.,
l' :
635-640.. Peoples, M.B. and Dallings, M.J. (1978). Degradation of ribulose 1-5 diphosphate carboxylaseby proteolytic enzymes from crude extracts of wheat leaves. Planta, 188: 153-160.
Pienkos. P.T., Shan V.K. and Brill, W.J •. (1977). Molybdenum cofactor from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc. Nat. Acad. Sci. U.S.A., 74: 5468-5471.
PHYSIOLOGICAL SIGNIFICANCE OF ZINC AND IllON 331
Rai, R., V. Prasad, Choudhary S.K. and Sinha. N.P. (1984). Iron nutrition and symbiotic nitrogen fixation of lentil (Lens cuJinaries) genotypes in calcareous soil. J. Plant Nutr., 5 : 905
,
i 913.Rawlings, I. Shah. V.K., ChisneIl, J.R. Brill, W.l. Zimmerman, R. E. Munk and W.H. Orme
Jobnson (1977). Novel metal cluster in the iron-molybdenum co-factor ofnitrogena~ (nitrogen-fixation). J. Bioi. Chem.,2.53: 1001-1004.
Scrutton, M.C., Wu, C.W. and Goldthwait, D.A. (1971). The presence and pOssible role of zinc
in the RNA polymerase obtained from E. coli. Proc. Nat. Acad. Sci., U.S., 68 : 2497 2501.
Shah, V.K., Cbisnell l.R. ahd Brill, W.l. (1977). lsnlation of an iron-molybdenum co-factor from nitrogenase (nitrogen-fixation). Proc. Nat. Acad. Sci., U.S.A., 74 : 3249-3253.
Shah, V.K., Chisnell J.R.land Brill, W.J. (1978). Acetylene reduction by the iron-molybdenum co-factor from nitrogenase. BiQchem. Biophys. Res. Commun., 81 : 232-236.
Smith, B.N. (1984). Iron in higher plants: storage and metabolic role. J. Plant Nutr., 7 : 759-766.
Takaki, H. and Kushizaki. M. (1970), Accumulation offree tryptophan and tryptnmine in zinc. deficient maize seedlings. Plant and Cell Physiol., Z : 793-804.
Tsui, C. (1948). The role of zinc in auxin synthesis in tomato plants. Amer. J. Bot., 3S : 172-179.
Vallee, B.L. and Wacker, W.E.C. (1970). In 'Proteins' (H. Neurath ed.) 2nd ed., S. Academic
Press, New York.
Vaishampayan, A. (1984). Copper -iron interactions. in a N1·fixing cyanobacterium. J. Plant Nutr.~
7: 567-573.
Voica, C. (1969). Influence of B, Mn, Cu, Zn and Mo on some of the phyyioiogical processes of seedlings of barley cv. Bruker. Studii Cere. BioI., Zl : 151-155.