ORIGINAL ARTICLE
Liver Transplantation for HCV Cirrhosis:
Improved Survival in Recent Years and
Increased Severity of Recurrent Disease in
Female Recipients: Results of a Long Term
Retrospective Study
Luca S. Belli,1Andrew K. Burroughs,2Patrizia Burra,3Alberto B. Alberti,1Dimitrios Samonakis,2 Calogero Camma`,4Luciano De Carlis,1Ernesto Minola,5Alberto Quaglia,6Claudio Zavaglia,1 Marcello Vangeli,1David Patch,2Amar Dhillon,6Umberto Cillo,3Maria Guido,7Stefano Fagiuoli,3 Alessandro Giacomoni,1Omar A. Slim,1Aldo Airoldi,1Sara Boninsegna,3Brian R. Davidson,2 Keith Rolles,2and Giovambattista Pinzello1
1Hepatology and Abdominal Organ Transplantation Unit, Niguarda Hospital, Milan, Italy;2Royal Free Hospital and School of Medicine, London, UK;3Department of Gastroenterological Sciences, University Hospital, Padua, Italy;4Istituto di Metodologie Diagnostiche Avanzate CNR, University Hospital, Palermo, Italy;5Department of Histopathology, Niguarda Hospital, Milan, Italy;6Royal Free Hospital, Department of Histopathology, London, UK;7Department of Histopathology, University Hospital, Padua, Italy
In recent years, a worsening outcome of hepatitis C virus (HCV)-positive recipients and a faster progression of recurrent disease to overt cirrhosis have been reported. Our aims were to 1) assess patient survival and development of severe recurrent disease (Ishak fibrosis score⬎3) in different transplant years; and 2) model the effects of pre- and post-liver transplantation (LT) variables on the severity of recurrent disease. A multicenter retrospective analysis was conducted on 502 consecutive HCV-positive transplant recipients between January 1990 and December 2002. Protocol liver biopsies were obtained at 1, 3, 5, 7, and 10 yr post-LT in almost 90% of the patients. All 502 patients were included in the overall survival analysis, while only the 354 patients with a follow-up longer than 1 yr were considered for the analysis of predictors of disease progression. The overall Kaplan-Meier survival rates were 78.7%, 66.3%, and 58.6%, at 12, 60, and 120 months, respectively, and a trend for a better patient survival over the years emerged from all 3 centers. The cumulative probability of developing HCV-related recurrent severe fibrosis (Ishak score 4-6) in the cohort of 354 patients who survived at least 1 yr remained unchanged over the years. Multivariate analysis indicated that older donors (P⫽0.0001) and female gender of recipient (P⫽0.02) were the 2 major risk factors for the development of severe recurrent disease, while the adoption of antilymphocytic preparations was associated with a less aggressive course (P⫽0.03). Two of these prognostic factors, donor age and recipient gender, are easily available before LT and their combination showed an important synergy, such that a female recipient not only had a much higher probability of severe recurrent disease than a male recipient but her risk increased with the increasing age of the donor, reaching almost 100% when the age of the donor was 60 or older. In conclusion, a trend for a better patient survival was observed in more recent years but the cumulative probability of developing severe recurrent disease remained unchanged. The combination of a female recipient receiving an older graft emerged as a strong risk factor for a severe recurrence. Liver Transpl 13:733-740, 2007.©2007 AASLD.
Received August 12, 2006; accepted December 8, 2006.
See Editorial on Page 641
Although recurrence of hepatitis C after liver transplan-tation (LT) is almost universal, the clinical outcome of
posttransplantation hepatitis C is highly variable, with as many as 30% of patients progressing to severe dis-ease within 5 yr after transplantation, while the major-ity have minimal or nonprogressive liver injury.1-3Once
the diagnosis of recurrent cirrhosis has been made, the
Abbreviations:HCV, hepatitis C virus; LT, liver transplantation; CI, confidence interval; HR, hazard ratio.
Address reprint requests to Luca S. Belli, MD, Department of Hepatology and Gastroenterology; Niguarda Hospital; Piazza Ospedale Maggiore 3, 20162, Milan, Italy. Telephone: 39-02-64442684; FAX: 39-02-64442788; E-mail: luca.belli@ospedaleniguarda.it; lucasaverio.belli@fastwebnet.it DOI 10.1002/lt.21093
risk of decompensation has been estimated to be ap-proximately 50% in 1 yr.3This course of the disease is
clearly accelerated when compared with that observed in the nontransplant population and decreases sur-vival. In this respect, data from the United Network for Organ Sharing registry indicate that LT in hepatitis C virus (HCV)-positive recipients is associated with an increased rate of death and graft failure as compared with patients who are HCV-negative.4In addition,
al-though individual host and viral characteristics have been associated with poor patient and graft survival,4-9
no model exists that can reliably identify those recipi-ents at greatest risk for either patient mortality or de-velopment of severe recurrent disease after transplan-tation. Moreover, a further complicating factor is that the rate of recurrent cirrhosis appears to have in-creased in recent years and the reasons for these worse results have been associated with the increasing age of donors and the use of more potent immunosuppressive protocols.10,11 Given this background, the present
study had 2 main aims:
1. to assess patient survival and fibrosis progression in different transplant years; and
2. to model the effects of pre- and posttransplantation variables on both patient survival and severity of the recurrent disease defined as the development of a Ishak fibrosis score⬎3.
To do this, we evaluated a large number of HCV-positive recipients transplanted in 3 European centers, each center having similar transplant activity over the years and adopting protocol liver biopsies during the follow-up.
MATERIALS AND METHODS
Study Population
Between January 1990 and December 2002, 502 con-secutive patients with HCV-related cirrhosis and with-out B or Delta coinfections were transplanted in 3 dif-ferent European Centers (Niguarda Hospital in Milan (center 1), Royal Free Hospital in London (center 2), and University Hospital in Padua (center 3). All 502 patients were included in the overall survival analysis, while only the 354 patients with a follow-up longer than 1 yr were considered for the analysis of predictors of both disease progression and patient survival from 1 yr on-ward. HCV infection was defined as positivity of serum HCV-ribonucleic acid by reverse transcription polymer-ase chain reaction.
Histological Follow-Up
Protocol liver biopsies were performed at least at 1, 3, and 5 yr posttransplantation, independently of liver biochemistry. Beyond this interval, a late liver biopsy was usually performed between 7 and 10 yr after trans-plantation. Considering only the scheduled liver biop-sies performed after the first posttransplantation year, a total of 847 biopsy specimens were available for his-tological analysis with a mean of 2.43 per patient
(me-dian 2, range 1-5). Liver biopsies were reviewed by 3 local pathologists (E.M. in Milan, A.Q. in London, and M.G. in Padua) who agreed to utilize the Ishak score for assessing the stage of fibrosis and the degree of necro-inflammatory activity.12 Protocol liver biopsies were
available in 342 of 354 (97%), 224 of 251 (89%), 156 of 175 (89%), 83 of 116 (72%), and 42 of 47 (89%) recipi-ents who were followed up for 1, 3, 5, 7, and 10 yr after orthotopic LT. Notably, overall expected liver biopsies at 3 centers were 90%, 84%, and 92%. All liver biopsies were blindly reevaluated at each site for the purpose of this study and the pathologists agreed on these 2 main points: 1) to recognize the parameter fibrosis S4-6 (Ishak fibrosis score 4-6); and 2) to consider as inade-quate liver biopsies with less than 6 complete portal spaces.
Immunosuppression
Milan
Induction immunosuppression did not change over the years and consisted of quadruple therapy with rabbit antithymocyte globulins (ATG; Fresenius, Bad Hom-burgh, Germany) for the first 5 days, azathioprine for the first 30 days, steroids that were generally with-drawn within 3 months from transplant, and cyclospor-ine maintacyclospor-ined long-term. Protocol biopsies were not used to evaluate rejection.
London
Induction protocols were changed over the years: for the first 5 yr of transplant activity, most patients re-ceived standard triple therapy (cyclosporine, azathio-prine, and steroids), whereas in the following years, tacrolimus was substituted for cyclosporine or tacroli-mus was used as monotherapy. Steroids were usually withdrawn within 6 months, whereas azathioprine was continued long-term unless side effects or complica-tions developed. Secondary drugs, including azathio-prine, mycophenolate mofetil, and steroids, were dis-continued within the first year after transplantation. Protocol biopsies were used to evaluate rejection.
Padua
Double-drug induction (cyclosporine or tacrolimus and corticosteroids) was the standard protocol. Steroids were withdrawn within 6 months from transplant and progressively earlier in recent years. Protocol biopsies were not used to evaluate rejection.
In case of renal impairment (defined as a plasma creatinine⬎1.5 mg/dL) all 3 centers adopted the policy of reducing target cyclosporine or tacrolimus levels, which were in general halved, while either azathioprine or mycophenolate acid were continued or added.
Virologic Data
The diagnosis of hepatitis C was based on the detection of anti-HCV and HCV-ribonucleic acid in the serum (HCV amplicor kit; Roche Diagnostic Systems,
Branch-burgh, NJ) prior to transplantation. HCV genotypes were typed according to different standardized meth-ods. In 20% of patients, genotyping results were not available mainly due to lack of serum samples. The viral load was not evaluated as a predictive factor, because the tests used to quantify viremia have changed sub-stantially over the years, and correctly stored serum samples were not usually available.
Statistics
Outcomes
Follow-up time was defined as the number of months from LT to clinical events, death, or the last contact with the patient up to December 31, 2003. Follow-up data were obtained through periodic controls at outpatient clinics. Clinical examination and routine laboratory in-vestigations were performed at least every 6 months in all cases. The primary outcome was survival. The sec-ondary outcome was severe fibrosis progression, de-fined as the development of an Ishak fibrosis score⬎3.
Statistical evaluation
Continuous variables were expressed as mean⫾ stan-dard deviation. The 2 and Student’s t-test were
per-formed as appropriate, allPvalues were 2-tailed. The Kaplan-Meier method was used to estimate the length of survival and of survival without severe fibrosis pro-gression. Different variables regarding the recipient, the donor, and the virus were considered for univariate analysis. Variables with aPvalue ⬍0.10 at univariate analysis were included in the final multivariate model. The following variables were evaluated by univariate analysis for both patient survival and development of stage 4-6 fibrosis. 1) Host risk factors: age at transplan-tation and gender, presence of hepatocellular carci-noma either known or incidental. 2) Viral factors: geno-type (genogeno-type 1 or 4 vs. non-1 non-4). 3) Donor-related variables: age, gender, and donor/recipient gender mis-match. In accord with data coming from the litera-ture,10donor age was also categorized in the following
cohorts:⬍50; 50-59, and⬎60 yr. 4) External risk fac-tors: including the type of immunosuppression (cyclo-sporine vs. tacrolimus; induction with antilymphocytic preparations; use of steroid pulses during the first 6 months; exposure yes/no and length of administration of azathioprine, steroids, and mycophenolate mofetil) and antiviral therapy with at least 6 months of inter-feron and ribavirin. 5) Year of transplantation both as a continuous variable and categorized as follows: 1990-1992; 1993-1994; 1995-1996; 1997-98; 1999-2000; and 2001-2002.
The Cox proportional hazard model was used to as-sess survival and survival without severe fibrosis pro-gression in a multiple repro-gression analysis. All analyses were conducted with SAS version 6.08 (SAS Institute, Cary, NC). For the Cox regression, the SAS PHREG procedure was used. AllPvalues were 2-tailed, and all the confidence intervals (CIs) were 95%. We verified that the assumption of proportional hazard in the Cox
model, was not violated; this was also checked with analytical and graphical methods. We also performed a test of trend in the hazard ratio by adding into the model a new variable representing the interaction effect between the prognostic variable and the follow-up time. If this variable is not statistically significant, this rep-resents a strong evidence that there is no trend (in-crease or de(in-crease) over time in the hazard ratio. To estimate expected survival time for a hypothetical pa-tient with a combination of prognostic factors, the esti-mate survival function was computed.
RESULTS
Patient Characteristics at Baseline
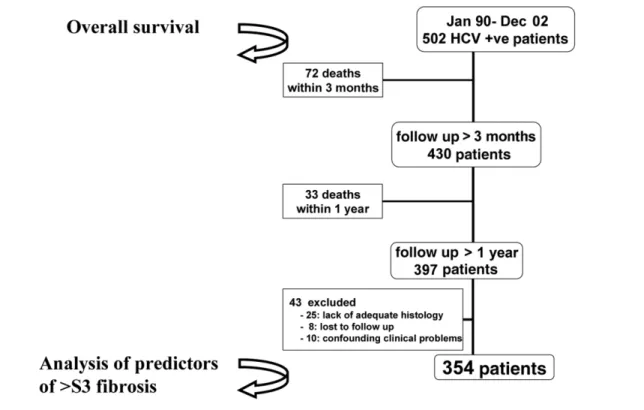
All 502 patients were included in the overall survival analysis while only the 354 patients with a follow-up longer than 1 yr were considered for the analysis of predictors of both disease progression and patient sur-vival from 1 yr onward (Fig. 1). Of the 148 patients excluded, 72 died within the first 3 postoperative months (early post-LT mortality), usually from a com-bination of poor graft function and sepsis and none from HCV recurrence. A total of 33 patients died be-tween 3 months and 1 yr from transplant and were excluded because they did not survive long enough to reach the time of the 1-yr protocol liver biopsy. The causes of death of these 33 patients were: recurrent hepatocellular carcinoma (6), vascular accidents (6), hematological neoplasms (3), chronic rejection (3), a combination of sepsis and poor graft function (12), HCV cholestatic recurrence (2), and cryptogenic cirrhosis (1). Of the remaining 43 excluded patients who survived beyond 1 yr, 33 were “dropouts,” either due to lack of adequate liver histology during follow-up (25 patients) or were lost to follow-up (8 patients). Finally, the re-maining 10 patients were excluded for the presence of ongoing confounding factors (4 patients with ongoing biliary problems, 3 with de novo hepatitis B virus, 2 with arterial occlusion, and 1 with portal thrombosis). The baseline characteristics of the subgroup of 354 patients eligible for the analysis of predictors are shown in Table 1. Notably, no differences were found between the baseline clinical features of the whole cohort of 502 patients and those of the 354 patients considered in the analysis of predictors.
Overall Survival Analysis on the Whole
Population of 502 Patients: Effect of Year of
Transplantation
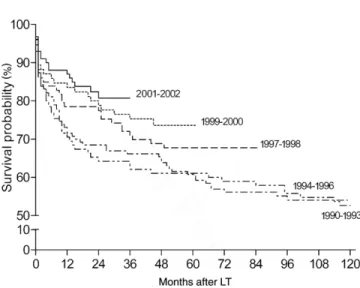
The overall survival rates were 78.7%, 66.3%, and 58.6%, at 12, 60, and 120 months, respectively, with-out a significant difference among the 3 participating centers (P⫽0.2 by log-rank test). In more detail, overall patient survival at 1, 5, and 10 yr after transplantation was: 74%, 64%, and 58% at Center 1; 78%, 68%, and 61% at Center 2; and 83%, 75%, and 65% at Center 3. A favorable trend over the years was observed when the survival analysis was performed according to
Kaplan-Meier (Fig. 2). We also arbitrarily split our pa-tient cohort into 2 subgroups (papa-tients transplanted before and after December 31, 1998) to make our re-sults comparable with those of the Valencia group that reported a very unfavorable outcome after December 31, 1998. When comparing patients receiving trans-plants before and after December 31, 1998, we ob-served a reduced mortality both at 3 and 12 months in patients receiving transplants after 1998 (P⫽0.09 and P⫽0.001) (Table 2).
Variables Associated With Patient Survival
(From 1 Yr) and With Severe Fibrosis
Progression
A total of 59 patients died during the follow-up period. Severe HCV recurrence was responsible for more than one-half of the deaths (33/59, 56%). The causes of death in the remaining 26 patients were as follows: de novo tumor (8), recurrent hepatocellular carcinoma (4), sepsis (3), chronic rejection (3), vascular accidents (3), and other various causes (5). Notably, the Ishak fibrosis scores in the 26 patients who died of causes unrelated to HCV ranged between 1 and 3.
A total of 89 of the 354 (25.1%) transplant recipients developed an Ishak fibrosis score 4-6 after a median time of 35 months posttransplantation. The overall 1-, 3-, 5-, and 10-yr cumulative incidence of Ishak fibrosis score 4-6 was 10%, 18%, 27%, and 32%, respectively. Notably, this cumulative incidence of Ishak fibrosis score 4-6 did not change significantly over the years (P⫽0.6).
Patient survival
By univariate analysis, 5 variables (presence of hepato-cellular carcinoma, advanced donor age, therapy of acute rejection, fibrosis progression beyond stage 3, and earlier year of transplantation [both as a continu-ous and categorical variable]) were significantly associ-ated with reduced patient survival and were included in a Cox proportional hazards model. Of these 5 variables, only 3 (advanced donor age, fibrosis progression be-yond stage 3, and earlier year of transplantation) re-mained significant after Cox multivariate analysis (Ta-ble 3).
Severe fibrosis progression
By univariate analysis, 5 variables (recipient female gender, advanced age of the donor, female gender of the donor, donor/recipient gender mismatch, and avoid-ance of induction therapy with antilymphocytic prepa-rations) were significantly associated with the develop-ment of Ishak fibrosis score 4-6 and were included in the Cox proportional hazards model. Of these 5 vari-ables, only 3 (recipient female gender, advanced age of the donor, and avoidance of antilymphocytic prepara-tions for induction) remained significant after Cox mul-tivariate analysis (Table 3).
The prognostic effect of donor age on fibrosis progres-sion was confirmed in all 3 subcohorts of each partici-pating center. As for female gender of the recipient, all 3 subgroups analyzed showed a similar “effect size” but no statistical significance (Center 1: hazard ratio [HR] 0.54, 95% CI 0.25-1.16,P⫽0.11; Center 2: HR 0.52, 95% CI 0.27-1.005,P⫽0.051; Center 3: HR 0.59, 95% Figure 1. Study population.
CI 0.22-1.158,P⫽0.29). The third variable “induction with antibody” could not be retested in the 3 separated subcohorts as it was mainly utilized in Center 1. Upon replacing Ishak fibrosis score 5-6 as the dependent variable in the multivariate analysis, we obtained a model in which donor age (HR 1.034; 95% CI 1.018-1.050) emerged as the only significant predictor while recipient gender (HR 0.62; 95% CI 0.38-1.022) and in-duction with rabbit antithymocyte globulins (HR 0.65; 95% CI 0.40-1.080) became of marginal statistical sig-nificance.
We finally combined the 2 significant prognostic fac-tors that can easily be obtained before transplantation (recipient gender and donor age) looking for possible interactions. The estimated probabilities of severe fibro-sis progression for hypothetical recipients according to their gender and age of their donors are shown in Figure 3. Notably, a female recipient receiving a graft from a 60-yr-old donor had an almost doubled risk of worsen-ing severity of hepatic fibrosis than a male recipient receiving a graft from the same 60-yr-old donor. Figure 2. Survival of the whole cohort of 502 patients:
Kaplan-Mayer analysis.
TABLE 1. Baseline Clinical Features of the 354 Patients Eligible for the Analysis of Predictors of Severe Fibrosis Development (Ishak Fibrosis Score⬎3) in the 3 Centers
Center 1 n⫽134 Center 2 n⫽129 Center 3 n⫽91 P
No. of early retransplantation (within 3 months from LT) (%) 9 (6.7) 7 (5.4) 4 (4.4) 0.75
Recipient age ( mean⫾SD) 50.7⫾7.3 50.9⫾8.8 51.6⫾8.4 0.46
Recipient gender (male/female) 97/37 98/31 66/25 0.77
HCC pre-LT (%) 51 (38.1) 35 (27.1) 22 (24.2) 0.05
Genotype (1-4/non-1 non-4) 83/45* 64/53* 30/8* 0.02
Donor gender (male/female) 91/43 77/48† 54/34† 0.68
Donor age (mean⫾SD) 38⫾17.4 41.8⫾13.5 37.1⫾16 0.98
Donor-recipient sex mismatch (%) 25/134 (18.7) 39/125‡(31.2) 31/88‡(35.2) 0.012
Year of LT 0.002 1990-1992 23 12 1 1993-1994 18 20 14 1995-1996 20 25 16 1997-1998 21 26 15 1999-2000 28 25 13 2001-2002 24 21 32
Primary immunosuppressant: CyA/Tacrolimus 130/4 70/59 49/42 0.001
Induction with antibodies (%) 134 (100) 5 (3.9) 2 (2.2) 0.001
Other immunosuppressant: AZA/MMF/no AZA no MMF 134/0/0 91/9/29 15/17/59 0.001
Months of AZA/MMF (mean⫾SD) 1⫾0 27.5⫾33.7 9.6⫾7.8 0.0001
Acute rejection therapy (%) 36 (26.9) 90 (69.8) 42 (46.1) 0.001
Type of acute rejection therapy 0.001
1-3 MP boluses (%) 9 (6.7) 56 (43.4) 29 (31.9)
⬎3 MP boluses (%) 27 (20.1) 29 (22.5) 9 (9.9)
OKT3 (%) 0 (0) 5 (3.9) 4 (4.4)
Months of maintenance with steroids (mean⫾SD) 4.8⫾7.1 3.8⫾8.8 3.1⫾0.8 0.13
IFN/RBV therapy post-LT (%) 43 (32.1) 0 (0)- 38 (41.8) 0.001
Follow up (mean⫾SD) 72⫾43.6 66⫾37.6 58⫾34.9 0.008
Recipient clinical status: dead (%) 19 (14.2) 27 (20.9) 13 (14.3) 0.27
Patients with Ishak fibrosis score⬎3 (%) 27 (20.1) 44 (34.1) 18 (19.8) 0.025 Abbreviations: HCC, hepatocellular carcinoma; CyA, cyclosporine; AZA, azathioprine; MMF, mycophenolate mofetil; MP, methylprednisolone; IFN, interferon; RBV, ribavirin; SD, standard deviation.
*6, 11, and 53 genotypes missing from the 3 centers, respectively. †4 and 3 donor gender missing.
DISCUSSION
Although the main limits of the present study, as in many other retrospective studies, are related to the heterogeneity of the posttransplantation immunosuppressive regimens and the presence of many other confounding variables, nev-ertheless, it has 2 major strengths as it considers a large cohort of HCV liver transplant recipients followed up for a long period of time and it evaluates fibrosis progression after LT using protocol liver biopsies scheduled up to 10 yr after transplantation. Our main findings can be summarized as
follows: 1) patients receiving transplants in more recent years have a significant improved outcome in spite of the increased utilization of older donors; 2) older donors and the female gender of the recipients are the 2 major risk factor for the development of severe recurrent disease, while the adop-tion of an inducadop-tion therapy with antibody preparaadop-tions is associated with a less aggressive course.
These observations stand in sharp contrast to what has been previously reported by some groups who showed a dramatic worsening in the outcome of HCV
TABLE 2. Mortalities at 3 and 12 Months Post-LT in the Overall Cohort of 502 Patients: Comparison Between LT Performed Before and After December 31, 1998
1990-1998 1999-2002 P
Mortality at 3 months (%) 52/318 (16) 20/184 (11) 0.09
Mortality at 12 months (%) 85/318 (27) 26/184 (14) 0.001
TABLE 3. Predictors of Death and of Development Severe Fibrosis (Ishak Score⬎3)
Predictor
Mortality Severe fibrosis
Univariate analysis Multivariate analysis Univariate analysis Multivariate analysis Hazard ratio (95% CI) Pvalue Hazard ratio (95% CI) Pvalue Hazard ratio (95% CI) Pvalue Hazard ratio (95% CI) Pvalue Center 1.10 (0.79-1.53) 0.54 1.11 (0.85-1.43) 0.42 Recipient age 1 (0.97-1.03) 0.83 0.99 (0.97-1.02) 0.81 Recipient gender 0.99 (0.55-1.78) 0.97 0.614 (0.39-0.95) 0.03 0.575 (0.36-0.98) 0.02 HCC 2.08 (1.24-3.49) 0.005 1.678 (0.98-2.86) 0.056 1.38 (0.89-2.14) 0.14 Genotype (1-4 vs. non-1 non-4) 0.97 (0.67-1.40) 0.88 1.48 (0.78-2.91) 0.22 Donor age 1.03 (1.01-1.04) 0.001 1.019 (1.00-1.04) 0.03 1.036 (1.02-1.05) 0.0001 1.035 (1.02-1.05) 0.0001 Donor gender 0.81 (0.48-1.37) 0.44 0.53 (0.35-0.82) 0.004 0.80 (0.49-1.30) 0.36 Donor/recipient gender mismatch 0.96 (0.54-1.71) 1 0.44 (0.22-3.87) 0.04 1 (0.61-1.64) 0.97 Primary CNI: CyA vs.
Tacrolimus 1.18 (0.8-1.67) 0.33 0.93 (0.68-1.26) 0.64 Induction with antibody 0.71 (0.41-1.22) 0.21 0.56 (0.35-0,89) 0.001 0.596 (0.37-0.97) 0.03 AZA or MMF vs. no AZA/MMF 1.10 (0.63-1.90) 0.73 1.062 (0.68-1.65) 0.78 Therapy of acute rejection 1.48 (1.18-1.85) 0.0006 1.22 (0.91-1.62) 0.18 1.19 (0.97-1.46) 0.08 0.06 (0.11-0.26) 0.6 IFN⫹RBV post-LT 0.65 (0.36-1.15) 0.14 0.92 (0.62-1.36) 0.68 Fibrosis; Ishak score
⬎3 (⬎S3) 6.22 (3.84-10.1) 0.0001 4.91 (2.91-8.28) 0.0001 Year of LT: continuous variable 0.87 (0.80-0.95) 0.003 0.89 (0.8-0.98) 0.02 1.04 (0.97-1.12) 0.2 Year of LT: 2000-2002 vs. 1990-1999 0.74 (0.61-0.90) 0.02 0.21 (0.06-0.74) 0.015 0.08 (0.93-1.26) 0.3
Abbreviations: HCC, hepatocellular carcinoma; CNI, calcineurin-inhibitor; CyA, cyclosprine; AZA, azathioprine; MMF, mycophenolate mofetil; IFN, interferon; RBV, ribavirine; CI, confidence interval.
patients transplanted after 1998,3,5,11with only a 60%
survival at 1 yr in 1 center.10,11One explanation given
was possibly the combined detrimental effect of the increasing age of the donors and the use of more potent induction immunosuppression, followed by a rapid re-constitution of the immune system with discontinua-tion of secondary drugs at earlier time points than was previously done. The present study confirms that the age of the donor is an important determinant of both patient survival and fibrosis progression.10,13,14
How-ever, the survival improved over the years despite the fact that the median age of donors in our cohort was even higher than that reported by the Valencia group and there did not appear to be a worsening of the fibro-sis progression over the years. The improved survival in the present study is in keeping with the recent analysis of the United Network for Organ Sharing database on 6,441 HCV patients transplanted after 1994,15showing
that graft and patient survival was not lower in recent years compared with earlier time periods. Moreover, another study based on strict histologic surveillance of 183 HCV recipients undergoing LT in Berlin over the last 15 yr did not see any increase in fibrosis progres-sion in patients transplanted after 1996 when com-pared with those transplanted before 1996.16Notably,
a very recent work from the Valencia group,17 which
considered patients transplanted between 2001 and 2004, reported a significant improvement in the out-come of HCV infected patients in line with this and many other experiences.
Our finding that the use of T-cell-depleting drugs in
the induction phase of immunosuppression is associ-ated with a protective effect on fibrosis progression is rather intriguing. These regimens, in fact, result in very low rates of acute cellular rejection. One can speculate that avoidance of cellular rejection may be crucial in these patients as it will spare the utilization of steroid pulses, which have been frequently associated with se-vere recurrent disease.18-20
A key result of our analysis is that female recipients have worse HCV-induced fibrosis progression than male recipients and, importantly, this finding was ob-served in all 3 subcohorts of patients from the 3 partic-ipating centers. Although to our knowledge this associ-ation has never been reported in the past, data from the United Network for Organ Sharing registry have clearly indicated that HCV-positive female recipients have worse survival than males.4 It is difficult to explain
such a negative association between female gender and fibrosis progression, particularly if we consider that these results are at odds with several studies of HCV progression in nontransplant patients, in which male gender and not female is associated with a worse out-come. However, postmenopausal females lose this ad-vantage in the pretransplantation setting, which may represent a better comparison with the posttransplan-tation population. Preliminary data in immunocompe-tent individuals with chronic hepatitis C suggest that steatosis is frequently encountered in postmenopausal women and is an important cofactor for disease pro-gression.21Since the majority of our female recipients
are in the postmenopausal state, this association needs Figure 3. Cumulative hazard of severe fibrosis after LT in female (top) and in male (bottom) recipients, by donor age.
to be reinvestigated in appropriately-designed studies. To evaluate the clinical relevance of our findings, we evaluated the estimated risk of severe recurrent disease due to the combination of 2 prognostic factors, both of which can be easily obtained before transplantation: recipient gender and donor age. The results demon-strate a very important synergy such that the allocation of an old donor (⬎60 yr) to a female recipient with hepatitis C leads to severe fibrosis progression within 5 yr from transplantation in about 90% of cases. On the contrary, the allocation of the same old donor to a male recipient allows much better results, with less than 50% of the male patients progressing beyond Ishak fibrosis score 3 at 5 yr from transplantation. Currently, prioritization of recipients for LT in the United States is based solely on recipient characteristics (Model for End-Stage Liver Disease score, which combines serum bilirubin, creatinine, and international normalized ra-tio of prothrombin time), which does not take into con-sideration the etiology of the disease in the recipient nor their gender. Neither does it considers any donor factor. Although it is too early to suggest changes of the allo-cation procedures based on our findings, which need to be confirmed by others, nevertheless this study strongly suggests that the combination of a female re-cipient receiving an old graft should be considered a strong risk factor for the development of severe recur-rence.
REFERENCES
1. Gane E, Portman BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996; 334:815-820.
2. Feray C, Caccamo L, Alexander GJ, Ducot B, Gugenheim J, Casanovas T, et al. Collaborative study on factors influ-encing the outcome after liver transplantation for hepatitis C. Gastroenterology 1999;117:619-625.
3. Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayon M, et al. HCV related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 2000; 32;673-684.
4. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;112:889-896.
5. Charlton M, Seaberg E, Wiesner R, Everarth J, Zetter-mann R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology 1998;28:823-830.
6. Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and
Kid-ney Diseases Liver Transplantation Database. Liver Transpl Surg 1995;5(Suppl 1):S107–S114.
7. Rosen HR, Chou S, Corless CL, Gretch DR, Flora KD, Boudousquie A, et al. Cytomegalovirus viremia. Risk fac-tor for allograft cirrhosis after liver transplantation for hepatitis C. Transplantation 1997;64:721-726.
8. Belli LS, Burra P, Poli F, Alberti AB, Silini E, Zavaglia C, et al. G. HLA-DRB1 donor-recipient mismatch affects the outcome of hepatitis C disease recurrence after liver trans-plantation. Gastroenterology 2006;130:695-702.
9. Sreekumar R, Gonzales-Koch A, Maor-Kendler Y, Batts K, Moreno-Luna L, Poterucha J, et al. Early identification of recipients with progressive histologic recurrence of hepa-titis C after liver transplantation. Hepatology 2000;32: 1125-1130.
10. Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV infected liver transplant recipients. Hepatology 2002;36:202-210. 11. Berenguer M, Crippin J, Gish R, Bass N, Bostrom A, Netto G, et al. A model to predict severe HCV related disease following liver transplantation. Hepatology 2003;38:34-41.
12. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk E. Histologic grading and staging of chronic hepatitis. J Hepatol 1995;22:696-699.
13. Hoofnagle JH, Lombardero M, Zettermann RK, Lake J, Porayko M, Everarth J, et al. Donor age and outcome of liver transplantation. Hepatology 1996;24:89-96. 14. Charlton M. The impact of advancing donor age on
histo-logic recurrence of hepatitis C: the perils of ignored ma-ternal advice. Liver Transpl 2003;9:535-537.
15. Russo WM, Galanko JA, Zacks SL, Beavers KL, Fried MW, Shrestha S. Impact of donor age and year of transplant on graft survival in liver transplant recipients with chronic hepatitis C. Am J Transplant 2004;4:1133-1138. 16. Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM,
Neuhaus R, et al. Fibrosis progression after liver trans-plantation in patients with recurrent hepatitis C. J Hepa-tol 2004;41:830-836.
17. Berenguer M, Aguilera V, Prieto M, San Juan F, Rayon JM, Benlloch S, Berenguer J. Significant improvement in the outcome of HCV-infected transplant recipients by avoid-ing rapid steroid taperavoid-ing and potent induction immuno-suppression. J Hepatol 2006;44:717-722.
18. Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology 1995;21:30-34.
19. Lake JR. The role of immunosuppression in recurrence of hepatitis C. Liver Transplantation 2003;9(Suppl 3):63-66. 20. Wiesner R, Sorrell M, Villamil F. Report of the first Inter-national Liver Transplantation Society Expert Panel Con-sensus Conference on liver transplantation and hepatitis C. Liver Transpl 2003;9(Suppl 3):1-9.
21. Camma` C, Bruno S, Di Marco V, Di Bona D, Rumi M, Vinci M, et al. Insulin resistance is associated with steatosis in non diabetic patients with genotype 1 chronic hepatitis C. Hepatology 2006;43:64-71.