Original Article
Prognostic value of the metastatic lymph node
ratio in patients with pancreatic cancer
Chengzhi He1, Huisong Chen2, Yingxin Wang1, Changqing Yang1, Hengjun Gao1,3
1Department of Gastroenterology, Tongji Institute of Digestive Diseases, Tongji Hospital, Tongji University School of
Medicine, Shanghai 200065, China; 2Department of Gastroenterology, Shanghai Ninth People’s Hospital,
Shang-hai Jiao Tong University School of Medicine, ShangShang-hai 201999, China; 3Shanghai Engineering Center for
Molecu-lar Medicine, National Engineering Center for Biochip at Shanghai, Shanghai 201203, China
Received August 29, 2019; Accepted November 25, 2019; Epub December 1, 2019; Published December 15, 2019
Abstract: The purpose of the study was to analyze the prognostic value of metastatic lymph node ratio (LNR) in pancreatic cancer (PC) patients undergoing surgical operation. We retrospectively reviewed 615 patients with PC who underwent surgical operation. The clinic-pathologic factors related to lymph node metastasis (LNM) were ana-lyzed. There are 251 patients with LNM (40.8%), 364 cases of patients without LNM (59.2%). We found that overall survival (OS) of PC was significantly correlated to histological type, degree of differentiation, clinical staging, LNM, and LNR (all P < 0.05). Multivariate Cox regression analysis demonstrated that LNR was an independent risk factor for postoperative survival rate of patients with PC (P=0.006), and the presence of LNM was not an independent factor for predicting poor prognosis. When age and gender were included in multivariate Cox proportional hazards, LNR was still the independent prognostic factor for the OS of patients with PC (P=0.013). The value of LNR in the prediction of the prognosis of PC was better than the number and presence of LNM. It provided some helpful advice for clinicians to formulating a reasonable treatment strategy.
Keywords: Lymphatic metastasis, outcomes, prognostic factors, pancreatic cancer
Introduction
Pancreatic cancer (PC) is a lethal disease owing to the biology of cancer high invasion and me- tastasis. A large number of studies have shown that cancer cell invasion and metastasis can occur in the early stage of PC [1, 2]. Cancer cell invade and metastasize mainly into the sur-rounding lymph nodes, nerves and retroperito-neal plexus, which is the main cause of easy relapse after the radical resection of PC. Stud- ies have demonstrated that lymph node metas-tasis (LNM) is the main way of cancer metasta-sis and regarded as an important risk factor affecting survival outcomes for patients with PC [3-5]. However, LNM remains poorly associ-ated with overall survival (OS) in patients with positive margins and distal resections [6]. The American Joint Committee on Cancer (AJ- CC)/Union for International Cancer Control (UICC) tumor, node, and metastasis (TNM) stag-ing system are widely recognized and followed
more accurate prognostic method than LNM status and the number of positive lymph node [14]. However, most previous studies involved small sample size and different studies have adopted various LNR cutoff points for grouping [9]. Obviously, there is no consensus among researchers regarding which LNR cutoff value is the best for predicting prognosis in patients with PC. Moreover, the relationship of LNR and OS in PC has not been well defined [15]. To further confirm this result, we retrospective-ly reviewed the clinical and pathological data of 615 patients with PC who underwent surgical operation, and focused on evaluating the
rela-tionships between LNR and OS by comparing with traditional pN staging. We also investigat-ed the associations between LNR and other clinicopathological factors.
Materials and methods
Patients
Retrospective analysis of 615 cases of patients with PC undergoing radical surgery from July 2004 to April 2013, stored in the biobank cen-ter in National Engineering Cencen-ter for Biochip at Shanghai. Written informed consent was re- ceived from all patients and the study was approved by the ethical committee of the bio-bank center in National Engineering Center for Biochip at Shanghai. The clinical and pathologi-cal features of patients were obtained from the surgical pathology report. We collected the fol-lowing data: age, sex, tumor site, tumor size, pathology grade, degree of differentiation, and information on OS. Detailed information of clini-cal pathologiclini-cal data of 615 patients is provid-ed in Table 1. The study was approved by the ethical committee of biobank center in National Engineering Center for Biochip at Shanghai.
Status of lymph node dissection and postop-erative follow-up
Lymph nodes were retrieved in surgical PC specimens by a panel of pathologists, and fixed by Formaldehyde solution, made into paraffin sections. The lymph nodes status of specimens was observed by experienced pathologists after H&E staining. All follow-up times were measured from the date of surgery to the patient’s death from PC. Follow-up was per-formed at the hospital outpatient clinic by tele-phone or letter. The deadline date of follow-up was October 2013 and the longest follow-up period was 89 months.
Statistical method
The associations between clinicopathological parameters and LNM, LNR were compared using the χ2-test. OS was calculated and sur-vival curves were plotted according to Kaplan-Meier method and compared using log-rank test. Hazard ratios (HRs) were calculated using univariate and multivariate Cox regression analysis. The significant factors in univariate models were further analyzed by the
multivari-Table 1. Clinicopathological characteristics of the pancreatic cancer
Characteristics No. of Patients % Age (years)
median 60
range 17-90
Gender
female 235 38.2
male 380 61.8
Stage
stage I 161 26.2
stage II 192 31.2
stage III 0 0
stage IV 8 1.3
missing data 254 41.3
Lymph node status
negative 364 59.2
positive 251 40.8
LNR
LNR=0 364 59.2
LNR < 0.25 112 18.2
LNR≥0.25 139 22.6
Tumor site
head 377 61.3
body and tail 176 28.6
others 62 10.1
Tumor size
median (cm) 4
range (cm) 0.5-21 Tumor differentiation
well 112 19.8
moderate 380 61.8
poor 20 3.3
[image:2.612.90.285.98.518.2]ate Cox’s analysis for independent prognostic value. The statistical analyses were performed using the SPSS software package (SPSS Inc, Chicago, IL, USA, version 17.0). All statistical tests were two-sided and P values < 0.05 were considered statistically significant.
Results
Lymph node information
The clinicopathological parameters and data of the follow-up of 615 patients with lymph nodes status of PC were collected (Table 1). Among these patients, 251 cases of patients (40.8%) with lymph node metastasis, 364 cases of patients (59.2%) without lymph node metasta-sis. According to the postoperative pathologic results, a total of 4887 lymph nodes were observed and the number of examined lymph nodes ranged from 1 to 32, with a mean of 8. Of the 251 cases of patients with LNM, a total number of 647 positive lymph nodes were
found, with a mean of 2.6. Spearman rank cor-relation analysis showed that the number of lymph node metastasis were positively corre-lated with the detectability of lymph nodes (P < 0.01).
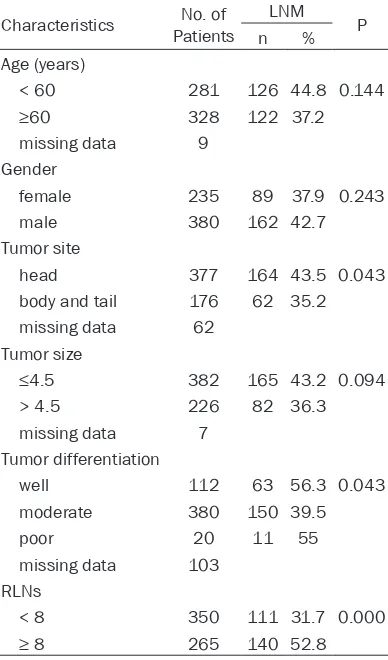
The relationship between the clinical patho-logic features and LNM, LNR
LNR was categorized into three groups (0, < 25% and ≥25%) according to the results of receiver-operating characteristic plots for det- ermination of the cut-off value. The relation-ships between the clinical pathological charac-teristics of 615 patients with PC and LNM, LNR are respectively shown in Tables 2 and 3. Our result shows that differentiation (P=0.043), number of retrieved lymph nodes (RLNs) (P < 0.01) are significantly correlated with LNM. However, gender, age, type of organization, pathological classification, and lesion size are not significantly associated with LNM (all P > 0.05). We find that age (P=0.008), tumor site (P=0.004), tumor size (P < 0.001) and RLNs (P < 0.001) have significant relationship with LNR.
The relationship between the clinical patholog-ic features and the prognosis univariate and multivariate survival analysis
Follow-up data was available in 111 cases, and the median survival period is 12 months, and 1 year, 3 years, 5 years survival rates were 49.3%, 33.1% and 29.7% respectively. Kaplan-Meier survival analysis showed that with LNM (P=0.019), LNR (P=0.001), AJCC TNM staging (P=0.037) were the prognostic factors in PC (Figure 1). However, gender, age, tumor differ-entiation, tumor location, tumor size, lymph node group number, RLNs had no significant association with survival prognosis.
[image:3.612.92.286.102.432.2]Multivariable COX regression analysis indicat-ed the LNR (P=0.016), the degree of differenti-ation (P=0.002) are independent risk factors for postoperative PC (Table 4). The presence of LNM was not an independent factor of progno-sis of PC survival. When age and gender were introduced into the equation, LNR was still an independent prognostic predictor for PC. This result suggests that the influence of LNR on the prognosis of patients with PC is significantly higher than LNM.
Table 2. Relationships between the clinical pathologic features and LNM
Characteristics PatientsNo. of LNM P
n %
Age (years)
< 60 281 126 44.8 0.144
≥60 328 122 37.2
missing data 9 Gender
female 235 89 37.9 0.243
male 380 162 42.7
Tumor site
head 377 164 43.5 0.043
body and tail 176 62 35.2 missing data 62
Tumor size
≤4.5 382 165 43.2 0.094
> 4.5 226 82 36.3
missing data 7 Tumor differentiation
well 112 63 56.3 0.043
moderate 380 150 39.5
poor 20 11 55
missing data 103 RLNs
< 8 350 111 31.7 0.000
Table 3. Relationships between the clinical pathologic features and LNR
Characteristics PatientsNo. of LNR P 0 < 25% ≥25% Age (years)
< 60 281 155 65 61 0.008
≥60 328 206 44 78
Gender
female 235 146 41 48 0.478
male 380 218 71 91
Tumor site
head 377 213 82 82 0.004
body and tail 176 114 20 42 Tumor size
≤4.5 382 217 93 72 0.000
>4.5 226 144 19 63
Tumor differentiation
well 112 59 27 36 0.082
moderate 380 230 70 80
poor 20 9 5 6
RLNs
< 8 350 239 21 90 0.000
≥8 265 125 91 49
Although the RLNs were not significantly asso-ciated with survival prognosis, the stratification analysis by AJCC/UICC stage for PC revealed that RLNs ( < 8 vs ≥8) mainly affected the prog-nosis of patients with stage I PC (P=0.041), while it does not influence the survival of patients with stage II. Moreover, we found that the 5-year survival rate of PC decreased signifi-cantly with increasing LNR when the number of lymph node detected was greater than or equal to 8 (P < 0.001) (Figure 2).
ROC curve analysis
ROC analysis was used to further evaluate the prognostic performance of the LNR, LNM, and RLNs for survival prediction in this study. The areas under the curve (AUCs) of LNR, LNM and RLNs were 0.621±0.05 (P=0.027), 0.610± 0.052 (P=0.046) and 0.468±0.054 (P=0.559) respectively. The result indicated that LNR is a better predictor of prognosis than LNM and RLNs in evaluating the prognosis of PC (Figure 3).
Discussion
In the present study, we retrospectively
ana-tumor samples of PC patients who under-went surgical resection. Survival analysis found that LNM and LNR were the influ-ence factors for the prognosis of patients with PC. Multivariable COX regression analysis indicated that the LNR was an independent risk factor for clinical prog-nosis. Furthermore, ROC analysis showed that LNR was a more powerful predictor for evaluating the prognosis of postopera-tive PC than LNM.
LNM was one of the most important prog-nostic factors for subsequent adjuvant treatments in patients with cancer. In the present study, the results showed that the OS of patients with LNM was significantly (P=0.019) shorter than that of patients without LNM. However, multivariable COX regression analysis failed to show LNM was an independent prognostic factor in PC. Moreover, some previous studies sug-gested that RLNs was a prognostic factor for survival [14, 16, 17]. However, a sys-tematic review with 17 studies including 4,883 patients showed that RLNs did not affect the prognosis of PC [15]. Our study found that RLNs and the number of positive lymph node were also not important factors for predicting clinical outcome in PC, which is con-sistent with some other research results [18, 19]. However, in the present study, the stratifi-cation analysis by AJCC/UICC stage revealed that RLNs ( < 8 vs ≥8) mainly affected the prog-nosis of patients with stage I PC (P=0.041), which was used to stratify patients into two groups that should be considered when select-ing treatment [20].
clini-Table 4. Univariate and multivariable Cox regression analysis of overall survival (n=183)
Features Univariate analysis Multivariable analysis
HR (95% CI) P value HR (95% CI) P value Tumor features
age (years), < 60 v ≥60 0.96 (0.60 to 1.54) 0.877 sex, female v male 0.85 (0.61 to 1.17) 0.314 tumor location, head v body and rear 1.43 (0.86 to 2.37) 0.172 tumor size, ≤4.5 v > 4.5 1.01 (0.62 to 1.61) 0.997
tumor differentiation, poor v moderate/well 0.53 (0.32 to 0.89) 0.015 0.42 (0.24 to 0.74) 0.002 AJCC stage, I v II, IV 1.54 (1.10 to 2.17) 0.013 1.12 (0.72 to 1.75) 0.619 LNM, positive v negative 1.69 (1.07 to 2.65) 0.023 0.66 (0.26 to 1.64) 0.370 LNR (0, < 0.25, ≥0.25) 1.58 (1.19 to 2.11) 0.002 2.45 (1.18 to 5.09) 0.016 lymph node group number 1.95 (0.54 to 1.69) 0.867
RLNs (< 8, ≥8) 0.67 (0.43 to 1.05) 0.080
Komatsu et al. [21] retrospectively analyzed 138 patients who underwent curative gastrec-tomy with lymphadenecgastrec-tomy,the univariate and multivariate analyses revealed that LNR [>/= 0.4, P < 0.001, HR 3.1 (95% CI 1.7-5.4)] was independent prognostic factor and was useful to evaluate the extent of local tumor clearance in pN3 gastric cancer. However, the role of LNR in predicting survival is contradictory. Mohan et al. [22] compared the LNR with the current N1/ N2 classification of Stage III colon cancer, found that the LNR had low specificity and sen-sitivity for predicting survival and did not pro-vide additional prognostic value to current staging for overall or disease-specific survival. Nakagawa et al. [23] analyzed data on 191 Patients who underwent gastrectomy with curative intent for remnant gastric cancer, the multivariable analyses revealed that pT (7th
[image:5.612.91.523.70.222.2] [image:5.612.92.526.288.460.2]contrary, LNR significantly was decreased when the number of lymph nodes detected was less than 8.
[image:6.612.92.521.70.446.2]Although LNR is better than lymph node metas-tasis status in reflecting the degree of nodal metastasis and prognosis of patients, studies on the boundary value of LNR exists a big differ-ence [26]. Aoyama et al. [9] used a lymph node ratio of 0.1 to be the optimal cut-off point for grouping based on the 3-year and 5-year sur-vival rates. Komatsu et al. [27] showed that the Figure 2. The stratification analysis by AJCC/UICC stage showed that RLNs (< 8 vs ≥8) mainly affected the prognosis of patients with stage I (P=0.041) (A), while it does not influence the survival of patients with stage II (P=0.262) (B). Kaplan-Meier analysis of LNR and OS after stratification by RLNs (< 8 vs ≥8) showed that no significant association was observed between LNR and OS when RLNs < 8 (P=0.271) (C), while high level of LNR correlated with worse survival when RLNs ≥8 (P=0.001) (D).
[image:6.612.91.286.526.719.2]LNR was an independent prognostic factor using multivariate analysis, and found that the best cut-off value was 0.2 to stratify the prog-nosis of gastric cancer patients. Vinh-Hung [28] et al. used the cutoff points of LNR 0.20 and 0.65 classified 17,685 breast cancer patients into three groups: low (≤0.20), intermediate ( > 0.20 and ≤0.65), and high-risk ( > 0.65). The result indicated that LNR was significantly cor-related with prognosis of postoperative inva-sive breast cancer. Some LNR cutoff values have been proposed based on quartiles, means and various other statistical derivations with-out any consensus [29-31]. In our study, a LNR of 0.25 was considered to be the optimal cut-off point for classification, the results showed that patients with a LNR≥0.25 had significantly shorter OS compared to those with a LNR < 0.25. Similar to our result, Ahmad et al. [30] used the median LNR of 0.25 to stratify patients into high LNR and low LNR groups and the result showed that LNR was an independent predictor of survival in patients undergoing liver resection in stage IV CRC and was associated with the extent of hepatic tumor burden. In summary, our study demonstrated that LNR was superior to TNM and AICC staging in pre-dicting clinical outcome and LNR≥0.25 was an independent adverse prognostic factor, which was very powerful and useful for prognostic assessment for patient with PC. Of course, vali-dation of this value in a similar setting is required. Moreover, extended lymph node dis-section or postoperative chemotherapy may be selected to improve the outcome for high LNR patients.
The study was approved by the ethical commit-tee of biobank center in National Engineering Center for Biochip at Shanghai. Written in- formed consent was received from all patients.
Acknowledgements
This study is supported by China National 863 Project Foundation for Cancer Genomics (Pan- creas Genomics) (Grant No: 1006AA02A302). Disclosure of conflict of interest
None.
Address correspondence to: Dr. Hengjun Gao, Department of Gastroenterology, Tongji Institute of
Digestive Diseases, Tongji Hospital, School of Medicine, Tongji University, Shanghai 200065, China. E-mail: hengjun_gao@shbiochip.com
References
[1] Baxter NN, Whitson BA and Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol 2007; 14: 1320-6.
[2] Du L, DeFoe M, Ruzinova MB, Olsen JR and Wang-Gillam A. Perioperative therapy for surgi-cally resectable pancreatic adenocarcinoma. Hematol Oncol Clin North Am 2015; 29: 717-26.
[3] Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD and Yeo CJ. 1423 pancreati-coduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006; 10: 1199-210; discussion 1210-1. [4] Schmidt CM, Powell ES, Yiannoutsos CT, How-ard TJ, Wiebke EA, Wiesenauer CA, BaumgHow-ard- Baumgard-ner JA, Cummings OW, Jacobson LE, Broadie TA, Canal DF, Goulet RJ Jr, Curie EA, Cardenes H, Watkins JM, Loehrer PJ, Lillemoe KD and Madura JA. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004; 139: 718-25; discussion 725-7.
[5] Ignjatovic I, Knezevic S, Knezevic D, Dugalic V, Micev M, Matic S, Ostojic S, Bogdanovic M, Pavlovic I and Jurisic V. Standard versus ex-tended lymphadenectomy in radical surgical treatment for pancreatic head carcinoma. J BUON 2017; 22: 232-8.
[6] Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, Bui JL, Yuan C, Qian ZR, Babic A, Da Silva A, Nowak JA, Khalaf N, Brais LK, Welch MW, Zellers CL, Ng K, Chang DT, Miksad RA, Bullock AJ, Tseng JF, Swanson RS, Clancy TE, Linehan DC, Findeis-Hosey JJ, Doyle LA, Horn-ick JL, Ogino S, Fuchs CS, Hezel AF, Koong AC and Wolpin BM. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer 2017; 117: 1874-82.
[8] Solak M, Turkoz FP, Keskin O, Aksoy S, Baba-can T, Sarici F, Kertmen N, Sever AR and Al-tundag K. The lymph node ratio as an indepen-dent prognostic factor for non-metastatic node-positive breast cancer recurrence and mortality. J BUON 2015; 20: 737-45.
[9] Aoyama T, Yamamoto N, Kamiya M, Murakawa M, Tamagawa H, Sawazaki S, Numata M, Shio-zawa M, Kobayashi S, Ueno M, Morimoto M, Yukawa N, Oshima T, Yoshikawa T, Rino Y, Ma-suda M and Morinaga S. The lymph node ratio is an independent prognostic factor in pancatic cancer patients who receive curative re-section followed by adjuvant chemotherapy. Anticancer Res 2018; 38: 4877-82.
[10] Jin C, Deng X, Li Y, He W, Yang X and Liu J. Lymph node ratio is an independent prognostic factor for rectal cancer after neoadjuvant ther-apy: a meta-analysis. J EvidBased Med 2018; 11: 169-175.
[11] Haager B, Wiesemann S, Passlick B and Schmid S. Prognostic value of lymph node ratio after induction therapy in stage IIIA/N2 non-small cell lung cancer: a monocentric clinical study. J Thorac Dis 2018; 10: 3225-31. [12] Joo JH, Kim YS and Nam JH. Prognostic
signifi-cance of lymph node ratio in node-positive cer-vical cancer patients. Medicine (Baltimore) 2018; 97: e11711.
[13] Sawayama H, Iwatsuki M, Kuroda D, Toihata T, Uchihara T, Koga Y, Yagi T, Kiyozumi Y, Eto T, Hiyoshi Y, Ishimoto T, Baba Y, Miyamoto Y, Yo-shida N and Baba H. The association of the lymph node ratio and serum carbohydrate anti-gen 19-9 with early recurrence after curative gastrectomy for gastric cancer. Surg Today 2018; 48: 994-1003.
[14] Conci S, Ruzzenente A, Sandri M, Bertuzzo F, Campagnaro T, Bagante F, Capelli P, D’Onofrio M, Piccino M, Dorna AE, Pedrazzani C, Iacono C and Guglielmi A. What is the most accurate lymph node staging method for perihilar chol-angiocarcinoma? Comparison of UICC/AJCC pN stage, number of metastatic lymph nodes, lymph node ratio, and log odds of metastatic lymph nodes. Eur J Surg Oncol 2017; 43: 743-50.
[15] Elshaer M, Gravante G, Kosmin M, Riaz A and Al-Bahrani A. A systematic review of the prog-nostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl 2017; 99: 101-6.
[16] Mirkin KA, Hollenbeak CS and Wong J. Greater lymph node retrieval and lymph node ratio im-pacts survival in resected pancreatic cancer. J Surg Res 2017; 220: 12-24.
[17] Contreras CM, Lin CP, Oster RA, Reddy S, Wang
atic cancer survival with greater lymph node retrieval in the National cancer data base. Am J Surgy 2017; 214: 442-9.
[18] Bouliaris K, Rachiotis G, Diamantis A, Christo-doulidis G, Polychronopoulou E and Tepetes K. Lymph node ratio as a prognostic factor in gas-tric cancer patients following D1 resection. Comparison with the current TNM staging sys-tem. Eur J Surg Oncol 2017; 43: 1350-6. [19] Dasari BV, Pasquali S, Vohra RS, Smith AM,
Taylor MA, Sutcliffe RP, Muiesan P, Roberts KJ, Isaac J and Mirza DF. Extended versus stan-dard lymphadenectomy for pancreatic head cancer: meta-analysis of randomized con-trolled trials. J Gastrointest Surg 2015; 19: 1725-32.
[20] Basturk O, Saka B, Balci S, Postlewait LM, Knight J, Goodman M,Kooby D, Sarmiento JM, El-Rayes B, Choi H, Bagci P, Krasinskas A, Quig-ley B, Reid MD, Akkas G, Maithel SK, Adsay V. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann Surg On-col 2015; 22 Suppl 3: S1187-95.
[21] Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Okamoto K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki A, Kuriu Y, Ikoma H, Na-kanishi M, Fujiwara H and Otsuji E. Positive lymph node ratio as an indicator of prognosis and local tumor clearance in N3 gastric can-cer. J Gastrointest Surg 2016; 20: 1565-71. [22] Mohan HM, Walsh C, Kennelly R, Ng CH,
O’Connell PR, Hyland JM,Hanly A, Martin S, Gibbons D, Sheahan K and Winter DC. The lymph node ratio does not provide additional prognostic information compared with the N1/ N2 classification in Stage III colon cancer. Colorectal Dis 2017; 19: 165-71.
[23] Nakagawa M, Choi YY, An JY, Hong JH, Kim JW, Kim HI, Cheong JH, Hyung WJ, Choi SH and Noh SH. Staging for remnant gastric cancer: the metastatic lymph node ratio vs. the UICC 7th edition system. Ann Surg Oncol 2016; 23: 4322-31.
[24] Healy MA, Reynolds E, Banerjee M and Wong SL. Lymph node ratio is less prognostic in mel-anoma when minimum node retrieval thresh-olds are not met. Ann Surg Oncol 2017; 24: 340-6.
[25] Duan XF, Tang P, Shang XB, Jiang HJ and Yu ZT. Metastatic to negative lymph node ratio dem-onstrates significant prognostic value in pa-tients with esophageal squamous cell carcino-ma after esophagectomy. Oncotarget 2017; 8: 86908-16.
node ratio as a prognostic factor for survival in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx 2018; 45: 846-53.
[27] Komatsu S, Ichikawa D, Nishimura M, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H and Otsuji E. Evaluation of prognostic value and stage migration effect using positive lymph node ratio in gastric cancer. Eur J Surg Oncol 2017; 43: 203-9.
[28] Vinh-Hung V, Joseph SA, Coutty N, Ly BH, Vlas-tos G and Nguyen NP. Age and axillary lymph node ratio in postmenopausal women with T1-T2 node positive breast cancer. Oncologist 2010; 15: 1050-62.
[29] Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, Friess H and Hölzel D. The prognostic value of lymph node ratio in a population-based collective of colorectal can-cer patients. Ann Surg 2010; 251: 1070-8.
[30] Ahmad A, Reha J, Saied A, Espat NJ, Somasun-dar P and Katz SC. Association of primary tu-mor lymph node ratio with burden of liver me-tastases and survival in stage IV colorectal cancer. Hepatobiliary Surg Nutr 2017; 6: 154-61.