Chronic
Protracted
Diarrhea
of Infancy:
A Nutritional
Disease
Clifford
W. Lo, MD, MPH,
and W. Allan
Walker,
MD
From the Pediatric Gastrointestinal and Nutrition Unit, Massachusetts General Hospital, Harvard Medical School, Boston; and Department of Nutrition and Food Science, Massachusetts Institute of Technology, Cambridge
ABSTRACT.
Diarrhea is an extremely common cause ofmorbidity in infancy. Occasionally, it becomes protracted, leading to a vicious cycle of malabsorption, malnutrition,
and failure to thrive. A number of causes of chronic
diarrhea in infancy are discussed, including
postinfec-tious enteritis, celiac sprue, cow’s milk allergy, and
par-asitic infection. Although many mechanisms may
con-tribute to diarrhea, a similar pathophysiologic syndrome
of mucosal atrophy, inflammation, and malabsorption
results. Attention should be paid to recognition of
mal-nutrition as well as etiologic diagnosis. Therapeutic
ef-forts should concentrate on nutritional rehabilitation,
through appropriate oral elemental formulas or total
parenteral nutrition. However, encouragement of
breast-feeding is probably a more effective way of preventing
this difficult problem. Pediatrics 1983;72:786-800;
chronic diarrhea, malabsorption, malnutrition, failure to
thrive.
“Like the diet prescribed by doctors which neither
re-stores the strength of the patient nor allows him to suc-cumb. “-Demosthenes (385-322 Bc)
Diarrhea! diseases continue to be the leading
cause of morbidity and mortality in the world today,
with estimates of 3 to 5 billion cases per year (an
average of roughly one per person per year).’ The
burden is especially severe on children less than the
age of 5 in Asia, Africa, and Latin America, with
approximately 500 million episodes of diarrhea
leading to 5 to 18 million deaths per year.2 Even in
industrialized countries, mortality of infants
hos-pitalized for diarrhea still exceeds 1 % , half of these
from complications of prolonged chronic
diar-Received for publication March 15, 1982; accepted March 23
1983.
Reprint requests to (W.A.W.) Pediatric Gastrointestinal and Nutrition Unit, Massachusetts General Hospital, Boston, MA
02114.
PEDIATRICS (ISSN 0031 4005). Copyright © 1983 by the American Academy of Pediatrics.
rhea”4 In the United States, diarrhea! diseases were
the leading cause of infant mortality until 50 years
ago,5 especially the dread summer “cholera
infan-tum” which appeared about the middle of the 18th
century6 and reached its peak at the end of the 19th
century. This occurred almost exclusively among
artificially fed babies, contributing to an overall
mortality of 80% to 90% in this group.5’3”
In 1968, Avery et a!7 defined intractable diarrhea
of infancy by the following criteria: (1) lasting more
than 2 weeks, (2) onset in infants less than 3
months of age, and (3) with three or more negative
cultures for Shigella, Salmonella, Escherichia coli,
and ova and parasites. Larcher et a!8 reported 82
cases of protracted diarrhea of four or more loose
stools per day for longer than 2 weeks with infants
suffering failure to thrive. Of these, 33% had celiac
disease, 12% had secondary disaccharidase
defi-ciency (possibly postinfectious), and 11% had cow’s
milk protein intolerance; in 28%, however, no
di-agnosis could be established. Overall mortality was
5%, much improved over the earlier experience of
Avery et al of 45% mortality, and this improvement
was attributed to the use of an elemental
chicken-based formula. Sunshine et al9 reviewed the subject
and cited 42 additional cases, in which the largest
category (48% ) consisted of a nonspecific
entero-colitis secondary to an infectious gastroenteritis or
cow’s milk protein intolerance, with a mortality of
28%. Other reports of persistent diarrhea in
infants’#{176}’ claim much reduced morbidity and
mor-tality with improved methods for delivery of oral or
intravenous alimentation.
Other reviews of chronic diarrhea in children’4’6 stress the wide range of etiologic and
pathophysio-logic mechanisms that may be involved in this
problematic syndrome. Much of the problem lies
with the lack of a common definition of diarrhea.
said 2,000 years ago to be “the discharge of
undi-gested food in a fluid state,” but more quantitative
definitions are elusive. In addition to the two liters
of fluid ingested daily by the average adult human,
seven additional liters of salivary, gastric, biliary,
pancreatic, and intestinal fluid are also secreted
into the intestine.’7 With a high degree of efficiency,
95% to 98% of the water, fat, carbohydrate, and
protein ingested or secreted into the intestinal
lu-men is normally reabsorbed. The consistency of
stools is not solely determined by the water content,
and the average stool weighing 100 to 200 g in
adults contains 60% to 85% water, whereas
diar-rheal stools may also contain 60% to 95% water.’8
Stool frequency in adults usually ranges from twice
weekly to twice daily and from one to four times
daily in children.’9 However, it is not uncommon to
see a healthy breast-fed infant pass six to eight or
more seedy, yellow stools per day. Likewise, the
usual stool weight of less than 10 g/kg of body
weight per day (up to 200 g maximum) is often
exceeded on a high-fiber diet, as observed in Africa.
Thus, neither stool frequency, stool weight, or stool
consistency is always reliable in determining
patho-logic diarrhea. Any attempt to assess the severity
of diarrhea in children, whether by mothers’ reports
or weighing of stools, should also include attention
to its acute or long-term effects such as
dehydra-tion, malabsorption, malnutrition, and failure to
thrive.
The syndrome of chronic nonspecific diarrhea is
distinguished from intractable diarrhea by lack of
any evidence of malabsorption, growth retardation,
or dehydration.20’2’ Occurring classically between 6
and 30 months of age, this syndrome is thought to
be the childhood counterpart of the all-too-common
irritable bowel syndrome22 and has a strong
psycho-logical component.23 It may be associated with
ele-vated levels of plasma prostaglandin24 possibly due
to decreased gut transit time and disordered gut
motility.25 After noticing a correlation with dietary fat restriction designed to prevent atherosclerosis,26
Cohen et a! recently advocated empiric treatment
by increasing dietary fat intake.27
Intractable diarrhea, on the other hand, is much
more serious because malabsorption of essential
nutrients leads to malnutrition and failure to thrive.
Indeed, intractable diarrhea may be seen as the
end-stage of chronic malabsorption, which may be
life-threatening if effective intervention is not
,available. Originally described by Aretaeus the
Cap-padocian in the first century AD, the “coeliac
dia-thesis” was associated with general emaciation, and
was noted by Samuel Gee (1839_1911)28 to be
“es-pecially apt to affect children between one and five
years old.” The malabsorption syndrome now
in-cludes a long list of specific diseases, some
exceed-ingly rare2933 (see Table 1). A comprehensive
dis-cussion of such causes is beyond the scope of this
review, and only the most common underlying
causes of intractable diarrhea, (1) celiac sprue, (2)
cow’s milk allergy, (3) giardiasis, and (4)
postin-fectious diarrhea, will be discussed here. Most of
the other causes will not be seen commonly,
al-though two autosomally recessive inherited
disor-ders, cystic fibrosis and congenital
sucrase-isomal-tase deficiency,33 should not be forgotten.
ETIOLOGY
Celiac
Sprue
Celiac sprue was observed to be related to wheat
in the diet, after certain Dutch children’s health
improved when bread was temporarily unavailable
during World War II. Thought to be autosomal
dominant with incomplete penetrance,34 celiac
sprue has a strong association with
histocompati-bility type HLA-B8. The prevalence of celiac
dis-ease is reported to be as high as 1/300 in Ireland,
but much lower in other parts of Europe and the
United States.35 “Gluten-sensitive enteropathy”
has been proposed as an alternative term,36
inas-much as challenges with the gluten protein produce
TABLE 1. Causes of Protracted Diarrhea
Postinfectious diarrhea
Shigella, Salmonella, Escherichia coli, Yersinia,
Cam-pylobacter
Rotavirus, Norwalk agent, adenovirus, calcivirus,
co-ronavirus, astrovirus, other enteroviruses Celiac sprue
Allergic gastroenteropathy
Cow’s milk allergy
Soy protein intolerance Pancreatic insufficiency
Cystic fibrosis
Shwachman-Diamond syndrome
Congenital enzyme deficiencies
Sucrose-isomaltase deficiency, enterokinase
defi-ciency, glucose-galactose malabsorption, lactase
de-ficiency, congenital chloridorrhea Parasitic infection
Giardiasis, strongyloidiasis, amebiasis, capillariasis, coccidiosis
Bacterial overgrowth, stasis syndromes
Clostridium difficile
Short-bowel syndromes
Drugs
Laxatives, phenolphthlalein, antibiotics, antacids,
sorbitol Miscellaneous
Intestinal lymphangiectasia, abetalipoproteinemia,
Wolman’s syndrome, Hirschsprung’s disease,
a characteristic (but nonspecific) intestinal villous
atrophy.37 Nevertheless, the basic pathogenesis of
the toxic reaction to the alcohol-soluble gliadin
fraction of gluten is still not completely clear.38
Celiac disease may represent a primary or
second-ary immune response to gluten. To support this
hypothesis, a wide range of immunologic
disturb-ances have been described, including
immunoglob-ulin deficiencies,39 a transfer factor defect,4#{176} the
presence of antireticulin antibodies,4’ food
anti-bodies and immune complexes,42’43 altered
cell-me-diated immunity,44 and leukocyte migration
inhi-bition factors,45 as well as associations with
der-matitis herpetiformis and other immunologic
dis-eases.46
The characteristic flat intestinal lesion of villous
atrophy, crypt hyperplasia, and cellular
inflamma-tory response after challenge and rechallenge with
gluten, is no longer thought to be specific;47 it occurs
also in cow’s milk intolerance,48’49 soy protein
in-tolerance,5#{176} eosinophilic gastroenteropathy,5’
im-munodeficiency,52 tropical sprue,53’54 bacterial
over-growth, giardiasis, acute gastroenteritis, and other
chronic diarrhea! dieases.55 Steatorrhea is
accom-panied by secondary disaccharidase deficiency,37’5#{176}
disturbed bile and pancreatic secretion, and
secre-tory imbalance.38 Although some patients may
re-main asymptomatic or have only transient gluten
intolerance, long-term dietary avoidance of gluten
(wheat, rye, barley, and oats) remains essential57’58
to prevent relapses of intractable diarrhea and
growth failure.
Cow’s Milk Allergy
Although recognized since the time of
Hippo-crates, cow’s milk intolerance has been a topic of
increasing interest as the effects of widespread use
of cow’s milk-based formulas in infant nutrition
have become apparent. The manifestations of cow’s
milk allergy are protean; in addition to diarrhea
and a variety of other gastrointestinal (GI)
symp-toms, they may include respiratory, dermatologic,
hematologic, neurologic, and cardiovascular signs
and symptoms. From a series of children with cow’s
milk allergy,59 Lebenthal60 lists presenting
symp-toms as diarrhea, 88%; vomiting, 44%; abdominal
pain or colic, 39%; atopic dermatitis, 33%; rhinitis,
31%; asthma, 31%; urticaria, 13%; and anaphylaxis,
12%. Cow’s milk allergy usually occurs before the
age of 6 months, but diagnosis is not always clearly
documented, leading to reports of incidence ranging
from 0.3% to 75%6162 This is partly due to the
difficulty of fulfilling the strict criteria of Goldman
et al:63 (1) symptoms subsiding after milk
elimina-tion; (2) symptoms occurring within 48 hours
fol-lowing a trial feeding of milk; (3) three such positive
challenges similar in onset, duration, and clinical
features; and (4) symptoms subsiding following
each challenge reaction. Inasmuch as many
work-ers64’65 feel that three challenges are impractical, an
alternative approach to diagnosis is serial intestinal
biopsies after a single challenge, yielding a mucosal
villous atrophy of varying severity which resolves
on a milk-free diet.48’66’67 However, in view of the
diverse pathogenesis and manifestations of the
dis-ease, abnormal morphologic findings during small
bowel biopsy are neither specific nor consistently
detectable.’69
A large number of immunologic tests have been
reported to be variably abnormal in cow’s milk
intolerance, including skin tests,7#{176} hemagglutinin
and milk precipitin antibodies,7’ secretory
coproan-tibodies, eosinophilia, elevated serum
immunoglob-ulin E (IgE) levels, and positive radioallergosorbent
test (RAST),72 immune complexes, leukocyte
his-tamine release, lymphoblast transformation, and
lymphokine production.73 However, most of these
tests have poor sensitivity and specificity, and no
single simple test that can easily be performed has
gained wide acceptance.
Indeed, a number of immunologic mechanisms
may be involved in the variety of clinical
manifes-tations. The local small intestinal response may be
mediated by antibodies of the secretory IgA class
and possibly IgG and 1gM.74 Colitis, occurring
dis-tally,75 may involve a systemic type II Arthus
re-sponse mediated by IgG and complement.76 Of
course, the term cow’s milk allergy suggests an
immediate IgE type I hypersensitivity.77 Delayed
hypersensitivity mediated by T lymphocytes might
explain late reactions, lymphokine release,
lympho-blast transformation, and eosinophilia.78
Cow’s milk allergy may be due to early exposure
to cow’s milk protein at a time when there is
excessive intestinal antigenic uptake of
macro-molecules;79’60 thus it may be prevented by
breast-feeding through the first months of life. Increased
macromolecular transport may also occur after an
episode of infectious diarrhea,82 producing a
sec-ondary cow’s milk intolerance.60M This may
pro-long the duration of mucosal damage during
refeed-ing, leading to secondary lactase deficiency, lactose
malabsorption, malnutrition, and failure to
thrive.m The main fractions of proteins in cow’s
milk, (-lactoglobulin, a-lactalbumin, albumin,
ca-sein, and rny-globulin, are all demonstrably antigenic,
and may be processed by partial digestion into as
many as 100 or more additional different antigenic
protein fragments.74
The reports of similar or concomitant reactions
to soy protein5”87’ suggest a relationship to a larger
category of GI food allergy89 that may even include
syn-drome usually disappears spontaneously by age 2
years,83 but affected children may suffer from other
allergic complaints. There is evidence that
diso-dium cromoglycate may be effective in
prophy-laxis,#{176}but the best prevention is avoidance of cow’s
milk, preferably by institution of breast-feeding.9’
Giardia and Other Parasites
Giardiasis is another condition that is becoming
more frequently recognized as a cause of chronic
diarrhea. Although more common in countries with
contaminated water sources, infection with Giardia
lamblia also occurs frequently in some parts of the
United States (such as along the Colorado River),
and worldwide reports of prevalence range from 1%
to as high as 3#{216}%#{149}9293However, most infections are
asymptomatic, and the clinical syndrome of
ma!-absorption, steatorrhea, and severe acute or chronic
diarrhea is not directly related to the number of
parasites or the severity of the mucosal injury.94’95
Thus, Giardia cysts might not be found in stools of
some symptomatic patients, and a small-bowel
bi-opsy, duodenal aspirate or entero-string test may
be necessary for positive diagnosisY
The pathogenesis of disease in giardiasis is still
unclear, and theories of mucosal invasion,
compe-tition for nutrients, toxin production, or a
para-sitic mechanical barrier to absorption have been
proposed.97’98 Mucosal damage with reduction of
brush border sucrase and lactase activity has been
shown.99 Patients with a variety of B-cell
immu-nodeficiencies, including nodular lymphoid
hyper-plasia and “hypogammaglobulinemic sprue,” have
a predilection for Giardia infections accompanied
by villous flattening and malabsorption,’#{176}#{176}”#{176}’again
illustrating an interaction between immunity,
in-fection, diarrhea, and malnutrition. Infection is
usually cured by quinacrine or metronidazole for
ten days, but relapses or treatment failures
occa-sionally OCCUr’#{176}2
Entamoeba histolytica is the other common
par-asite that can cause a chronic dysentary or diarrhea;
the case fatality rate may be as high as 27%.b03
Invasive amebiasis results from a variable virulence
affected by bacterial flora and diet of the host.’#{176}4
Trophozoites attach to the colonic mucosa and
release cytotoxic factors and cytoplasmic enzymes
to create characteristic flask-shaped ulcers. The
range of the disease is variable, from asymptomatic
to ulcerative postdysenteric colitis, peritonitis,
strictures, ameboma, cutaneous infections, and
liver abscess’05 Treatment of amebiasis depends on
the form of the disease, but metronidazole (750 mg
three times daily for five to ten days) is suggested
for dysentery as well as extraintestinal amebiasis.’#{176}
Other parasites that may occasionally produce
chronic diarrhea and malabsorption include
Stron-gyloides stercoralis, Isopora belli, Capillaria
philip-pinensis, and hookworm,29 and even the ubiquitous Ascaris lumbricoides’#{176}7; all cause some degree of
mucosal damage.
Postinfectious Diarrhea
Postinfectious diarrhea is probably the most
common cause of intractable diarrhea, especially in
developing countries, but much remains to be
un-derstood about the progression of disease and
pro-longation of diarrhea. It is now clear that the
uni-tarian idea of infectious diarrhea as a bacillary
dysentery caused by Shigella, Salmonella, or E Coli
is far from the entire truth.’#{176} Only between 4%
and 33% of diarrhea! stools yield these specific
bacterial pathogens.’#{176} The virulence of these
bac-teria has been classically attributed to mucosal
adherence and proliferation and invasion of the
mucosal cell, which produce an inflammatory
ileo-colitis with loss of water, blood, and mucus from
the damaged mucosa.”#{176}”2 However, at least in
some E coli and Shigella infections, there is also production of enterotoxin, which initiates secretory
diarrhea in the proximal small intestine, such as in
cholera.”3”6 Indeed, it may be more relevant to
look for enterotoxigenic E coli strains than the
probably outdated list of enteropathogenic E coli
‘7
Recently, other bacterial and viral pathogens
have been receiving increased attention as causes
of infectious diarrheas in children.”’2#{176} Yersinia
enterocolitica produces a bloody diarrhea when it
invades the intestinal mucosa, but it may also
pro-duce an enterotoxin.’2’’24 Occasionally, it can cause
a chronic inflammation of the bowel, appendicitis,
or a chronic diarrhea lasting for months.’25
Cam-pylobacter fetus likewise produces a febrile illness
with bloody diarrhea’26”27 may mimic ulcerative
colitis,’28 and has been associated with consumption
of unpasteurized milk.’29
However, the real advance in the diagnosis of infectious diarrhea has come in the identification
of viral agents, especially rotavirus.’30’33 Although
many viruses, including adeno-, corona-, echo-, and
myxoviruses had been isolated from patients with
diarrhea,’34 it was not until a 27-nm particle in
diarrheal stool filtrate from an epidemic in
Nor-walk, OH was used to infect volunteers that a viral
etiology for diarrhea was confirmed.’35 In addition
to the Norwalk agent, a 70-nm rotavirus has been
identified as one of the most common causes of
diarrhea in children less than 3 years of age.’35
Originally detected by immune electron
micro-scopy’37”38 because of difficulty in cell culture
counterimmunoelectrophoresis,’41”42 and especially
ELISA (enzyme-linked immunosorbent
as-say),’43”44 have disclosed a surprisingly high
fre-quency. Rotavirus has been detected in stools of up
to 40% to 70% of infants with gastroenteritis in
both developing countries and in the United
States.’45 Indeed, one study showed that 64% of
children had evidence of exposure by age 3 years
and 85% by age 5 years.’46 Rotavirus, although
mainly affecting children less than 3 years of age,
has also been found in epidemics among
school-children’47 and adults.’48
PATHOGENESIS
Viral gastroenteritis causes structural
abnormal-ities similar to those found with celiac disease, with
villous atrophy, crypt hypertrophy, and
inflamma-tory infiltrate.’49’52 Animal studies show viral
in-vasion of the intestinal epithelium, with replication
and shedding of the villous enterocytes. These are
replaced by premature migration of
less-differen-tiated crypt cells, which causes an altered mucosal
surface and derangement of sodium-glucose
trans-port.’53 A severe decrease in effective absorptive
area after viral gastroenteritis has been
demon-strated by careful examination of small-bowel
biop-sies.’54 Disaccharidase activity, particularly
lac-tase,’55”56 located on the microvillous brush border,
is consequently impaired, and secondary
ma!-absorption of lactose may persist for some
time.’57”” Undigested carbohydrates cause large
volumes of water to enter the lumen of the bowel
in an osmotic diarrhea.’59 The presence of
unab-sorbed sugars in the lumen also encourages a
pro-liferation of enteric microflora in the small
intes-tine,’60”6’ which causes further malabsorption and
diarrhea.’62 In the colon, bacterial fermentation of
undigested sugars produces lactic acid, releases
hy-drogen, and lowers the pH of the stool.’63
In diarrhea caused by enterotoxigenic E coli
or cholera, active secretion of large quantities
of sodium chloride and water is stimulated by
cAMP.’’67 Other causes of secretory diarrhea in
children include congenital chloridorrhea,1
non-beta islet cell hyperplasia producing vasoactive
in-testinal peptide’69 and hormone-producing
tu-mors.’7#{176} Although cAMP may not be involved in
rotavirus gastroenteritis or celiac sprue, secretory
diarrhea also occurs, perhaps because of the
prolif-eration of “leaky” immature crypt cells.’7”72
Malabsorption of fat in protracted diarrhea
usu-ally produces obvious steatorrhea, but may also
occur without clinical evidence.’73 In some cases,
this may be due to bile salt malabsorption in the
enterohepatic circulation’74’76 or their
deconjuga-tion by bacteria;’77 bile salt depletion leads to
in-adequate micellar formation.
Protein malabsorption is another major factor’78
and may be due to reduced enterokinase and trypsin
activity’79 or protein-losing enteropathy’tm with loss
of plasma proteins’8’ as well as sloughing of
intes-tinal mucosa.’82 If negative nitrogen balance
per-sists because of prolonged reliance on protein-free
oral or intravenous glucose-electrolyte solutions,
weight loss, malnutrition, and failure to thrive will
eventually ensue. Protein-calorie malnutrition, in
turn, further prolongs diarrhea, disaccharide
into!-erance,’83 and ma!absorption of fats, protein,
car-bohydrate, and vitaminsMm (see Table 2). Indeed,
the small intestinal mucosa in malnutrition
typi-cally ranges from nonspecific shortening of villi in
marasmus to a sprue-like flat intestinal lesion in
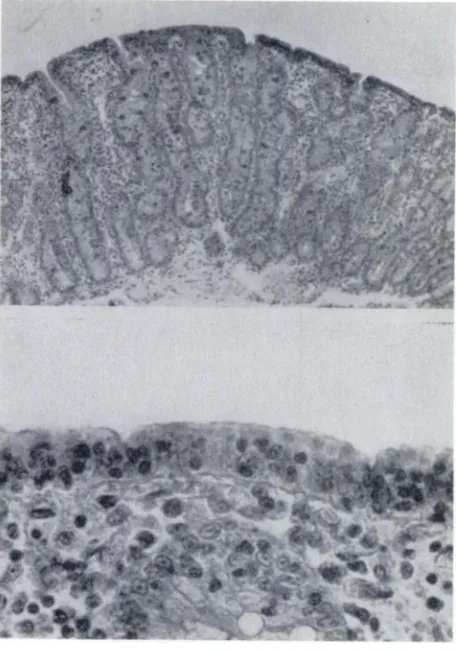
kwashiorkor (Fig Thus, malnutrition not
only contributes to delayed recovery from
intrac-table diarrhea but is the major consequence of it.
Secondary malnutrition, with respect to protein or
other specific nutrients, leads to impaired host
de-fenses, particularly in decreased cell-mediated
im-munity, secretory antibodies, phagocyte function,
and complement levels’89’94 and thus increases
sus-ceptibility to further infection.19520’ This vicious
cycle of diarrhea, malabsorption, malnutrition,
im-paired immunity, and infection202’203 has serious
effects not only on immediate morbidity but also
on long-term behavioral and intellectual
devel-opment204208 (see Fig 2).
DIAGNOSIS
The great majority of acute infectious diarrheas
resolve after a few days of treatment with
glucose-electrolyte solutions. Occasionally, however, the
diarrhea fails to resolve despite many changes of
formula. In this case, the value of a detailed dietary
history is apparent. Not infrequently, a mother who
is breast-feeding is counseled to change to an
arti-ficial formula, and things can go from bad to worse.
Diarrhea can occur when an infant is receiving
breast milk, but most studies document a strikingly
lower incidence in breast-fed babies.2#{176}2’5 Sorting
TABLE 2. Gastrointestinal Changes in Malnutrition*
Subtotal villous atrophy
Decreased functional surface area
Disrupted mucosal barrier Enzymes
Antibodies
I
MucinsDecreased crypt cell turnover
Migration of cells
Altered intestinal flora
Relative pancreatic insufficiency
* From Walker WA: Cellular and immune changes in the
gastrointestinal tract, in Winick M (ed): Nutrition and
Fig 1. Flattened mucosa similar to that produced by
celiac disease in kwashiorkor. Top, Low-power view
shows absence of villi and flat surface. There is moderate
infiltration of lamina propria with lymphocytes and
plasma cells (periodic acid-Schiff). Bottom, High-power
view shows low surface epithelium with irregularly
dis-tributed nuclei. Brush border is irregular and sparse
(hematoxylin and eosin). Reproduced with permission
from 0. Brunser, A. Reid, F. Monckeberg, et a!: Jejunal
biopsies in infant malnutrition: With special reference to mitotic index. Pediatrics 1966;38:608.
out the various formula changes and their effects
on stool frequency can take some time and requires
some familiarity with the composition of infant
formulas.
A careful history of travel, illnesses, medications,
development, family history, and especially social
situation can sometimes provide the only clue to
the origin of the diarrhea. Giardiasis or amebiasis
can be contracted during travel through an endemic
area, such as on a camping trip in the Rocky
Moun-tains. Cystic fibrosis or congenital carbohydrate
intolerance216 may be suggested by a positive family
history. Ampicillin and most other antibiotics have
been implicated in diarrhea and colitis, now known
to be due to an overgrowth of Clostridium
diffi-cile.21722#{176}Not infrequently, a disturbed social
situ-ation may be the clue to diarrhea induced by the
mother or a relative surreptitiously administering
a laxative to the infant.
Much important information is gained by
plot-ting the infant’s growth curve with serial
measure-ments of weight, height, and weight for height. The
widely used Gomez classification22’ defines mild
(grade I) malnutrition as between 75% and 90% of
standard weight for age; moderate (grade II) as
between 60% and 75%; and severe (grade III) as
less than 60% weight for age. Values less than 80%
of standard weight for height, or values below the
fifth percentile of the new National Center for
Health Statistics (NCHS) standards have been
widely used as the criteria for chronic malnutrition.
Other anthropometric measurements in common
use include the mid-arm circumference (for muscle
mass) and the triceps fatfold (for subcutaneous fat),
but unless these measurements are performed with
standardized equipment and careful technique, they
are subject to wide intraobserver variation
espe-cially in infants less than 1 year of age.222
On physical examination, the general appearance
of a child with chronic diarrhea can often separate
those with benign nonspecific loose stools and those
with chronic malabsorption and malnutrition. The
clinical picture of severe marasmus includes
leth-argy, emaciation, muscle wasting, hypotonia, and
sunken cheeks,223 but these signs may be more
subtle in mild or moderate malnutrition.
Occasion-ally, edema, a distended abdomen, and “flaky paint”
dermatitis are seen, classic signs of kwashiorkor.224
Pallor may reflect an anemia, most likely iron
de-ficiency, but could also be due to a deficiency of
vitamin C, B6, B,2, folate, or vitamin E. Clinical
signs of specific nutrient deficiencies are not
com-mon in the United States, but include areas of rapid
cell turnover, such as the skin, hair, mucous
mem-branes, bones, as well as the intestinal mucosa. A
dermatitis might indicate a deficiency of riboflavin,
niacin, protein, zinc, or essential fatty acids.
Pete-chiae or purpura may be manifestations of vitamin
A or C deficiency. Glossitis, cheilosis, and angular
stomatitis are nonspecific signs that may
accom-pany lack of niacin or riboflavin. Bone deformities
such as costochondral beading (“rachitic rosary”),
craniotabes, or epiphyseal swelling may be signs of
rickets (vitamin D deficiency) or scurvy (lack of
vitamin C).
Pale, thin, brittle hair may result from protein or
essential fatty acid deficiency. Eye findings include
Bitot’s spots, xerophthalmia, and keratomalacia
(vitamin A deficiency) and cornea! vascu!arization
(ariboflavinosis). Neurologic signs may be found in
deficiencies of folate, vitamin B,2, thiamin, pyridox-me, or niacin.
Occasionally, physical signs may suggest the
cause of the diarrhea. Nasal polyps or rectal
pro-lapse are characteristic features of cystic fibrosis.
Fig 2. Hypothetical pathogenesis for nutritional cause of intractable diarrhea of infancy.
protean manifestations of neurob!astoma. Eczema
is associated with cow’s milk allergy and ce!iac
disease. Infants with acute dehydration from
gas-troenteritis, superimposed on their chronic
malnu-trition, will show loss of skin turgor with “tenting”
of the skin, sunken eyes, dry lips, and concentrated
urine. Such a state requires prompt attention to
fluid needs.
Laboratory Findings
Laboratory examination of the stool is often
overlooked, but usually provides far more
informa-tion than most biochemical serum tests in the
di-agnosis of chronic diarrhea. With no equipment
other than a microscope, the liquid portion of the
stool sample can easily and quickly be tested for
weight, acidity, sugars (reducing substances), occult
blood, fat globules, leukocytes, ova, and parasites.
A stool pH less than 6.0 suggests bacterial action
on malabsorbed carbohydrates; however, this may
be a normal finding in breast-fed infants.
Malab-sorption of reducing sugars (glucose, lactose, but
not sucrose) can be detected by greater than 0.5%
reading by a Clinitest tablet dropped into equal
portions of liquid stool and water. If the infant is
receiving a sucrose formula (as is the case with soy
formula), the mixture must be heated with 0.1 N
hydrochloric acid for a few minutes to hydrolyze
the sugar. A positive stool guaiac test suggests some
source of GI bleeding, but can also be positive if the
diet contains meat. Excessive fat globules can be
seen easily under low-power microscope, but this
may be normal in young infants whose coefficient
of absorption on a fecal fat collection may be as
low as 85% instead of the 95% expected in adults.
Fecal leukocytes, seen with a methylene blue stain,
usually indicate bacillary dysentery caused by E
coli, Salmonella, Yersinia, or especially Shigella.225
The same methylene blue stain may reveal
char-acteristic Giardia trophozoites, amebic cysts, or
he!-minth ova.
Biochemical tests of nutritional status tend to be
too expensive and limited for most situations, but
a good initial screen might include a complete blood
count (CBC) with differential and red cell
mor-phology, serum electrolytes, total protein, and
a!-bumin. In a hospital setting, serum carotene,
cal-cium, phosphorus, iron, transferrin, zinc, copper,
thyroxine, cholesterol, triglycerides, or liver
func-tion tests (SGPT, SGOT, bilirubin, alkaline
phos-phatase, 5‘-nucleotidase, -y-glutamyl
transpepti-dase, and prothrombin time) might be among those
considered for further investigation. A sweat
chlo-ride test should be performed at a reliable center as
this test, when done properly, is a sensitive and
specific test for cystic fibrosis. Stool cultures,
mu!-tiple samples for ova and parasites, and a 72-hour
collection for fecal fat can be done on an inpatient
or outpatient basis, although this may be difficult
in infants.
To assess carbohydrate absorption, oral
carbo-hydrate tolerance tests may be given with 2 g of
lactose, sucrose, or glucose per kilogram of body
weight, up to 50 g in children or 100 g in adults, or
5 to 25 g of xylose.226 Traditionally, increased stool
output with presence of sugar, symptoms of
CHRONIC DIARRHEA
MANAGEMENT
L
ACE DIARRHJNo
dehydration
Total parenteral
nutrition
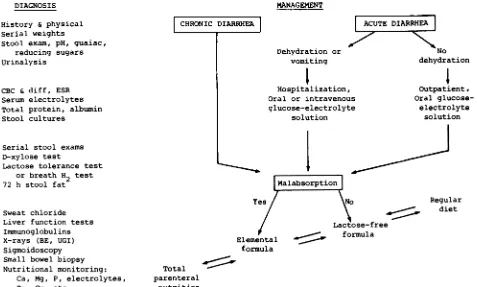
mulas should be selected according to persistence or
resolution of carbohydrate malabsorption, progressing
along a graded sequence from intravenous to elemental
to lactose-free to regular diets as malabsorption resolves,
or vice versa if it persists. Abbreviations used are: BE,
barium enema; UGI, upper gastrointestinal.
glucose concentration at 30, 60, or 90 minutes have
been used as bases for a positive test. Breath
hy-drogen analysis allows a quantitative, noninvasive
method of detecting carbohydrate
malabsorp-tion.227229 Lactose or sucrose that is ingested but
malabsorbed in the small intestine progresses to
the colon where it is metabolized by bacteria,
re-leasing excess quantities of hydrogen gas, which
can be detected in the breath. However, various
factors such as stool flora and pH changes can affect
breath hydrogen, making its diagnostic use in acute
diarrhea unreliable. Fat malabsorption can also be
measured using radioactive ‘4C23#{176}or stable
‘3C-labeled substrates. Unfortunately, this latter
tech-nique requires a mass spectrometer for breath
anal-ysis and is only available presently at a few research
centers.
One of the most important tools for investigation
of chronic diarrhea and failure to thrive is
hospi-ta!ization for observation of stool pattern and
weight gain. It is best to leave the infant on the diet
currently producing the diarrhea. Daily weights,
careful input and output records, and serial
mea-surements of stool weight, pH, and reducing
sub-stances, will quantitatively document the severity
of the diarrhea and ma!absorption. Occasionally,
an upper GI contrast series with small bowel
follow-through, a barium enema, ultrasound studies, a
small bowel biopsy and duodenal aspirate,
sigmoid-oscopy, or colonoscopy may be necessary to reveal
the underlying cause (Fig 3).
Management
The basic principles of the treatment of diarrhea
are nutritional, replenishing not only sodium,
po-tassium, and water losses, but also, protein, calorie,
and other nutrient stores. Although fluid
rehydra-tion was known as a treatment for cholera as early
as 150 years ago, high mortality continued until the
use of intravenous sodium and potassium salt
so-lutions gained widespread acceptance 100 years
later.23’234 Although complex calculations of
so-dium and potassium maintenance and deficit
re-quirements were 3236 less attention was paid
to protein and calorie needs, and infants were
sub-jected to a prolonged fast for “bowel rest.” This
concept has been 32 and recent
evi-dence suggests that both gut villous morphology
and disaccharidase activity improved faster when
DIAGNOSIS
History & physical Serial weights
Stool exam, pH, guaiac, reducing sugars Urinalysis
CBC & diff, ESR Serum electrolytes Total protein, albumin Stool cultures
Serial stool exams
D-xylose test
Lactose tolerance test or breath H2 test
72 h stool fat
Sweat chloride
Liver function tests
Iminunoglobulins
X-rays (BE, UGI) Sigmoidoscopy
Small bowel biopsy Nutritional monitoring:
Ca, Mg, P, electrolytes, Zn, Cu, etc.
Fig 3. Diagnostic tests and treatment alternatives in
management of chronic diarrhea. Diagnostic tests are listed by groups in suggested order ranging from initial outpatient screening tests to more involved inpatient
procedures. Not all tests need be or should be performed unless specifically indicated. Therapeutic changes of
for-Dehydration or
vomiting
Ir Hospitalization,
Oral or intravenous
glucose-electrolyte
solution
t
1otio1
YS/
Elemental formula
actose-free formula
Outpatient,
Oral
glucose-electrolyte solution
Regular
Dextrose
Carbohydrate Fat Protein kcal/mL mosm/L
* Abbreviation used is: MCT, medium-chain triglyceride.
Nonfat cow’s milk 0.67 260
stimulated by early enteral feedings than with
pro-longed “bowel rest” and intravenous
alimenta-tion.239242
Oral glucose-electrolyte solutions have been used
increasingly as initial therapy in diarrhea and
de-hydration due to a wide variety of infectious
agents.243246 They contain simple monosaccharides
and minerals which are usually absorbed by active
transport across partly damaged gut mucosal
sur-faces without normal villi.247249 However, the
ca-loric value of clear liquids is low, and they contain
no protein source that can be used to heal damaged
gut epithelium.25#{176} Therefore, clear liquids alone
should not be used for more than a few days without
the provision of additional calorie or nitrogen
sources.
Oral elemental diets containing simply
monosac-charides, amino acids, and safflower oil (eg,
Preges-timi!) are essentially completely predigested and
are usually well tolerated by infants no matter how
badly the gut is 2252 However, as their
high osmolarity may provoke an osmotic diarrhea,
elemental diets must usually be diluted for use in
infants. Other elemental formulas containing
ca-sein hydrolysates (short-chain polypeptides),
me-dium-chain triglycerides (eight- to ten-carbon
syn-thetic fatty acids which can be absorbed without
bile acids or micelles), and sucrose or glucose
pol-ymers instead of lactose, can be tailored to the
individual patient’s absorptive capacities as tested
by tolerance or breath tests253255 (Table 3). These
elemental formulas, claimed to be hypoallergenic,
are also useful if the problem involves milk or soy
protein allergy. If bo!us feedings cannot be
toler-ated, a slow continuous drip through a nasogastric
tube may be tried.
TABLE 3. Formula Composition*
Although oral elemental formulas will be
suffi-cient in most cases, occasionally total parenteral
nutrition may be necessary if diarrhea and
malab-sorption 2256 Peripheral administration
of protein dextrose solutions with fat emulsions
have been used to provide maintenance calories.’3
However, this does not allow the large amount of
extra calories to be administered, up to 200 kcal/
kg/d or more, which an extremely malnourished
infant or child might require for growth. Therefore,
placement of a central venous catheter for infusion
of hypertonic solutions becomes necessary for
treat-ment of prolonged diarrhea or severe malabsorption
problems.257259 The presence of an indwelling
cath-eter for prolonged periods has raised appropriate
concern over the high rates of infection observed in
early 2626k but the use of Silastic
cathe-262263 strict aseptic technique, and increased
experience has lowered complications to acceptable
levels.
Drug treatment of chronic diarrhea in children is
usually not warranted. No advantage has been
found in giving antibiotics in diarrhea due to E coli,
Salmonella, or nonspecific infections22; Shigelkz
is the specific exception. Intestinal paralytic agents,
such as diphenoxylate-atropine (Lomotil), may
re-lieve symptoms by decreasing fluid transit through
the gut, but without reducing secretion. The excess
water will instead tend to pool in distended loops
of bowel, masking dehydration and delaying the
usual excretion of infectious organisms.267
Adsorb-ents such as kaolin-pectin suspension (Kaopectate)
may actually cause increased sodium and potassium
losses.2 Bismuth subsalicylate (Pepto-Bismol) has
recently been shown to be of use in traveler’s
diar-rhea, possibly by enhancing the mucosal barrier or
Glucose-electrolyte
so-lutions
Pedialyte Lytren
Elemental formulas
Vivonex Pregestimil
Nutramigen Portagen
Lactose-free formulas
Isomil ProSobee Nursoy
Regular infant formulas
Similac Enfami! SMA
Glucose
Glucose polymers
Sucrose Sucrose
Glucose polymers
Lactose
Safflower oil
60% Corn oil, 40% MCT
Corn oil
90% MCT
Soy oil
80% Soy oil, 20%
coconut oil
Amino acids Casein hydrolysate
Casein hydro!ysate
Casein hydrolysate
Soy protein
0.20 400
1.00 550
0.67 300
0.67 400
0.67 200
by affecting prostag!andins,26927#{176} but the large
quantities necessary may put young infants at risk
for toxic salicylism. Other common antidiarrheal
agents are probably useless and may actually
pro-long diarrhea and be hazardous in young
chil-dren.27’
Prevention
Rehabilitation from severe intractable diarrhea
with protein-calorie malnutrition requires a long,
expensive hospitalization, with not inconsiderable
morbidity and mortality.272274 If diarrhea does
de-velop, proper attention to the infant’s nutritional
needs by the mother and the physician and
avoid-ance of prolonged iatrogenic starvation on clear
liquids or diluted formulas will prevent further
de-terioration into the vicious cycle of malnutrition,
infection, and malabsorption (Fig 1).
Increasing evidence is being accumulated on the
anti-infective properties of breast milk,275277 and
specific protection against Salmonella,278
rotavi-rus,279 and E coli’#{176}has been demonstrated. Even in
the United States, fewer hospitalizations and
infec-tious illnesses were found in infants breast-fed for
prolonged periods.2”215 Obviously, cow’s milk
in-tolerance will not be a problem in exclusively
breast-fed infants, but there is some evidence that
other allergic illness is also prevented.208
SUMMARY
Diarrhea! diseases are a major health problem in
the world today. Even in Western countries, such
morbidity and mortality result from complications
of prolonged intractable diarrhea. Although
diar-rhea is difficult to define quantitatively, intractable
diarrhea is distinguished from the chronic
nonspe-cific diarrhea syndrome by malabsorption,
malnu-trition, and failure to thrive. Most commonly
caused by allergic (celiac sprue, cow’s milk allergy)
or infectious (postinfectious enterocolitis,
giardi-asis) disease, a similar pathophysiologic syndrome
often develops. Mucosal villous atrophy, crypt
pro-liferation, and inflammation lead to carbohydrate,
fat, and protein malabsorption. The resulting
diar-rhea may involve several mechanisms including
osmotic and secretory diarrhea, bacterial
over-growth, and disordered motility. Eventually,
ma!-nutrition and failure to thrive may develop into a
vicious cycle with failure to heal mucosal damage
and further debilitation. Diagnosis of chronic
diar-rheal disease should include a careful dietary
his-tory and attention to signs of malnutrition and
tests of specific malabsorption. Management is
chiefly directed at providing adequate nutrition
through oral elemental diets or total parenteral
nutrition. However, long rehabilitation may be
nec-essary if early intervention is not instituted.
Pre-vention of the original causes can be most
effec-tively implemented by encouraging breast-feeding.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes
of Health Grants AM16269, GM 21700, and HD 12437.
Dr Lo is supported by Public Health Service Training
Grant T32AM07070-07.
REFERENCES
1. Walsh JA, Warren KS: Selective primary health care: An interim strategy for disease control in developing countries. N Engi J Med 1979;301:967
2. Rhode JE, Northup RS: Taking science where the
diar-rhoea is. Ciba Found Symp 1976;42:339
3. Tripp JH, Wilmers MJ, Wharton BA: Gastroenteritis: A continuing problem of child health in Britain. Lancet 1977;2:233
4. Ironside AG, Tuxford AF, Heyworth B: A survey of infan-tile gastroenteritis. Br Med J 1970;3:20
5. Cone TE: History of American Pediatrics. Boston, Little, Brown and Co, 1979
6. Faber K, McIntosh R: History of the American Pediatric
Society 1887-1965. New York, McGraw-Hill, 1966 7. Avery GB, Villavicencio 0, Lilly JR, et al: Intractable
diarrhea in early infancy. Pediatrics 1968;41:712 8. Larcher VF, Shepherd R, Francis DEM, et al: Protracted
diarrhoea in infancy. Arch Dis Child 1977;52:597
9. Sunshine P, Sinatra FR, Mitchell CH: Intractable
diar-rhoea of infancy. Clin Gastroenterol 1977;6:445
10. Keating JP, Ternberg JL: Amino acid-hypertonic glucose treatment for intractable diarrhea in infants. Am J Dis Child 1971;122:226
1 1. Lloyd-Still JD, Shwachman H, Filler RM: Protracted diar-rhea of infancy treated by intravenous alimentation. Am J Dis Child 1973;125:359
12. Hyman CJ, Reiter J, Rodman J, et al: Parenteral and oral alimentation in the treatment of the nonspecific protracted diarrheal syndrome of infancy. J Pediatr 1971;78:17 13. Banister A, Matin-Siddiqi SA, Hatcher GW, et al:
Intra-venous feeding of young infants with persistent diarrhoea.
Acta Paediatr Scand 1975;64:732
14. Poley JR: Chronic diarrhea in infants and children. South Med J 1973;66:1035
15. Gryboski JD: Chronic diarrhea. Curr ProblPediatr 1979;9:5
16. Gall DG, Hamilton R: Chronic diarrhea in childhood. Pe-diatr Clin North Am 1974;21:1001
17. Phillips SF: Diarrhea: A broad perspective. Viewpoints Dig Dis 1975;7:5
18. Phillips SF: Diarrhea: A current view of the
pathophysiol-ogy. Gastroenterology 1972;63:495
19. Mansourian PG, Sayers BM, Newell KW, et al: A pattern
analysis of weanling diarrhoea! disease of infants.
mt
JEpidemiol 1975;4:173
20. Cohlan SQ: Chronic nonspecific diarrhea in infants and
children treated with diiodohydroxyquinoline. Pediatrics 1956;18:424
21. Davidson M, Wasserman R: The irritable colon of child-hood (chronic nonspecific diarrhea syndrome). J Pediatr 1966;69:1027
22. Drossman DA, Powell DW, Sessions JT: The irritable bowel syndrome. Gastroenterology 1977;73:811
23. Wender EH, Palmer FB, Herbst JJ, et al: Behavioral characteristics of children with chronic nonspecific
diar-rhea. Am J Psychiatry 1976;133:1
25. Snape WJ, Carison GM, Matarazzo SA, et al: Evidence that abnormal myoelectrical activity produces colonic
mo-tor dysfunction in the irritable bowel syndrome. Gastroen-terology1977;72:383
26. Cohen SA, Hendricks KM, Eastham EJ, et al: Chronic nonspecific diarrhea: A complication of dietary fat restric-tion. Am J Dis Child 1979;133:490
27. Cohen S, Lake AM, Mathis RK, et al: Perspective on chronic nonspecific diarrhea: Dietary management. Pedi-atrics 1978;61:808
28. David B, Walker-Smith J: Samuel Gee, Aretaeus, and the coeliac affection. Br Med J 1974;2:45
29. Anderson CM: Malabsorption in children. Clin Gastroen-terology 1977;6:355
30. Ament ME: Malabsorption syndromes in infancy and childhood. J Pediatr 1972;81:685,867
31. Sleisenger MH: Malabsorption syndrome. N EngI J Med 1969;281:l111
32. Sleisenger MH, Brandborg LL: Malabsorption. Major Probi
Intern Med 1977;13:1
33. Ament ME, Perera DR, Esther U: Sucrase-isomaltase deficiency: A frequently misdiagnosed disease. J Pediatr
1973;83:721
34. MacDonald WC, Dobbins WO, Rubin CE: Studies of the familial nature of celiac sprue using biopsy of the small intestine. N EngI J Med 1965;272:448
35. Lebenthal E, Branski D: Childhood celiac disease: A reap-praisal. J Pediatr 1981;98:681
36. Katz AJ, Falchuk ZM: Current concepts in gluten sensi-tive enteropathy (celiac sprue). Pediatr Clin North Am 1975;22:767
37. Hamilton JR, Lynch MJ, Reilly BJ: Active coeliac disease in childhood.
Q
J Med 1969;38:13538. Strober W, Falchuk ZM, Rogentine GN, et al: The patho-genesis of gluten-sensitive enteropathy. Ann Intern Med 1975;83:242
39. Brown DL, Cooper AG, Hepner GW: 1gM metabolism in
coeliac disease. Lancet 1969;1:858
40. Hood J, Mason AMS: Diffuse pulmonary disease with transfer defect occurring with coeliac disease. Lancet
1970;1:445
41. Seah PP, Fry L, Rossiter MA, et al: Anti-reticulum anti-bodies in childhood coeliac disease. Lancet 1975;2:681 42. Kivel RM, Kearns DH, Liebowitz D: Significance of
anti-bodies to dietary proteins in the serums of patients with nontropical sprue. N Engi J Med 1964;271:769
43. Shiner M, Ballard J: Antigen-antibody reactions in jejunal mucosa in childhood celiac disease after gluten challenge. Lancet 1972;1:1202
44. Marks J, Young 5: Skin tests for celiac disease. Laricet 1978;2:1303
45. Ashkenazi A, Levin 5, Idar D, et al: Immunological assay for the diagnosis of coeliac disease: Interaction between purified gluten fractions. Pediatr Res 1980;14:776
46. Cooper BT, Holmes GKT, Cooke WT: Coeliac disease and
immunological disorders. Br Med J 1978;1:537
47. Katz AJ, Grand RJ: All that flattens is not “sprue.” Gas-troenterology 1979;76:375
48. Kuitunen P, Rapola J, Savilahti E, et al: Response of the
jejunal mucosa to cow’s milk in the malabsorption syn-drome with cow’s milk intolerance. Acta Paediatr Scand
1973;62:585
49. Fontaine JL, Navarro J: Small intestinal biopsy in cow’s milk protein allergy in infancy. Arch Dis Child 1975;50:357 50. Ament ME, Rubin CE: Soy protein: Another cause of the
flat intestinal lesion. Gastroenterology 1972;62:227 51. Klein NC, Hargrove L, Sleisenger MH, et al: Eosinophilic
gastroenteritis. Medicine 1970;49:299
52. Ament ME: Immunodeficiency syndromes and gastrointes-tinal disease. Pediatr Clin North Am 1975;22:807
53. Santiago-Borrero PJ, Maldonado N, Horta E: Tropical
sprue in children. J Pediatr 1970;76:470
54. Lindenbaum J: Tropical enteropathy. Gastroenterology
1973;64:637
55. Katz AJ, Falchuk ZM: Definitive diagnosis of
gluten-sen-sitive enteropathy use of an in vitro organ culture model. Gastroenterology 1978;75:695
56. Plotkin GR, Isselbacher KJ: Secondary disaccharidase de-ficiency in adult celiac disease (nontropical sprue) and other malabsorption states. N Engl J Med 1964;271:1033 57. Hamilton JR, McNeill LK: Childhood celiac disease:
Re-sponse of treated patients to a small uniform daily dose of wheat gluten. J Pediatr 1972;81:885
58. Collins JR, Isselbacher KJ: Treatment of adult celiac
dis-ease (nontropical sprue). NEnglJMed 1964;271:1153
59. Bahna SL, Heiner DC: Allergies to Milk. New York, Grune
& Stratton, 1980
60. Lebenthal E: Cow’s milk protein allergy. Pediatr Clin North
Am 1975;22:827
61. Bahna SL, Heiner DC: Cow’s milk allergy. Adv Pediatr
1978;25:1
62. Gerrard JW, MacKenzie JWA, Goluboff N, et al: Cow’s milk allergy: Prevalence and manifestations in an
unse-lected series of newborns. Acta Paediatr Scand 1973(suppl
234)
63. Goldman SA, Anderson DW, Sellers WA, et al: Milk
al-lergy: I. Oral challenge with milk and isolated milk proteins
in allergic children. Pediatrics 1963;32:425
64. Walker-Smith J: Cow’s milk protein intolerance: Transient food intolerance of infancy. Arch Dis Child 1975;50:347 65. Walker-Smith J, Harrison M, Kilby A, et al: Cow’s milk
sensitive enteropathy. Arch Dis Child 1978;53:375
66. Iyngkaran N, Robinson MH, Prathap K, et al: Cow’s milk protein-sensitive enteropathy: Combined clinical and his-tological criteria for diagnosis. Arch Dis Child 1978;53:20 67. Shiner M, Ballard J, Smith ME: The small intestinal
mucosa in cow’s milk allergy. Lancet 1975;1:136
68. Shiner M, Ballard J, Brook CGD, et al: Intestinal biopsy in the diagnosis of cow’s milk protein intolerance without acute symptoms. Lancet 1975;2:1O60
69. Hill DJ, Davidson GP, Cameron DJS, et al: The spectrum
of cow’s milk allergy in childhood. Acta Paediatr Scand
1979;68:847
70. Goldman AS, Sellers WA, Halpern SR, et al: Milk allergy: II. Skin testing ofallergic and normal children with purified milk proteins. Pediatrics 1963;32:572
71. Freier 5, Kletter B, Gery I, et al: Intolerance to milk protein. J Pediatr 1969;75:623
72. Wraith DG, Merrett J, Roth A, et al: Recognition of food-allergic patients and their allergens by the RAST technique
for clinical investigation. Clin Allergy 1979;9:25
73. Bahna SL: Control of milk allergy: A challenge for physi-cians, mothers, and industry. Ann Allergy 1978;41:1 74. Eastham EJ, Walker WA: Effect of cow’s milk on the
gastrointestinal tract: A persistent dilemma for the pedia-trician. Pediatrics 1968;40:354
75. Gryboski JD: Gastrointestinal milk allergy in infants. Pe-diatrics 1968;40:354
76. Matthews TS, Soothill JF: Complement activation after milk feeding in children with cow’s milk allergy. Lancet
1970;2:893
77. Visakorpi JK: Milk allergy and the gastrointestinal tract in children, in Asquith P (ed): Immunology of the Gastroin-testinal Tract. London, Churchill Livingston, 1979 78. Juto P, Stranegard 0: T lymphocytes and blood eosinophils
in early infancy in relation to heredity for allergy and type
of feeding. J Allergy Clin Immunol 1979;64:38
79. Walker WA, Isselbacher KJ: Uptake and transport of mac-romolecules by the intestine. Gastroenterology 1974;67:531 80. Galant SP: Biological and clinical significance of the gut
as a barrier to penetration of macromolecules. Clin Pediatr
1976;15:731
81. Rubino A: Absorption of amino acids and peptides during development. Mod Probi Paediatr 1975;15:201
82. Gruskay FL, Cooke RE: The gastrointestinal absorption of unaltered protein in normal infants and in infants recover-ing from diarrhea. Pediatrics 1955;16:763
1976;1: 1501
84. Iyngkaran N, David K, Robinson MJ, et al: Cow’s milk protein-sensitive enteropathy. Arch Dis Child 1979;54:39 85. Kuitunen P, Visakorpi JK, Savilahti E, et al:
Malabsorp-tion syndrome with cow’s milk intolerance: Clinical find-ings and course in 54 cases. Arch Dis Child 1975;50:351 86. Lee VA, Lorenz K: The nutritional and physiological
im-pact of milk in human nutrition. CRC Crit Rev Food Sci Nutr 1979;11:41
87. Eastham EJ, Lichauco T, Grady MI, et al: Antigenicity of infant formulas: Role of immature intestine on protein permeability. J Pediatr 1978;93:561
88. Whitington PF, Gibson R: Soy protein intolerance: Four patients with concomitant cow’s milk intolerance. Pediat-rics 1977;59:730
89. Freier S: Pediatric gastrointestinal allergy. Clin Allergy 1973;3 (suppl):5957
90. Freier S, Berger H: Disodium cromoglycate in gastrointes-tinal protein intolerance. Lancet 1973;1:913
91. Hide DW, Guyer BM: Clinical manifestations of allergy related to breast and cow’s milk feeding. Arch Dis Child
1981;56:172
92. Peterson HJ: Giardiasis (lambliasis). Scand J Gastroenterol
1972;7(suppl):14
93. Eastham FJ, Douglas AP, Watson AJ: Diagnosis of Giardia lamblia infection as a cause of diarrhea. Lancet 1976;2:950 94. Wolfe MS: Giardiasis. N EngI J Med 1978;298:319 95. Wolfe MS: Giardiasis. Pediatr Clin North Am 1979;26:295 96. Ament ME: Diagnosis and treatment of giardiasis. J
Pe-diatr 1972;80:633
97. Meyer EA, Radulescu 5: Giardia and giardiasis. Adv Par-asitol1979;17:1
98. Tandon BN, Tandon RK, Satpathy BK: Giardia and ste-atorrhea. Gut 1977;18:176
99. Hartong WA, Courley WK, Arvanitakis C: Giardiasis: Clin-ical spectrum and functional-structural abnormalities of the small intestinal mucosa. Gastroenterology 1979;77:61 100. Katz AJ, Rosen FS: Gastrointestinal complications of
im-munodeficiency syndromes. Ciba Found Symp 1977;46:243 101. Raizman RE: Giardiasis: An overview for the clinician. Am
J Dig Dis 1976;21:1070
102. Burke JA: Giardiasis in childhood. Am J Dis Child
1975;129:1304
103. Adams EB, MacLedo IN: Invasive amebiasis: I. Amebic dysentary and its complications. Medicine 1977;56:315 104. Juniper K: Amoebiasis. Clin Gastroenterol 1978;7:3 105. Adams FB, MacLeod IN: Invasive amebiasis: II. Amebic
liver abscess and its complications. Medicine 1978;56:325 106. Krogstad DJ, Spencer HC, Healy GR: Amebiasis. N EngI
J Med 1978;298:262
107. Tripathy K, Dugue E, Bolanos 0, et al: Malabsorption syndrome in ascariasis. Am J Clin Nutr 1972;25:1276 108. Jelliffe DB: The etiology of diarrhea in early childhood. J
Pediatr 1966;68:792
109. Harries JT: The problem of bacterial diarrhoea: Acute Diarrhoea in Childhood. Ciba Found Symp 1976;42:3 1 10. Gianella RA: Pathogenesis of Salmonella and Shigella
diar-rhea, in Etiology Pathophysiology and Treatment of Acute
Gastroenteritis. Report of the 74th Ross Conference on
Pediatric Research. Columbus, OH, Ross Laboratories,
1978
1 1 1. Grady GF, Keusch GT: Pathogenesis ofbacterial diarrheas. N Engi J Med 1971;285:831, 891
112. McNeish AS, Turner P, Fleming J, et al: Mucosal adher-ence of human enteropathogenic Escherichia coli. Lancet 1975;2:946
113. Donta ST, Wallace RB, Whipp SC, et al: Enterotoxigenic Escherichia coli and diarrheal disease in Mexican children. J Infect Dis 1977;135:482
1 14. O’Brien AD, Gentry MK, Thompson MR, et al: Shigellosis
and Escherichia coli diarrhea: Relative importance of
in-vasive and toxigenic mechanisms. Am J Clin Nutr
1979;32:229
115. Formal SB, Gemski P, Giannella RA, et al: Studies on the pathogenesis of enteric infections caused by invasive
bac-teria. Ciba Found Symp 1976;42:27
1 16. Carpenter CCJ: Cholera and other enterotoxin-related diarrheal diseases. J Infect Dis 1972;126:551
1 17. Gangarosa EJ: The Escherichia coli controversy: The mci-dence of enterotoxigenic and enteroinvasive E coli diar-rheas compared to the classic serotypes, in Etiology, Path-ophysiology and Treatment ofAcute Gastroenteritis. Report of the 74th Ross Conference on Pediatric Research. Colum-bus, OH, Ross Laboratories, 1978
118. Dupont HL, Pickering LK: Infections of the Gastrointes-tinal Tract. New York Plenum Press, 1980
119. Edelman R, Levine MM: Acute diarrheal infections in infants: II. Bacterial and viral causes. Hosp Pract
1980;15:97
120. Feldman WE: Infectious causes of acute gastroenteritis in pediatric patients. Cont Educ, Jan 1981; p 19
121. Kohl 5: Yersinia enterocolitica: A significant “new”
patho-gen. Hosp Pract 1978;13:81
122. Kohl 5, Jacobson JA, Nahmias A: Yersinia enterocolitica infections in children. J Pediatr 1976;89:77
123. Maki M, Vesikari T, Rantala I, et al: Yersinosis in children. Arch Dis Child 1980;55:861
124. Rodriguez WJ, Controni G, Cohen GJ, et al: Yersinia enterocolitica enteritis in children. JAMA 1979;242:1978
125. Kohl 5: Yersinia enterocolitica infections in children.
Pe-diatr Clin North Am 1979;26:433
126. Blaser MJ, Berkowitz ID, LaForce FM, et al: Campylobac-ter enteritis: Clinical and epidemiologic features. Ann In-tern Med 1979;91:179
127. Torphy DE, Bond WW: Campylobacter fetus infections in children. Pediatrics 1979;64:898
128. Lambert ME, Scholfield PF, Ironside AG, et al: Campylo-bacter colitis. Br Med J 1979;1:857
129. Robinson DA, Edgar WJ, Gibson GL, et al: Campylobacter enteritis associated with consumption of unpasteurized milk. Br Med J 1979;1:1171
130. Estes MK, Graham DY: Epidemic viral gastroenteritis. Am
J Med 1979;66:1001
131. Hamilton JR, Gall DG, Kerzner B, et al: Recent develop-ments in viral gastroenteritis. Pediatr Clin North Am
1975;22:747
132. Dupont HL, Portnoy BL, Conklin RH: Viral agents and diarrheal illness. Annu Rev Med 1977;28:167
133. Blacklow NR, Cukor G: Viral gastroenteritis. N Engl J Med 1981;304:397
134. Weindling AM, Walker-Smith JA, Bird R: Micro-orga-nisms in outpatient infantile gastroenteritis. Arch Dis Child
1980;55:185
135. Blacklow NR, Dolin R, Fedson DS, et al: Acute infectious nonbacterial gastroenteritis: Etiology and pathogenesis.
Ann Intern Med 1972;76:993
136. Steinhoff MC: Rotavirus: The first five years. J Pediatr
1980;96:61 1
137. Bishop RF, Davidson GP, Holmes IH, et al: Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 1973;2:1281 138. Flewett TH, Bryden AS, Davies H: Virus particles in
gastroenteritis. Lancet 1973;2:1497
139. Bryden AS, Davies HA, Thouless ME, et al: Diagnosis of rotavirus infection by cell culture. J Med Microbial
1977;10:121
140. Zissis G, Lambert JP, DeKegel D: Routine diagnosis of human rotaviruses in stools. J Clin Pat/wI 1978;31:175 141. Cook DA, Zbitnew A, Dempster G, et al: Detection of
antibody to rotavirus by counterimmunoelectrophoresis in human serum, colostrum, and milk. J Pediatr 1978;93:967 142. Kapikian AZ: Identification and serology of Rotavirus, Norwalk and Norwalk-like Agent, in Etiology, Pathophys-iology, and Treatment of Acute Gastroenteritis. Report of the 74th Ross Conference on Pediatric Research. Columbus
OH, Ross Laboratories, 1978
143. Yolken H: ELISA: Enzyme-linked immunosorbent assay. Hosp Pract 1978;13:121