SPECIAL ARTICLE
Ischemic Perinatal Stroke: Summary of a Workshop
Sponsored by the National Institute of Child Health
and Human Development and the National Institute
of Neurological Disorders and Stroke
Tonse N. K. Raju, MD, DCHa, Karin B. Nelson, MDb, Donna Ferriero, MDc, John Kylan Lynch, DO, MPHb, and the NICHD-NINDS Perinatal Stroke Workshop Participants
aNational Institute of Child Health and Human Development,bNational Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland; cDepartments of Neurology and Pediatrics, University of California, San Francisco, California
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
Ischemic perinatal stroke is a disorder associated with significant long-term neu-rologic morbidity. With an estimated incidence of 1 in 2300 to 5000 births, stroke is more likely to occur in the perinatal period than at any time in childhood. The incidence of ischemic perinatal stroke ranks second only to that of strokes in the elderly population. Although ischemic perinatal stroke is a well-recognized disor-der, many aspects remain to be studied. There is no consensus on its terminology, definition, or classification. Several risk factors have been identified, but their precise roles in causing stroke are not well understood. There are no reliable predictors of ischemic perinatal stroke on which to base prevention or treatment strategies. To review these important issues and propose a research agenda, the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke convened a workshop in August 2006. This article provides a summary of the workshop.
www.pediatrics.org/cgi/doi/10.1542/ peds.2007-0336
doi:10.1542/peds.2007-0336
Key Words
cerebral palsy, diseases of pregnancy, focal cerebral infarction, congenital hemiplegia, hypoxic ischemic encephalopathy, intracranial hemorrhage, newborn, porencephaly, seizures, thrombotic disorders
Abbreviations
IPS—ischemic perinatal stroke CP— cerebral palsy MCA—middle cerebral artery NICHD—National Institute of Child Health and Human Development
NINDS—National Institute of Neurological Disorders and Stroke
PPIS—presumed perinatal ischemic stroke CT— computed tomography
DWI— diffusion-weighted imaging
Accepted for publication Apr 11, 2007
Address correspondence to Tonse N. K. Raju, MD, DCH, 6100 Executive Blvd, Room 4B03, Bethesda, MD 20892. E-mail: rajut@mail.nih. gov
S
TROKES THAT OCCURin the perinatal period are not rare; in fact, the perinatal period ranks second only to adult age groups in the incidence of stroke. It is estimated that the incidence of ischemic perinatal stroke (IPS) ranges between 1 in 2300 to 1 in 5000 births1–8andaccounts for 30% of children affected with hemiplegic cerebral palsy (CP) who were born at term or late-preterm gestations.4 Thus, IPS is the leading known
cause for CP.
IPS is slightly more frequent in boys and in black infants compared with white infants. The reasons for these differential risks are not known. The left middle cerebral artery (MCA) is the most common vessel in-volved, with the left cerebral hemisphere the most com-mon region affected. Thus, congenital hemiplegia from IPS is seen more often on the right than the left limbs. Other long-term risks from IPS include seizure disorders and delayed or impaired language development. Al-though IPS is primarily a neurologic disorder, children with this condition require expertise from a number of specialties including neurology, neuroradiology, hema-tology, perinatal/neonatal medicine, genetics, general pediatrics, and developmental and rehabilitation medi-cine for assessment, treatment, and follow-up care.
Although IPS is common, much remains to be learned about it. To review this topic and establish a research agenda, the National Institute of Child Health and Hu-man Development (NICHD) and the National Institute of Neurological Disorders and Stroke (NINDS) organized a workshop in August 2006. This article provides a sum-mary of the workshop. Because many comprehensive reviews have been published,1–8 we focus on 4 key
is-sues: terminology, definition, and classification; risk fac-tors and their role in the causal pathway; diagnosis; and research priorities.
TERMINOLOGY, DEFINITION, AND CLASSIFICATION
A variety of events during the perinatal period can lead to occlusion of cerebral arterial or venous structures with focal disruption of blood supply to the brain, which causes a “stroke.” Because this workshop was about IPS, the most common variety of stroke in late-preterm and term infants, we did not focus our discussions on other conditions such as cerebral arteriovenous malforma-tions, intraparenchymal, periventricular, and intraven-tricular hemorrhages, focal patterns of cerebral damage resulting from diffuse ischemia including watershed in-farctions seen in infants with perinatal hypoxic ischemic encephalopathy, or periventricular leukomalacia of pre-term infants.
Authors have used differing terminologies and defi-nitions for both stroke and perinatal period, resulting in numerous terms to describe this condition. Some of the terms used include perinatal stroke,8–10perinatal arterial
stroke,11,12perinatal and neonatal ischemic stroke,13
ar-terial ischemic stroke,14fetal stroke,15and presumed
pre-natal or peripre-natal arterial ischemic stroke.16
Adhering to a consistent terminology and definition, however, would enrich the quality of research. There-fore, we chose the term IPS and defined it as “a group of heterogeneous conditions in which there is focal disrup-tion of cerebral blood flow secondary to arterial or cere-bral venous thrombosis or embolization, between 20 weeks of fetal life through the 28th postnatal day, con-firmed by neuroimaging or neuropathologic studies.”
Because the timing of the vascular event leading to IPS is almost always unknown, it was suggested that the classification of IPS be based on the gestational or post-natal age at diagnosis. The suggested subcategories were (1) fetal ischemic stroke, diagnosed before birth by using fetal imaging methods or in stillbirths on the basis of neuropathologic examination, (2) neonatal ischemic stroke, diagnosed after birth and on or before the 28th postnatal day (including in preterm infants), and (3) presumed perinatal ischemic stroke (PPIS), diagnosed in infants⬎28 days of age in whom it is presumed (but not certain) that the ischemic event occurred sometime be-tween the 20th week of fetal life through the 28th postnatal day.
Rationale for the Suggested Definition and Classification Scheme
The definition of “perinatal period” follows the standard used for compiling national perinatal statistics in the United States. The phrase “ischemic stroke” emphasizes that the nature of pathology is one of ischemia, most often from vascular (arterial or venous) thrombosis. Cat-egorization based on the age at diagnosis implies that one knows only the age at which a stroke diagnosis has been made and that, in most cases, the gestational age at which the stroke occurred cannot be ascertained with any degree of certainty. The PPIS subcategory allows for the inclusion of cases diagnosed after the neonatal pe-riod, yet they are “presumed” (but not certain) to have occurred during the perinatal period.
It is further stressed that in all categories of IPS (in-cluding PPIS), the age at stroke cannot be established with any degree of certainty and can only be conjectured to have occurred between the 20th week of fetal life through the 28th postnatal day.
Limitations of the Suggested Definition and Classification
be the difficulty in establishing the timing of the infarc-tion.
Even neuropathologic studies cannot always distin-guish initially ischemic lesions from initially hemor-rhagic lesions, especially if stroke occurred long before death. Venous infarcts are often hemorrhagic to begin with, and ischemic infarcts may become hemorrhagic after reperfusion. Moreover, a single patient may have many vascular pathologies. Mortality from IPS is rare, and the autopsy rates have been declining; thus, relying solely on neuropathology for the diagnosis of IPS can lead to an underestimation of IPS prevalence while over-estimating its severe forms.
Other limitations of the proposal are the timing of the diagnosis: the current proposal for fetal stroke excludes vasoocclusive strokes that might occur before 20 weeks of gestation. However, little is known about such condi-tions, mostly because pathologic evaluations are rarely conducted in fetal deaths before 20 weeks. Patients with silent strokes that occur after the 28th day of age may manifest signs and symptoms later in infancy or child-hood. With the current definition, one can erroneously diagnose PPIS in such infants, although their stroke oc-curred after the perinatal period.
The Special Case of IPS in the Preterm Infant
Diagnosis of ischemic stroke in preterm infants poses a special challenge, because other forms of vascular pa-thologies are more common in preterm infants. More-over, diagnosis of vasoocclusive strokes may be difficult without expertise in interpreting neuroimaging studies. Almost all reports on IPS have focused on term or late-preterm (previously known as near-term) infants. How-ever, Lee et al found that 15% of infants with IPS in a general population were born at ⬍36 weeks of
gesta-tion.17–19 Using a hospital-based data set of ⬎10 years,
Benders et al19identified arterial IPS in 31 infants born at
⬍37 weeks of gestation. All diagnoses were made by using cranial ultrasound and confirmed by using MRI. In 25 (81%) patients, the stroke was found in the territory of the MCA. Twin-to-twin transfusion syndrome, hypo-glycemia, and abnormal fetal heart patterns were found to be associated with IPS.
RISK FACTORS FOR IPS AND THEIR ROLE IN THE CAUSAL PATHWAY
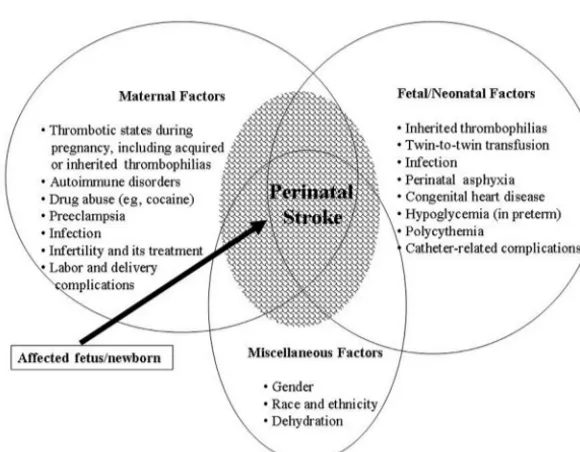
Figure 1 shows the complex, multifactorial relationship of risk factors identified in cases of IPS.16,20–29 Despite
their association, however, the causal pathways and the nature of their interactions leading to IPS are poorly un-derstood. Therefore, the observed risk factors (Table 1) cannot be inferred as definitive etiologies of IPS. Most of these risk factors were derived from descriptive epidemio-logic studies; thus, only tentative conclusions can be drawn about their causal relationship to IPS.
Compared with strokes in other age groups, IPS has many unique features. The normal physiologic processes in pregnancy lead to hypercoagulability of the blood even in healthy women. Pregnancy, therefore, is con-sidered to be a natural prothrombotic state, with as many as 67 per 100 000 pregnant women developing cerebral infarctions.30Increased proclivity for clot
forma-tion during pregnancy is secondary to accelerated plate-let-vessel interaction related to various hemostatic fac-tors, accelerated thrombin generation, and decreased thrombolysis. Although a number of inherited and ac-quired thrombophilias in the mother and/or the fetus (Table 2) can cause enhanced clot formation (in the maternal or fetal vasculature or in the uteroplacental unit), it should be stressed that an overwhelming
ma-FIGURE 1
jority of pregnant women with thrombophilia are healthy and have a very low incidence of stroke in their offspring.
Thrombotic episodes on the fetal side of the placenta can potentially lead to an embolic phenomenon in the fetal brain because of the patency of the foramen ovale and the right-to-left direction of blood flow in the fetal ductus arterious. Some investigators have proposed that in patients with symptomatic IPS, screening be con-ducted for several prothrombotic defects as done in the pediatric cases of stroke noted in Table 2. However, the cost/benefit analysis of such evaluations in all cases of IPS has not been studied.
Endothelial disruption associated with preeclampsia can also function as a prothrombotic stimulus. Diffuse and increased expression of tissue factors and thrombin early in the fetal life seems to play multiple roles in cell
proliferation, differentiation, and signaling, with the fe-tus maintaining a delicate equilibrium between bleeding and thrombosis. This balance may shift toward excessive thrombotic or bleeding tendency depending on which segment of the coagulation cascade is perturbed.
Other unique features of IPS include the proinflam-matory status of pregnancy, which increases the poten-tial for prothrombotic interactions between inflamma-tory and coagulation pathways. Superimposed infections can exacerbate these tendencies and promote throm-botic episodes in either maternal or fetal circulation, particularly when there is concomitant maternal or fetal thrombophilia.
Other conditions associated with increased IPS in-clude fetal polycythemia, twin-to-twin transfusion syn-drome, persistent pulmonary hypertension, prolonged maintenance of catheters in the systemic or umbilical vessels, congenital heart disease with right-to-left shunting, arteriovenous malformations, and dehydration. Some nonspecific factors associated with increased incidence of IPS are male gender, non-Hispanic black ethnicity, and maternal autoimmune conditions.
DIAGNOSTIC ISSUES
Newborn infants with stroke most often present with focal or generalized seizures and sometimes with apnea, hypotonia, or episodes of duskiness, irritability, and poor feeding. Neuroimaging studies remain the most impor-tant tools for the confirmatory diagnosis of IPS14,31–35
(Table 3).
Although cranial ultrasound helps in detecting a stroke in the first few days of life, one may miss cerebral ischemic lesions, especially those that are located more anteriorly or posteriorly.32,33 Even with computed
to-mography (CT) one may also miss IPS lesions, especially small lesions, during the first 24 hours after infarction. Therefore, conventional T2-weighted MRI, magnetic resonance angiography, and diffusion-weighted imaging (DWI) remain the principal methods for establishing the diagnosis of IPS.34,35
The time between the onset of symptoms and MRI is crucial for establishing the diagnosis with these tech-niques. Because of the increased water content of the unmyelinated brain, compared with the mature brain, T2 findings in IPS can be subtle during the first several days. Many studies have confirmed that ischemic tissue in the newborn brain can be detected by using DWI with high sensitivity during the first 2 days after the appearance of the symptoms. DWI sensitivity declines after 5 days of the appearance of symptoms. However, as the lesions become less visible on DWI, they become more visible on the conventional T1- and T2-weighted MRI.14,31,34,35
Conventional T1- and T2-weighted MRI and DWI allows precise delineation of the arterial distribution of the lesions, which helps in differentiating IPS from
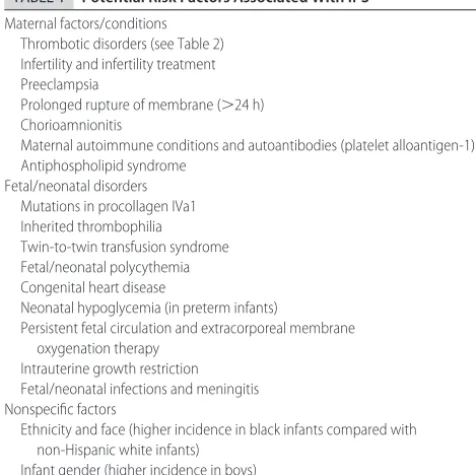
wa-TABLE 1 Potential Risk Factors Associated With IPS
Maternal factors/conditions Thrombotic disorders (see Table 2) Infertility and infertility treatment Preeclampsia
Prolonged rupture of membrane (⬎24 h) Chorioamnionitis
Maternal autoimmune conditions and autoantibodies (platelet alloantigen-1) Antiphospholipid syndrome
Fetal/neonatal disorders Mutations in procollagen IVa1 Inherited thrombophilia Twin-to-twin transfusion syndrome Fetal/neonatal polycythemia Congenital heart disease
Neonatal hypoglycemia (in preterm infants)
Persistent fetal circulation and extracorporeal membrane oxygenation therapy
Intrauterine growth restriction Fetal/neonatal infections and meningitis Nonspecific factors
Ethnicity and face (higher incidence in black infants compared with non-Hispanic white infants)
Infant gender (higher incidence in boys)
TABLE 2 Screening for Risk Factors: Acquired or Inherited Thrombotic Disorder in Pediatric Patients With Ischemic Stroke
Plasma/Protein Based DNA Based
Activated protein C resistance Factor V G1691A
Protein C activity/antigen Prothrombin G20210A
Free and total protein S antigen Antithrombin activity/antigen Lipoprotein (a)
Fasting homocysteine
Lupus anticoagulant/antiphospholipid antibodies Fibrinogen (Clauss)
Plasminogen Factor VIIIC
Some experts make these recommendations for screening in pediatric stroke cases38; however,
tershed or other nonvasoocclusive ischemic lesions. MRI features also enable prognostication, especially of motor outcomes. Vascular ischemic lesions in the MCA distri-bution, which affect tissue in the hemisphere, the pos-terior limb of the internal capsule, and the basal ganglia (lenticulostriate vessels), tend to cause hemiplegia irre-spective of the size of the infarct. Involvement of the cerebral peduncles seen on early DWI is also associated with the development of hemiplegia.34,35
Ancillary Studies
Evaluation for risk factors includes testing for prothrom-botic conditions. However, there is no consensus on how many of these tests are to be performed in IPS cases. Some experts advocate routine testing of all close family members of pediatric patients with stroke, and in posi-tive cases, the members of extended family are included. The cost/benefit ratio of such investigations (especially
those listed in Table 2) and its utility in counseling parents of infants with IPS have not been studied.
Other ancillary investigations in patients with IPS include tests for congenital heart malformations, assess-ment of vascular anatomy (especially in the neck), and evaluation of thrombotic lesions in systemic vessels.36
Autopsy and Evaluation of the Placenta
An autopsy can be very useful in defining the timing and the evolution of the vascular event that led to stroke.37
A complete autopsy, including examination of the heart, aorta, extracranial vessels, placenta, and umbilical cord,38,39is necessary for evaluation of potential sources
of thromboemboli and systemic vascular abnormalities. During the neuropathologic examination, the status of the intracranial arterial and sinovenous systems, gyral abnormalities, and the vascular distribution of all cere-bral lesions should be documented. Microscopic sections
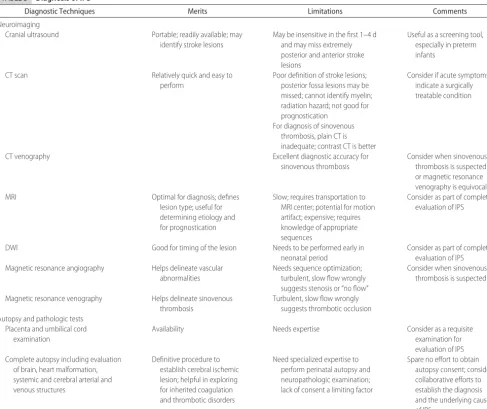
TABLE 3 Diagnosis of IPS
Diagnostic Techniques Merits Limitations Comments
Neuroimaging
Cranial ultrasound Portable; readily available; may
identify stroke lesions
May be insensitive in the first 1–4 d and may miss extremely posterior and anterior stroke lesions
Useful as a screening tool, especially in preterm infants
CT scan Relatively quick and easy to
perform
Poor definition of stroke lesions; posterior fossa lesions may be missed; cannot identify myelin; radiation hazard; not good for prognostication
Consider if acute symptoms indicate a surgically treatable condition
For diagnosis of sinovenous thrombosis, plain CT is inadequate; contrast CT is better
CT venography Excellent diagnostic accuracy for
sinovenous thrombosis
Consider when sinovenous thrombosis is suspected or magnetic resonance venography is equivocal
MRI Optimal for diagnosis; defines
lesion type; useful for determining etiology and for prognostication
Slow; requires transportation to MRI center; potential for motion artifact; expensive; requires knowledge of appropriate sequences
Consider as part of complete evaluation of IPS
DWI Good for timing of the lesion Needs to be performed early in
neonatal period
Consider as part of complete evaluation of IPS Magnetic resonance angiography Helps delineate vascular
abnormalities
Needs sequence optimization; turbulent, slow flow wrongly suggests stenosis or “no flow”
Consider when sinovenous thrombosis is suspected
Magnetic resonance venography Helps delineate sinovenous thrombosis
Turbulent, slow flow wrongly suggests thrombotic occlusion Autopsy and pathologic tests
Placenta and umbilical cord examination
Availability Needs expertise Consider as a requisite
examination for evaluation of IPS Complete autopsy including evaluation
of brain, heart malformation, systemic and cerebral arterial and venous structures
Definitive procedure to establish cerebral ischemic lesion; helpful in exploring for inherited coagulation and thrombotic disorders
Need specialized expertise to perform perinatal autopsy and neuropathologic examination; lack of consent a limiting factor
should include the core, penumbra, and margin of iden-tified lesions, corresponding contralateral regions, and involved blood vessels. Macroscopic and microscopic ex-aminations of the placenta and the umbilical cord need to be undertaken.
CURRENT AND EVOLVING THERAPIES
Several experimental therapies for IPS are being evalu-ated.40,41However, at present, treatment of infants with
IPS remains focused on symptom relief, and the roles of thrombolytic therapies being studied in adult strokes have yet to be tested in infants.
LONG-TERM OUTCOME
Many studies have been published on the long-term outcome of IPS survivors.42–51 However, accurate
out-come data are difficult to summarize because of varying characteristics of the cohorts, definitions, measures, and the duration of follow-up. However, some broad con-clusions can be drawn. Neurologic deficits or epilepsy occur in 50% to 75% of survivors, with sensorimotor deficits being the most common.2,11,16,43–51IPS is the most
identified cause of congenital hemiplegia.1,47More than
80% of infants with PPIS have hemiparesis.2,11,16,49
Defi-cits in language, vision, cognition, and behavior occur in 20% to 60% of IPS survivors.11,16,44,49 In most infants
with IPS, focal neurologic deficits tend to emerge after early infancy, and new deficits can continue to evolve over the several years of childhood. Thus, long-term follow-up with standardized measures are required, be-cause IPS survivors tend to grow into their deficits, and the extent of disability is not fully appreciated until they reach school age.
RESEARCH GAPS AND PRIORITIES
Numerous research gaps were identified from epidemi-ology to therapy of IPS (Table 4). Often, available funds dictate the research priorities and agenda. However, 2 areas were considered as needing urgent attention by the research community and the funding agencies: (1) developing uniform standards and criteria for IPS diag-nosis using neuroimaging methods and studying their changes over time and (2) developing consistent termi-nology and subcategorization with precise definitions.
SUMMARY AND CONCLUSIONS
Over the past decade, IPS has emerged as an important cause of brain injury in the perinatal period and remains a leading cause of CP. The timing of stroke in IPS can almost never be established with certainty. Much re-mains to be learned to decipher the causal pathways and interactions among the risk factors identified in the mother and her infant with IPS. Because IPS is a multi-factorial disorder that spans many specialties, profes-sional societies may need to develop strategies to educate
health care workers to understand this condition and to help in planning multispecialty follow-up care.
ACKNOWLEDGMENTS
We offer special thanks to Gabrielle deVeber, MD (Di-rector, Children’s Stroke Program Division of Neurology, Hospital for Sick Children, Toronto, Ontario, Canada) for her immense contributions to the field of pediatric stroke and child neurology. She also provided valuable sugges-tions during the preparation of this article. The organiz-ers thank the Office of Rare Diseases at the National Institutes of Health (NIH) for supporting this workshop. We thank all the following speakers, discussants, ses-sion moderators, and participants: Stephen Ashwal, MD (Loma Linda University School of Medicine, Loma Linda, CA), Taeun Chang, MD (Children’s National Medical Center, Washington, DC), Jieli Chen, MD (Henry Ford Hospital, Detroit, MI), Anne Comi, MD (Kennedy Krieger Institute, Baltimore, MD), Frances Cowan, MD, PhD (Imperial College London, Hammer-smith Hospital, London, United Kingdom), Linda de Vries, MD (Wilhelmina Children’s Hospital, UMC Utrecht, Utrecht, Netherlands), David Edwards, FMedSci (Imperial College London), Donna Ferriero, MD (Uni-versity of California, San Francisco, CA), Pankaj Ganguly (National Heart, Lung, and Blood Institute, NIH, Be-thesda, MD), Murray Goldstein, DO, MPH (United Ce-rebral Palsy Research and Educational Foundation,
Be-TABLE 4 Research Opportunities
Epidemiological research
Develop precise terminology, definition, and classification Research on incidence and prevalence rates in defined populations Establish criteria for including (or not including) various types of vascular
pathologies into the diagnosis of perinatal stroke Risk factors
Identify risk factors for perinatal stroke
Explore the causal pathways between risk factors and perinatal stroke Diagnosis
Develop standardized criteria for diagnostic imaging
Establish neuroimaging-monitoring protocols for frequency and modalities during the acute and follow-up periods
Develop neuroimaging criteria for prognostication
Establish criteria for timing of the vascular events based on neuroimaging findings
Establish criteria for comprehensive autopsy studies for confirming the diagnosis of perinatal stroke and exploring underlying causes Establish criteria for the evaluation of the placenta and the umbilical cord
when IPS has been diagnosed Ancillary studies
Establish a minimum panel of tests to explore the existence of inherited and acquired thrombophilias
Explore the value of tests to explore inherited and acquired thrombophilias in the mother and other family members
Therapies
Explore new therapies (eg, thrombolytic medications) Public health
thesda, MD), Meredith Golomb, MD, MSC (Indiana University School of Medicine, Indianapolis, IN), Marjo-rie Grafe, MD, PhD (Oregon Health and Science Univer-sity, Portland, OR), Pierre Gressens, MD, PhD (Hospital Robert Debre, Paris, France), Gary Hankins, MD (Uni-versity of Texas Medical Branch, Galveston, TX), James Hanson, MD (NICHD, NIH, Bethesda, MD), Marcus Her-mansen, MD, Dartmouth Medical School, Nashua, NH), Rosemary Higgins, MD (NICHD, NIH), Deborah Hirtz, MD (NINDS, NIH, Bethesda, MD), Jill Hunter, MBBS (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Rebecca Ichord, MD (Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA), John Ilekis, PhD (NICHD, NIH), Terrie Inder, MD, PhD (Washington University School of Medicine, St Louis Children’s Hospital, St Louis, MO), Janna Journeycake, MD, University of Texas Southwestern Medical Center, Children’s Medical Center Dallas, Dallas, TX), Gili Kenet, MD (Tel Aviv University, Sheba Medical Center, Tel HaShomer, Is-rael), Adam Kirton, MD (Hospital for Sick Children, Toronto, Ontario, Canada), Michael Kupferminc, MD (Lis Maternity Hospital Tel Aviv Sourasky Medical Cen-ter, Tel Aviv University, Givataim, Israel), Warren Lo, MD (Ohio State University School of Medicine, Colum-bus, OH), Charles Lockwood, MD (Yale University School of Medicine, New Haven, CT), John K. Lynch, DO, MPH (NINDS, NIH), Karin Nelson, MD (NINDS, NIH), Dwight Nissley, PhD (Children’s Hemiplegia and Stroke Association and Gene Regulation and Chromo-some Biology Laboratory/National Cancer Institute-Fre-derick, FreInstitute-Fre-derick, MD), U. Nowak-Gottl, MD (University Children’s Hospital, Munster, Germany), Mary Ellen Palko, MS (Children’s Hemiplegia and Stroke Associa-tion and Gene RegulaAssocia-tion and Chromosome Biology Laboratory/National Cancer Institute-Frederick), Nigel Paneth, MD, MPH (Michigan State University, East Lan-sing, MI), Steven Pavlakis, MD (Mount Sinai School of Medicine, Maimonides Infants and Children’s Hospital, Brooklyn, NY), Jeffery Perlman, MBChB (Weill Cornell Medical College, New York, NY), Tonse N. K. Raju, MD, DCH (NICHD, NIH), Uma Reddy, MD, MPH (NICHD, NIH), Raymond Redline, MD (Case School of Medicine, Cleveland, OH), E. Steve Roach, MD (Ohio State Uni-versity School of Medicine), Robert Silver, MD (Univer-sity of Utah Health Sciences Center, Salt Lake City, UT), Faye Silverstein, MD (University of Michigan, Ann Ar-bor, MI), Catherine Spong, MD (NICHD, NIH), Ann Stark, MD (Baylor College of Medicine, Texas Children’s Hospital), Marian Willinger, PhD (NICHD, NIH), and Yvonne Wu, MD, MPH (University of California, San Francisco, CA).
REFERENCES
1. Nelson KB, Lynch JK. Stroke in newborn infants.Lancet Neurol. 2004;3:150 –158
2. Wu YW, Lynch JK, Nelson KB. Perinatal arterial stroke: un-derstanding mechanisms and outcomes.Semin Neurol.2005;25: 424 – 434
3. Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, New-man TB. Perinatal stroke in children with motor impairment: a population-based study.Pediatrics.2004;114:612– 619 4. Wu, YW, Linda CE, Henning LH, et al. Neuroimaging
abnor-malities in infants with congenital hemiparesis.Pediatr Neurol. 2006;35:191–196
5. Hunt RW, Inder TE. Perinatal and neonatal ischaemic stroke: a review.Thromb Res.2006;118:39 – 48
6. Perlman JM. Brain injury in the term infant.Semin Perinatol. 2004;28:415– 424
7. Kirton A, deVeber G. Cerebral palsy secondary to perinatal ischemic stroke.Clin Perinatol.2006;33:367–386
8. Lynch JK, Nelson KB. Epidemiology of perinatal stroke.Curr Opin Pediatr.2001;13:499 –505
9. Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, Ferriero DM. Perinatal stroke in term infants with neonatal encepha-lopathy.Neurology.2004;62:2088 –2091
10. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke work-shop on perinatal and childhood stroke.Pediatrics.2002;109: 116 –123
11. Lee J, Croen LA, Lindan C, et al. Predictors of outcome in perinatal arterial stroke: a population-based study.Ann Neurol. 2005;58:303–308
12. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant.JAMA.2005;293:723–729
13. Golomb MR. The contribution of prothrombotic disorders to peri- and neonatal ischemic stroke.Semin Thromb Hemost.2003; 29:415–24
14. Cowan FM, de Vries LS. The internal capsule in neonatal imaging.Semin Fetal Neonatal Med.2005;10:461– 474
15. Ozduman K, Pober BR, Barnes P, et al. Fetal stroke.Pediatr Neurol.2004;30:151–162
16. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol.2001;50:163–168
17. de Vries LS, Roelants-van Rijn AM, Rademaker KJ, Van Haas-tert IC, Beek FJ, Groenendaal F. Unilateral parenchymal hae-morrhagic infarction in the preterm infant.Eur J Paediatr Neu-rol.2001;5:139 –149
18. de Vries LS, Van der Grond J, Van Haastert IC, Groenendaal F. Prediction of outcome in new-born infants with arterial isch-aemic stroke using diffusion-weighted magnetic resonance im-aging.Neuropediatrics.2005;36:12–20
19. Benders MJ, Groenendaal F, Uiterwaal CS, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the preterm infant.Stroke.2007;38:1759 –1765
20. Chalmers EA. Perinatal stroke: risk factors and management. Br J Haematol.2005;130:333–343
21. Golomb MR, Williams LS, Garg BP. Perinatal stroke in twins without co-twin demise.Pediatr Neurol.2006;35:75–77 22. Debus O, Koch HG, Kurlemann G, et al. Factor V Leiden and
genetic defects of thrombophilia in childhood porencephaly. Arch Dis Child Fetal Neonatal Ed.1998;78:F121–F124
23. Gu¨nther G, Junker R, Stra¨ter R, et al. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic pro-thrombotic risk factors [published correction appears inStroke. 2001;32:279].Stroke.2000;31:2437–2241
24. Heller C, Becker S, Scharrer I, Kreuz W. Prothrombotic risk factors in childhood stroke and venous thrombosis.Eur J Pedi-atr.1999;158(suppl 3):S117–S121
antiphospholipid antibodies are significant risk factors for isch-emic stroke in children.Stroke.2000;31:1283–1288
26. Manco-Johnson MJ, Nuss R, Key N, et al. Lupus anticoagulant and protein S deficiency in children with postvaricella purpura fulminans or thrombosis.J Pediatr.1996;128:319 –323 27. McColl MD, Chalmers EA, Thomas A, et al. Factor V Leiden,
prothrombin 20210GA and the MTHFR C677T mutations in childhood stroke.Thromb Haemost.1999;81:690 – 694 28. Zenz W, Bodo Z, Plotho J, et al. Factor V Leiden and
prothrom-bin gene G20210A variant in children with stroke. Thromb Haemost.1998;80:763–766
29. Kurnik K, Kosch A, Stra¨ter R, et al. Recurrent thromboembo-lism in infants and children suffering from symptomatic neo-natal arterial stroke: a prospective follow-up study. Stroke. 2003;34:2887–2892
30. Jaigobin C, Silver FL. Stroke and pregnancy.Stroke.2000;31: 2948 –2951
31. Khong PL, Lam BC, Tung HK, Wong V, Chan FL, Ooi GC. MRI of neonatal encephalopathy.Clin Radiol.2003;58:833– 844 32. Golomb MR, Dick PT, MacGregor DL, Armstrong DC, deVeber
GA. Cranial ultrasonography has a low sensitivity for detecting arterial ischemic stroke in term neonates.J Child Neurol.2003; 18:98 –103
33. Cowan F, Mercuri E, Groenendaal F, et al. Does cranial ultra-sound imaging identify arterial cerebral infarction in term ne-onates?Arch Dis Child Fetal Neonatal Ed.2005;90:F252–F256 34. Roelants-van Rijn AM, Nikkels PG, Groenendaal F, et al.
Neo-natal diffusion-weighted MR imaging: relation with histopa-thology or follow-up MR examination.Neuropediatrics. 2001; 32:286 –294
35. Bydder GM, Rutherford MA, Cowan FM. Diffusion-weighted imaging in neonates.Childs Nerv Syst.2001;17:190 –194 36. Beattie LM, Butler SJ, Goudie DE. Pathways of neonatal stroke
and subclavian steal syndrome.Arch Dis Child Fetal Neonatal Ed. 2006;91:F204 –F207
37. Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol.2005;20:940 –949
38. Redline RW. Placental pathology and cerebral palsy.Clin Peri-natol.2006;33:503–516
39. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment.Am J Obstet Gynecol.2005; 192:452– 457
40. Kennea NL, Mehmet H. Perinatal applications of neural stem cells.Best Pract Res Clin Obstet Gynaecol.2004;18:977–994 41. Balduini W, Carloni S, Mazzoni E, Cimino M. New therapeutic
strategies in perinatal stroke. Curr Drug Targets CNS Neurol Disord.2004;3:315–323
42. deVeber G, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis.J Child Neurol.2000;15:316 –324 43. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous
thrombosis in children.N Engl J Med.2001;345:417– 423 44. Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb
MR. Cerebral sinovenous thrombosis in the neonate.Arch Neu-rol.2006;63:405– 409
45. Sreenan C, Bhargava R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term out-come.J Pediatr.2000;137:351–355
46. Mercuri E, Barnett A, Rutherford M, et al. Neonatal cerebral infarction and neuromotor outcome at school age.Pediatrics. 2004;113:95–100
47. Mercuri E, Rutherford M, Cowan F, et al. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic res-onance imaging study.Pediatrics.1999;103:39 – 46
48. Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94.Acta Paediatr.2001;90: 271–277
49. Trauner DA, Chase C, Walker P, Wulfeck B. Neurologic profiles of infants and children after perinatal stroke.Pediatr Neurol. 1993;9:383–386
50. Toet MC, Groenendaal F, Osredkar D, van Huffelen AC, de Vries LS. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol. 2005;32: 241–247
DOI: 10.1542/peds.2007-0336
2007;120;609
Pediatrics
Tonse N.K. Raju, Karin B. Nelson, Donna Ferriero and John Kylan Lynch
Neurological Disorders and Stroke
Institute of Child Health and Human Development and the National Institute of
Ischemic Perinatal Stroke: Summary of a Workshop Sponsored by the National
Services
Updated Information &
http://pediatrics.aappublications.org/content/120/3/609
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/120/3/609#BIBL
This article cites 50 articles, 13 of which you can access for free at:
Subspecialty Collections
sub
http://www.aappublications.org/cgi/collection/neurologic_disorders_ Neurologic Disorders
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2007-0336
2007;120;609
Pediatrics
Tonse N.K. Raju, Karin B. Nelson, Donna Ferriero and John Kylan Lynch
Neurological Disorders and Stroke
Institute of Child Health and Human Development and the National Institute of
Ischemic Perinatal Stroke: Summary of a Workshop Sponsored by the National
http://pediatrics.aappublications.org/content/120/3/609
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.