Prothrombotic Disorders and Abnormal Neurodevelopmental Outcome in
Infants With Neonatal Cerebral Infarction
Eugenio Mercuri, MD*; Frances Cowan, PhD*; Girish Gupte, MD*; Richard Manning, BSc‡; Mike Laffan, MD‡; Mary Rutherford, MD§; A. David Edwards, MD*§; Lilly Dubowitz, MD*; and
Irene Roberts, MD‡
ABSTRACT. Background and Purpose. The aim of this study was to evaluate the occurrence of prothrombotic disorders in a well-characterized cohort of infants with neonatal stroke and to document any association of pro-thrombotic disorders with the type of infarct seen on magnetic resonance imaging (MRI) and clinical outcome.
Methods. Twenty-four infants with perinatal cerebral infarction confirmed by neonatal MRI were enrolled in the study. All the infants and, when possible, both par-ents were tested to identify inherited and acquired pro-thrombotic disorders.
Results. None of the infants had a significant bleed-ing diathesis, but 10 (42%) had at least 1 prothrombotic risk factor. Five children showed heterozygosity for fac-tor V Leiden, and 6 had high facfac-tor VIIIc concentrations. There was a striking association between the occurrence of these abnormalities and both the presence of cerebral hemorrhage on MRI and poor neurologic outcome. Eight of the 11 patients (73%) with hemiplegia or global devel-opmental delay had factor V Leiden and/or raised factor VIIIc, whereas only 1 of the 13 patients (8%) with normal outcome had any prothrombotic risk factors. In particu-lar, all 5 infants with factor V Leiden had hemiplegia, compared with only 4 of the 19 infants without factor V Leiden (21%).
Conclusions. These data suggest that the presence of prothrombotic risk factors and, in particular, of the factor V Leiden mutation, is significantly associated with poor outcome after perinatal cerebral infarction. Pediatrics
2001;107:1400 –1404;newborn, stroke, coagulation.
ABBREVIATIONS. MRI, magnetic resonance imaging; T1, longi-tudinal relaxation time.
A
s a consequence of the wider availability of brain imaging in the newborn, neonatal cere-bral infarction has become an increasingly recognized entity, and recent population-based data report an incidence of 1 in 4000 term infants.1,2In the past, these lesions were mainly diagnosed on post-mortem examination and were always associated with severe conditions, such as sepsis, congenitalheart disease, disseminated intravascular coagula-tion, or severe asphyxia. In the last decade, however, several studies using early and serial imaging have demonstrated that infarcts can also be observed in the absence of severe antenatal and/or perinatal events.3,4
Although neonatal imaging has dramatically im-proved the detection of infarcts and may also pro-vide information on the timing of the insult,5 the cause of these lesions is usually unknown. By con-trast, there is increasing evidence that inherited or acquired prothrombotic disorders may play a signif-icant role in patients who have suffered a stroke. In particular, the incidence of factor V Leiden mutation and/or increased factor VIII have been found to be significantly associated with increased risk for thrombosis.6 –13 The majority of these studies, how-ever, reported strokes occurring in childhood or adulthood6 –10and only a small number of newborns with infarction have been studied.11–14
The aim of this study was to assess the thrombo-philic status in a cohort of infants who showed cere-bral infarction on neonatal magnetic resonance im-aging (MRI) and to establish: 1) the prevalence of factor V Leiden mutation and of increased factor VIII in these infants, and 2) whether the presence of these risk factors is related to the type and extent of lesions on MRI scans or to outcome.
METHODS
The study has been approved by the Hammersmith Hospital Trust Research Ethics Committee. Twenty-four infants who showed evidence of cerebral infarction on neonatal MRI were enrolled in this prospective study. In 23 of the 24 infants, the infarct was detected after convulsions in the first days after birth; in 1 infant, the lesion was an incidental finding when the infant was scanned on day 2 as part of a normal control group. All infants had normal Apgar scores at 5 minutes (ⱖ8) and normal cord pH (⬎7.2). Twenty-one of the 24 were white and 3 were Asian.
MRI
The infants were imaged on a 1.0 Tesla Picker HPQ system using conventional T1-weighted spin echo (860/20 ms), inversion recovery (3800/30/950), and T2-weighted spin echo (3000/120 ms) sequences. Although infants have been serially studied, in this study, we only assessed the scans performed between 1 and 4 weeks after birth, when perinatally acquired lesions are at their most obvious. The scans were assessed by an independent ob-server (M.R.) who was blind to the hematologic data.
The infarcts were classified according to the arterial distribu-tion of the lesions. This was based on the locadistribu-tion, extent, and shape of the lesions.4Infarcts were characterized as being in the
From the *Departments of Paediatrics and ‡Haematology and the §Medical Research Council Clinical Sciences Centre, Imperial College, Hammersmith Campus, London, United Kingdom.
Received for publication Jun 26, 2000; accepted Dec 4, 2000.
Reprint requests to (E.M.) Department of Paediatrics, Hammersmith Hos-pital, Du Cane Road, London, W12 OHN, United Kingdom. E-mail: emercuri@ic.ac.uk
territory of the main arteries or in a border zone distribution. The infarcts in the territory of the main arteries were further subdi-vided according to whether they occurred in the main branch or in 1 of the cortical branches of the artery.
The lesions were also classified according to whether the isch-emic lesion was associated with hemorrhage, either within the ischemic lesion or in other sites.
Outcome
Follow-up assessments were performed at 6, 12, 18, and 24 months of age, and then at least yearly after that. All the infants were assessed with a structured neurologic examination15and on
the developmental scales of Griffiths.16
Coagulation and Thrombophilia Profile
The coagulation profile included prothrombin time, activated partial thromboplastin time, thrombin time, platelet count, fibrin-ogen, and von Willebrand factor antigen. The prothrombotic screen included measurement of factor VIIIc, protein C, protein S, and antithrombin. In addition, the presence of the factor V Leiden mutation and the G 3 A transition at position 20210 of the prothrombin gene were screened using polymerase chain reac-tion-based mutation analysis. Blood groups were determined as factor VIII levels are related to the blood group systems of A, AB, B, and O.
Blood samples from children and parents were drawn into 0.105 M trisodium citrate bottles for coagulation tests and ethyl-enediaminetetraacetic acid bottles for full blood counts, blood film, and blood grouping. Blood samples were immediately cen-trifuged at 4°C for 10 minutes at 3000 rpm; platelet-poor plasma was separated and either tested immediately or stored in aliquots at ⫺80°C for testing. Ethylenediaminetetraacetic acid samples were frozen at⫺40°C for DNA analysis. Coagulation screens were performed on fresh platelet poor plasma on the ACL 300 R (In-strumentation Laboratory, Lexington, MA) and KC10 (Brownes Ltd, Reading, UK) instruments before January 1997 and on Sys-mex CA 6000 (SysSys-mex UK Ltd, Milton Keynes, UK) after January 1997. All the children born after May 1996 were tested within the first 2 weeks after birth (n⫽13). The children born before that date (n⫽11) were tested at 1 of their follow-ups.
Hematologic results were classified as normal or abnormal according to age-specific normative data.17,18
Statistics
Data were analyzed using 2 tests with Fisher’s exact test,
where appropriate. Testing the level of significance was set at 0.01 because of multiple significances.
RESULTS MRI
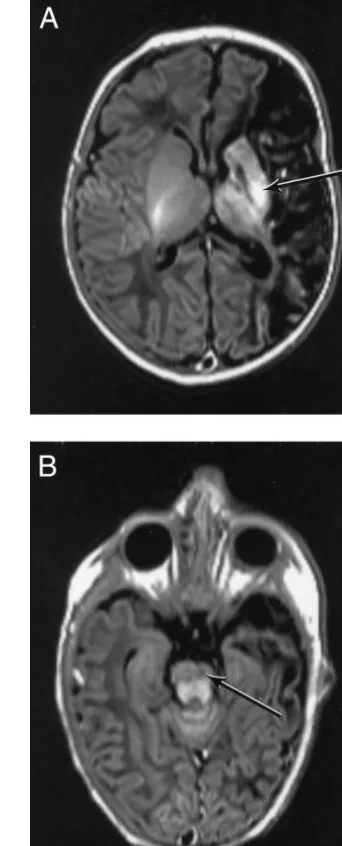
Twenty-two of the 24 children had arterial infarc-tion in the distribuinfarc-tion of the middle cerebral artery and 2 had border zone lesions. Of the 22 children with infarction of the middle cerebral artery, 5 had an infarction in the main branch and 17 in 1 of the cortical branches. Eleven of the 24 children also showed signal changes on T1 and transverse relax-ation time images consistent with hemorrhage, which was always in other sites (Figs 1 and 2). De-tails of the site and size of the lesions are shown in Table 1.
Outcome
All children were at least 24 months old at the time of their final assessment (range: 2–7 years). Thirteen children had a normal outcome, 9 had a hemiplegia, and 2 had global developmental delay but no signs of hemiplegia. In all 9 children with hemiplegia, the clinical signs suggestive of hemiplegia were already present before the age of 2 years.
Coagulation and Thrombophilia Profile
None of the 24 children studied had thrombocyto-penia or any significant abnormality in the coagula-tion profile, and there was no evidence of dissemi-nated intravascular coagulation.
Thrombophilia screening was normal in 14/24 pa-tients (58%). In the 10 papa-tients with abnormal results, the abnormalities were increased factor VIIIc (n⫽6) and heterozygous factor V Leiden (n ⫽ 5). One pa-tient had both increased factor VIIIc and heterozy-gous factor V Leiden. Table 1 shows the details of the individual findings. Parental samples were available for 4 of the 5 children with factor V Leiden heterozy-gosity. In all 4, 1 of the parents (the father in 2 and the mother in the remaining 2), was also heterozy-gous for factor V Leiden. Of the 6 children with
increased factor VIIIc, parental samples were avail-able for 11 parents and were normal in 10 (1 mother had a raised factor VIII when tested 11 months post-natally). In 4 of the 6 children, the increased factor VIIIC was detected at follow-up. Antithrombin, pro-tein C, and propro-tein S were normal in all of the infants tested and in their parents. There was no evidence of the G20210A mutation of the prothrombin gene in any of the children tested.
Correlation Between Hematologic Factors and MRI Findings
Hematologic Factors and Extent and Site of Lesion
There was no association between the extent of the lesions and abnormal hematologic factors. Both nor-mal and abnornor-mal factor VIIIc and factor V Leiden
heterozygosities were found in lesions in the terri-tory of cortical and main branch arteries. Both chil-dren with border zone lesions had increased factor VIIIc.
Hematologic Factors and Hemorrhage
Factor VIIIc was increased both in children with purely ischemic lesions and in those with additional hemorrhage (4/13 and 2/11). Factor V Leiden het-erozygosity was found in 5/11 children with a hem-orrhage and in none of the infants with purely isch-emic lesions (2,P⫽.006; Fisher’s exact test,P⫽.01).
Outcome and Hematologic Factors
Eight of the 11 children (73%) with hemiplegia or developmental delay had at least 1 abnormality of their thrombophilic profile, whereas only 2 of the 13 (8%) with normal outcome had abnormal thrombo-philic profile (2, P ⫽ .005). Factor V Leiden het-erozygosity was significantly associated with the presence of a hemiplegia (Fisher’s exact test, P ⫽ .003). All 5 children with factor V Leiden developed a hemiplegia, compared with only 4 of the 20 chil-dren without this mutation.
Of the 6 patients with increased Factor VIIIc, 3 had hemiplegia and 1 had global delay, whereas 2 had a normal outcome. The difference was not statistically significant. Table 1 shows individual details of out-come and of the coagulation and thrombophilia pro-file.
DISCUSSION
The results of this study showed that ⬃40% of infants with neonatal cerebral infarction had pro-thrombotic risk factors. Specifically, 5 infants were heterozygous for factor V Leiden, 1 of whom also had a raised factor VIIIc, and 5 infants had raised factor VIIIc as an isolated finding.
The prevalence of factor V Leiden mutation in our cohort (24%) was substantially higher than that re-ported in the normal population, which has been estimated between 2.7% and 10% in Europe and North America. In the United Kingdom, the fre-quency of factor V Leiden mutation is 3.4%.19Factor V Leiden has previously been reported in childhood and adult stroke9and is well-known as a risk factor for venous thrombosis.20 –22Recently, it has also been found in neonatal porencephalopathy and in other newborn infants with arterial thrombosis.11,23
This study is the first to demonstrate a relationship between the presence of factor V Leiden and both the nature and outcome of a major vascular occlusive event. All 5 infants in this study with factor V Leiden had residual hemiplegia (100%), whereas only 4 of the 19 infants without factor V Leiden (21%) had hemiplegia.
The reasons for this observation are not clear. In our cohort, factor V Leiden was not specifically as-sociated with the size of the infarct because it was found in both main branch and cortical branch dis-tributions. All of the infants with factor V Leiden, however, showed additional hemorrhagic lesions, which were found in only 30% of infants without factor V Leiden. We have previously reported that
the presence of MRI abnormalities within the basal ganglia, the cerebral hemisphere, and in the posterior limb of the internal capsule predicted the develop-ment of hemiplegia.4 All the infants with factor V Leiden in the present study had MRI abnormalities in these 3 areas. It is, therefore, possible that al-though the presence of prothrombotic risk factors is not associated with the absolute size of an infarct, they may influence both its nature and the combina-tion of sites involved, thereby leading to a high risk of hemiplegia. Factor V Leiden might equally mod-ulate the chance of reperfusion after ischemia or the ability of neural tissue to repair after the insult.
The association between factor V Leiden and hemi-plegia support recent findings by Debus et al23 and Nelson et al.24Both studies looked retrospectively at children with cerebral palsy and/or neurodevelop-mental delay and found a high incidence of pro-thrombotic factors. Debus et al23 also found that
⬎25% of the infants in their cohort had deficiencies of proteins C and S. These were normal in our cohort. This may reflect both sampling error and different gene pools among the study populations.
The other primary finding in the present study was the presence of a raised factor VIIIc level in 6 of the 22 infants in whom this was measured (27%). This is similar to the prevalence of raised factor VIIIc (⬎1.5 IU/mL) among adults with venous thrombosis in the Leiden Thrombophilia Study (25%)25 and in patients with ischemic cerebrovascular disease and compares with an expected prevalence of raised fac-tor VIIIc in the normal healthy population of 11%. Unlike these studies, we did not demonstrate an increased level of von Willebrand factor, which has also been associated with an increased risk of cere-bral infarction.25
Family studies were available for 11 of the parents of the 6 infants with a raised factor VIIIc but only 1
mother had a moderately raised factor VIIIc, which could possibly suggest a genetic basis for the raised factor VIIIc. Factor VIII is well known to behave as an acute phase reactant and so it is possible that increased factor VIII may, in some cases, be a result of rather than a cause of the stroke. However, of the 6 children with raised factor VIIIc, 4 maintained raised factor VIIIc levels beyond the neonatal period, 1 returned to normal at 5 months of age, and 1 was not retested. Normal levels of antithrombin, protein C, and protein S in all the parents tested make it seem unlikely that an anticoagulant deficiency has been missed as a result of the difficulty in applying normal ranges to young children.
These data indicate that the role of elevated factor VIIIc in neonatal stroke is worthy of additional in-vestigation. Three of the 6 infants who had abnormal outcome but did not have the factor V Leiden muta-tion had increased factor VIIIc (⬎1.5 IU/mL). Taken together, our results show that infants with a neona-tal stroke but a normal factor VIIIc and factor V Leiden seem more likely to have a favorable long-term outcome; only 3/14 (21%) having hemiplegia or developmental delay. In contrast, the presence of factor V Leiden and/or a raised Factor VIIIc in in-fants with neonatal stroke is associated with a high risk (80%) of poor neurologic outcome.
The high prevalence of factor V Leiden in our cohort also suggests that genetically determined ab-normalities of thrombophilia play a role in the cause of cerebral infarction. These lesions are probably at-tributable to a multifactorial cause rather than to a single factor. Although by using early and serial imaging we were able to time the insult to the peri-natal period, none of our children had severe acute perinatal events that could be clearly implicated as responsible for the lesion. It is, therefore, likely that genetic factors increase the risk for thrombosis,
TABLE 1. Coagulation Factors, MRI findings, and Outcome
Age Fib VIII vW PC PS FVL Distribution Hemorrhage Outcome
1 7 d 13.48 E E E E Borderzone (R⬎L) — N
2 1 d, 5 mo E 1⫺/1.62 E E E Borderzone (L⬎R) — Hemiplegia
3 1 d, 6 y E E E E E E L main branch — Hemiplegia
4 2.5 y E E E E E E L cortical branch — N
5 18 d, 8 mo E E E E E E L cortical branch — N
6 6 mo E E E E E E L cortical branch — N
7 3 d, 2 y E E E E E L cortical branch — N
8 7 d, 3 y E 1⫺/1.72 E E E E L cortical branch — N
9 2 d E E E L cortical branch — N
10 3.7 y E 11.63 E E E E R cortical branch — Global delay
11 6 mo E E E E E E L cortical branch — N
12 7 d E E E E E E L cortical branch — N
13 9 mo E E E E E E L cortical branch — N
14 9 d, 3 y E 12.5/1.8 E E E E L cortical branch Basal ganglia Global delay
15 2 mo E E E E L cortical branch Small intraventricular hemorrhage N
16 2 d, 15 mo E E E E E E L cortical branch Basal ganglia/punctate N
17 5 d, 3 y E E E E E E L cortical branch Parietal white matter Hemiplegia
18 7 d, 2 mo E E E E R main branch Periventricular N
19 6 mo E E E E E E L main branch Punctate white matter Hemiplegia
20 5 y E E E E E Het L main branch Parietal white matter Hemiplegia
21 6 d, 2 mo 13.6/5.5 E E Het L main branch Mesencephalon/subarachnoid Hemiplegia
22 5 mo E E E E E Het L cortical branch White matter/basal ganglia Hemiplegia
23 4 mo E E E E E Het L cortical branch Basal ganglia Hemiplegia
24 14 d E E E E E Het L cortical branch Basal ganglia Hemiplegia
Fib indicates fibrinogen; VIII, factor VIIIc; vW, von Willebrand factor; PC, protein C; PS, protein S, FVL, factor V Leiden;E, normal;2,
which can then be triggered even by otherwise minor adverse perinatal events.
This study highlights the need to assess all infants with focal hypoxic-ischemic and/or hemorrhagic brain injury for prothrombotic risk factors. The re-sults have implications for prognosis and likely de-velopment of cerebral palsy, as well as for the pos-sibility of future thrombotic events. Preventive measures and counseling of families may be indi-cated.
REFERENCES
1. Estan J, Hope P. Unilateral neonatal cerebral infarction in full term infants.Arch Dis Child. 1997;76:F88 –F93
2. De Vries L, Groenendal F, Eken P, van Haastert IC, Rademakers KJ, Meiners LC. Infarcts in the vascular distribution of the middle cerebral artery in preterm and full-term infants.Neuropediatrics. 1997;28:88 –96 3. Mercuri E, Cowan FM, Rutherford MA, Acolet D, Pennock J, Dubowitz
L. Ischaemic and haemorrhagic brain lesions in infants with seizures and normal Apgar scores.Arch Dis Child. 1995;73:F67–F74
4. Mercuri E, Rutherford M, Cowan F, et al. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electro-encephalogram, and magnetic resonance imaging study. Pediatrics. 1999;103:39 – 46
5. Cowan FM, Pennock JM, Harahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic-ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neu-ropediatrics. 1994;4:172–175
6. Nowak-Gottl U, Strater R, Dubbers A, Oleszuk-Raschke K, Vielhaber H. Ischaemic stroke in infancy and childhood: role of the Arg to Gln mutation in the factor V gene.Blood Coagul Fibrinolysis. 1996;7:684 – 688 7. Sanchez J, Roman J, de la Torre MJ, Velasco F, Torres A. Low prevalence of the factor V Leiden mutation among patients with ischemic stroke.
Haemostasis. 1997;27:9 –15
8. Becker S, Heller CH, Gropp F, Scharrer I, Kreuz W. Thrombophilic disorders in children with cerebral infarction. Lancet. 1998;352: 1756 –1757
9. Ganesan V, McShane MA, Liesner R, Cookson J, Hann I, Kirkham FJ. Inherited prothrombotic states and ischaemic stroke in childhood.
J Neurol Neurosurg Psychiatry. 1998;65:508 –511
10. Cardo Esther V, Campistol J, Artuch R, Colome C, Pineda M. Evaluation
of hyperhomocysteinaemia in children with stroke.Eur J Paediatr Neu-rol. 1999;3:113–117
11. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis.Ann Neurol. 1997;42:372–375 12. Sebire G. Factor V Leiden as a cause of hemiplegic cerebral palsy,
neonatal stroke, and placental thrombosis?Ann Neurol. 1998;44:426 – 427 13. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55: 1539 –1543
14. Hagstrom JN, Walter J, Bluebond-Langner R, Amatniek JC, Manno CS, High KA. Prevalence of the factor V Leiden mutation in children and neonates with thromboembolic disease.J Pediatr. 1998;133:777–781 15. Dubowitz L, Dubowitz V, Mercuri E.The Neurological Assessment of the
Preterm and Full-Term Infant. London, United Kingdom: McKeith Press; 1999
16. Griffiths R.The Abilities of Babies:An Association for Research in Infant and Child Development. Piscataway, NJ: Amersham Pharmacia Biotech; 1976 17. Andrew M, Paes B, Milner R, et al. The development of the human
coagulation system in the full-term infant.Blood. 1987;70:165–172 18. Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L.
Matu-ration of the hemostatic system during childhood. Blood. 1992;80: 1998 –2005
19. Rosendaal FR. Venous thrombosis: a multicausal disease.Lancet. 1993; 353:1167–1173
20. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke and venous thrombosis in apparently healthy men.N Engl J Med. 1995;332:912–917
21. Rees DC. The population genetics of factor V Leiden.Br J Haematol. 1996;95:579 –586
22. Sifontes MT, Nuss R, Jacobson LJ, Griffin JH, Manco-Johnson MJ. Thrombosis in otherwise well children with the factor V Leiden muta-tion.J Pediatr. 1996;128:324 –328
23. Debus O, Koch HG, Kurlemann G, et al. Factor V Leiden and genetic defects of thrombophilia in childhood porencephaly.Arch Dis Child. 1998;78:F121–F124
24. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665– 675
25. Koster T, Blann AD, Briet E, Vanderbrouke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep vein thrombosis.Lancet. 1995;345:152–155
SCHOOL TESTING IS EASY—INTERPRETATION DIFFICULT
. . . It seems reasonable to have tests identify schools that don’t improve. But we may put too much faith in scores that are less accurate than we think. . . Thomas J. Kane, an economist at the Hoover Institution in Palo Alto, California, and Douglas O. Staiger, an economics professor at Dartmouth College, have studied the accu-racy of North Carolina’s tests. When schools there have above average score gains, teachers get bonuses. . . But Dr Kane says even tiny sampling errors can keep scores from reflecting true performance. . . Dr Kane says that in typical North Carolina elementary schools, nearly one third of the variance in school reading gains is a result of the “luck of the draw”. . . A few extreme scores can more easily distort an average. . . Small schools with big gains may seem more effective. But small schools also more often post tiny gains or even losses. A small school’s results may have more to do with sampling error than school quality. . . David Rogosa, a professor of education statistics at Stanford University, analyzed error rates in California, where teachers at schools with rising scores will soon receive bonuses as high as $25,000 each. . . Professor Rogosa estimated that if awards were based on school averages alone, over one fourth of the schools with no gains would still qualify.
Rothstein R.New York Times.January 24, 2001
Noted by JFL, MD
DOI: 10.1542/peds.107.6.1400
2001;107;1400
Pediatrics
Mary Rutherford, A. David Edwards, Lilly Dubowitz and Irene Roberts
Eugenio Mercuri, Frances Cowan, Girish Gupte, Richard Manning, Mike Laffan,
Infants With Neonatal Cerebral Infarction
Prothrombotic Disorders and Abnormal Neurodevelopmental Outcome in
Services
Updated Information &
http://pediatrics.aappublications.org/content/107/6/1400
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/107/6/1400#BIBL
This article cites 23 articles, 4 of which you can access for free at:
Subspecialty Collections
ub
http://www.aappublications.org/cgi/collection/neurologic_disorders_s
Neurologic Disorders
http://www.aappublications.org/cgi/collection/neurology_sub
Neurology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.107.6.1400
2001;107;1400
Pediatrics
Mary Rutherford, A. David Edwards, Lilly Dubowitz and Irene Roberts
Eugenio Mercuri, Frances Cowan, Girish Gupte, Richard Manning, Mike Laffan,
Infants With Neonatal Cerebral Infarction
Prothrombotic Disorders and Abnormal Neurodevelopmental Outcome in
http://pediatrics.aappublications.org/content/107/6/1400
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.