355
OSMOTIC AND IONIC REGULATION IN THE SHORE
CRAB, CARCINUS MAENAS, WITH NOTES ON THE
BLOOD CONCENTRATIONS OF GAMMARUS
LOCUSTA AND LIGIA OCEANICA
BY J. B. BATEMAN
1.
(From the Marine Biological Laboratory, Plymouth.)
(Received 5th March, 1933.)
I. EXPERIMENTS ON CARCINUS.
IN the body fluids of marine invertebrates the concentrations of the various ions are usually different from those in the surrounding sea water, although diffusion between the two media can certainly take place, and although in most cases the total osmotic pressure is the same. In Carcinus mamas in ordinary sea water this approximate osmotic equality exists: in diluted sea water, however, the blood can be maintained at a considerably higher osmotic pressure than the medium. It has been suggested (A. V. Hill, 1931) that the gill membrane is impermeable to water, though permeable to salts. If so, then the steady state in Carcinus living in dilute sea water is merely the result of ionic regulation, which is usual, extended in an unusual degree to Na' and Cl' ions. If water cannot penetrate, then osmotic forces in the ordinary sense will not affect the regulation at all.
If the membrane is permeable to water, a specific resistance to water movement must exist, as postulated by Schlieper (19296), and the resultant observed ionic regulation might be affected by the presence of an indiffusible but osmotically active non-electrolyte in the external medium. An attempt is made in this paper to decide between these possibilities, and a further object of the work was to study the manner in which the regulation of a given ion—say Cl'—is modified by the presence of an added electrolyte such as NaNO3 or Na2SO4. The results obtained
have been complicated by the unpredictable specific effects of the substances added, and no generalisation is possible. The facts are given, therefore, as an empirical study of a living membrane with peculiar and interesting properties, without any attempt at a detailed interpretation.
An effort is made, also, to obtain data for a rough calculation of the work done in the regulatory process in Carcinus, and for a further analysis of the process; and some exploratory experiments—on the effect of cyanide, pH, temperature, and on the osmotic properties of the isolated gill—have been made, suggested by superficial analogies with other secretory processes such as the production of urine, the recovery of nerve and the accumulation of ions in plant cells.
356 J. B. BATEMAN
Symbols and units,
(1) The suffixes i and 0 indicate that values refer respectively to the body fluids and to the outside solution.
(2) c = total concentration, determined by vapour pressure measurement, ex-pressed as the molarity (moles per 1000 gm. water) of the aqueous NaCl solution with the same vapour pressure.
(3) Concentrations of individual substances, unless otherwise stated, are given as moles per 1000 gm. water; "concentration" is indicated by square brackets— e.g. [Cl].
(4) Ac, A [Cl], etc., always refer to the difference in concentration between internal and external media, being positive when the internal concentration is greater than the external.
(5) Thus with the above units Ac and A [Cl] can be at once compared, so that Ac — A [Cl] always gives a measure of the total difference in concentration between substances other than Cl in the internal and external media.
(6) Numerical data are for brevity usually given as mean values, with mention of the number of experiments performed, and a "mean error" calculated from the formula V'Ld'^/n (n — 1). It is not intended, however, to have any exact significance as a mean error, since the number of experiments is usually too small. It is merely a convenient way of indicating the amount of scatter among the results.
Experimental methods.
The crabs were obtained from the Cattewater, Plymouth Sound. They were generally kept for some days in the aquarium tanks, where they could feed, then for a further period without food in large accumulator jars containing 10-20 litres of aquarium tank water, and they were starved during all experiments. The com-position of the tank water is not strictly controlled. It is rather more concentrated than outside sea water, with c usually about 1-063 M. [Ca] is about 0-015 M, compared with 0-0098 M for outside sea water (Cooper, 1932). The pH is about 7-8, but is somewhat variable (Atkins, 1922).
Normal [Cl] and c for crab's blood.
Table I gives the mean results of Cl determinations, with a few vapour pressure measurements. Total water 0-900 for centrifuged blood and 0-964 for tank water were assumed. For crabs in tank water and 7/8 tank water A [Cl] is small and variable in sign. The observations of previous workers that blood [Cl] is less than that of sea water is due apparently to the units used being mg. NaCl per gm. or
Table I. Chloride and vapour-pressure measurements on crabs living in various concentrations of sea water.
Units and symbols are as denned on p. 356.
Date .(193O April April May-June May July Oct. July May July July July External solution Tank water +
0-27 M NaCl Tank water
4-o - i 3 7 M N a C l Tank water + o-o86MNaCl Tank water
>>
7/8 tank water
3/4 tank water
5/8 tank water
1/2 tank water
n 3 5 6 4 3 4 3 4 2 3 3 Times of immersion 45-48 hours 96 hours— 3 weeks 25 hours-4 weeks — — — — — [a], — — — 0-641 ± O-O2O O-6l7 ± O-OO7 0-574 ± 0010 0-522 ±0-014 0-463 ± 0-017 0-489 ± 0020 0-446 ± 0-009 0-396 ± 0017 [Gl]0 — — — 0-621 0-617 0-585 0516 O'457 0-452 0366 0-282 A[C1] — — — + O-O2O + o-oo — O-OII + 0-006 + 0-006 + 0-037 + 0-080 + 0-114 Ac + 0-0055 ± 0-004 + 0-0062 ± 0-003 — 0-0027 ± 0-006 + 0-0009 ± 0-003 + O-OO2 ± O002 + 0-048 ±O-OI3 + OO70 ± 0-004 + 0-I2I ± O-OO5
Ac - A [Cl]
—
—
—
+ 0-0009
— 0 0 0 4
+ O-OII
— o-oio
+ 0-007
Table I I . Blood chloride concentrations of several crabs, expressed as moles Cl per 1000 gm. water.
Observer
Quinton (1912) Duval (1924) Bethe (1929)
Bethe and Berger (1931) Bialaszewicz (1930) Dakin and Edmonds (1931) Bethe and Berger (1931)
[Cl]* 0598 0-571 o-68o 0-607 0-700 0-474 0-489 [Cl]0 0-590 0-587 0 6 5 0 0571 0-665 0-581 O'57i
[Cl]t--[C1]O
[image:3.533.43.454.209.594.2]per c.c. of blood. Table II, in which some of these are converted into moles per 1000 gm. water, shows that the difference is not real in the case of Carcinus and Maia, but that it is significant in the mangrove crab, Heloecius, and in Eriocheir sinensis. These are both brackish water forms and capable of living in fresh water. Similarly the grapsoid crabs studied by Baumberger and Olmsted (1928) appear to regulate to some extent in sea water.
Blood concentration of crabs living in air.
Carcinus can survive for some days in air, and it was interesting incidentally to find the resulting changes in blood vapour pressure. Six crabs were kept in dry jars for 8 days. At the end of this time two, the smallest, were dead, one was nearly dead, and three were comatose but became lively on stimulation. The blood of these three was plentiful and normal in appearance. The mean value of c, determined by comparison with 0-025 M NaCl, was 0-815 M. This behaviour of the blood is different from that observed in littoral crabs living almost continually on land (Pearse, 1930-1, 1931).
Blood vapour pressure and [Cl] for crabs in diluted and concentrated media.
For comparison with the effect of foreign substances it was necessary to have parallel determinations for crabs in diluted tank water. The crabs were kept for 1-7 days in the solutions (Table I). The total concentration differences have been observed before; by Fredericq (1901), Quinton (1912), Duval (1925), Schlieper (1929 a) and Margaria (1931). The [Cl] values agree well with these, and show that other ions or organic molecules in the body fluids play no consistent part in producing the osmotic differences, although the discrepancies occasionally observed cannot be due to experimental error. The subject needs further study with reference to moult age.
In sea water concentrated by NaCl, Duval's result (1925) is confirmed: there is here no osmotic regulation. The highest concentration of added NaCl tolerated for long periods was 0-17 M; 0-25 M was toxic. This is roughly the increase in blood concentration occurring in crabs dying in air. The Australian crabs are apparently different: Heloecius cordiformis, in concentrated sea water, can maintain its blood at a lower concentration than that of the external medium (Dakin and Edmonds, 1931).
Rate of change of concentration on immersion in diluted or concentrated media.
occurs in concentrated media. Crabs from tank water were put for 1-5 hours in tank water + 0-46 M NaCl, and the resulting change of blood vapour pressure determined. For comparison, other crabs from tank water were kept for. the same period in 1/4 tank water, giving an equal and opposite initial concentration gradient. The results (Table III) are expressed as the concentration difference between blood and the original external solution (tank water).
Table I I I . Rates of change of blood concentration in crabs from diluted and concentrated sea water.
The crabs were kept in tank water for 5 days before the experiment.
Concen-tration of tank water
c
0602
o-6n
Experimental external solution
1/4 tank water Tank water + 0-46 M NaCl
n
4 4
co
0149 1-070
Initial
concen-tration gradient
(c - c0)
+ O-4S3 - °-459
Change in c{
after i | hours
0049 ± 0-003
O-IIO ± 0-007
Change in weight
(%)
+ i'9 ±0-5 — 0 9 ±0-3
A legitimate objection to such experiments is that the physiologically unbalanced sea water + NaCl solution may alter the condition of the gill membrane. This is not easily disproved, but the following facts are against such a supposition: (a) The large difference between rates of dilution and concentration is not paralleled by different weight changes. (b) In several additional experiments it was found that the blood of crabs immersed in diluted sea water became diluted at the same rate as that of crabs in an isotonic pure NaCl solution.
The effect of cyanide.
If the steady state in Carcinus has some oxidative mechanism, it might be affected by cyanide, as are analogous processes. And, if water regulation occurs, cyanide should cause increased weight changes.
(i) Four crabs after 4 days in 1/2 tank water were put into 1/2 tank water + M/10,000 KCN. After 20 hours, two dead, one in poor condition—slow in reacting, abdomen extended—and one normal. Controls in 1/2 tank water were all normal. Mean Ac was 0-113 ± 0-003 M.
(ii) Four crabs from 1/2 tank water put into 1/2 tank water + M/20,000 KCN. After 19 hours, one dead, one normal, two rather unreactive. Mean Ac was 0-092 ± o-oioM. The blood smelt distinctly of HCN. Controls in 1/2 tank water gave 0-109 ± 0-009 M, but a further set gave 0-148 ± 0-006, while the mean in Table I, data of a year earlier, is o-i2 ± 0-005. The cause of these large variations is not clear, but the effect of cyanide is certainly not striking, even in lethal concentrations. Sometimes the absence of any effect could be due to impaired circulation, but often the crabs behaved normally.
(iii) Seven crabs from tank water were put for 2 hours in tank water + M/i5,ooo KCN and then for 1-5 hours in 1/4 tank water + Mj 15,000 KCN. The mean decrease in c at the end of the experiment was 0-057 ± 0-006 M, and the mean increase in weight o-8 ± 0-2 per cent. The crabs were all in good condition; the largest amount of dilution, 0-077, w a s shown by a soft crab, apparently in normal condition.
(iv) A further experiment like (iii). 4 hours tank water + M/15,000 KCN. 1-5 hours in 1/2 tank water + M/15,000 KCN. Decrease in c 0-041 M, increase in weight I-I per cent, n = 2.
360 J. B. BATEMAN
The physiological effect of cyanide was peculiar. The animals very soon lost their postural reflexes and seemed to be in a stupor, but continued stimulation would awaken them and they would then remain very active for an hour or two.
The rate of dilution in cyanide is slightly greater than the normal, and together with the steady state data one may conclude that cyanide has some effect, but that in these experiments the steady state was not abolished, nor did the rate of change of concentration approach that observed with crabs in concentrated sea water. The weight changes are quite close to those in Table III.
The effect of foreign electrolytes on Cl distribution and vapour pressure.
(i) Sodium sulphate. [Cl] and Ac determinations were made with blood from crabs in Na2SO4 solutions, and the amount of sulphate in the blood deduced from
them (Table IV). The last column, Ac — A [Cl], represents the osmotic equivalent, in terms of an NaCl solution, of the difference in sulphate concentration between blood and external medium.
Table IV. The effects of several foreign electrolytes on the steady state.
External medium previous to experiment Tank water 3/4 tank water 1/2 tank water Tank water Tank water Tank water Tank water Tank water
or 1/2 tank water 1/2 tank water Tank water Tank water Experimental solution
Tank water + 0-22 M NaaSO4.1oH.jO 3/4 tank water + 0-15 M Na2SO4.
ioH2O 1/2 tank water +
o-ioMNajSO4.
ioH20
Tank water + 0-15 M MgSO4.7H2O
1/2 tank water +
oisMMgSOj.
7H2O
Tank water + 0-15 M NaNO8
3/4 tank water +
1/2 tank water + 0-15 M NaNO3
1/4 tank water +
o - i s M N a N O ,
Tank water + 0-15 M NaAc.3H2O
1/2 tank water + o-15MNaAc.3H.1O
co
0-84
0-62
0 4 2
0-69
0-39
O 7 9
0-63
0-46
0-31
o-75
0 4 5
"Mean t (hours) 2 1 19 28 27 37 26 26 4 0 23 26 46 n 2 4 3 4 3 5 4 16 2 4 2 0-725 ± O ± 0-035 0-289 db 0-008
0 6 2 8 db 0-006 0-430
± 0 - 0 1 8
0-587 db 0-005 0-458 db 0-003 0-356 db 0-006 0-274 db 0-019 0-675 ±O-OI2 0-417 ± OO41 [Cl]o 0-663 0-497 0-282 0-645 0-286 0-627 0479 0303 0-142 0618 0-290 A[C1] + 0-062 + 0-044 + 0-007 — 0-017 + 0-144
— 0 - 0 4 0
— O-O2I + OO53 + O-I32 + OO57 + 0-127 Ac — 0-048 ± 0-019 + 0003 ± 0-021 + 0-060 ± 0-009 — 0-007 ± 0-004 + 0-073 ± 0017 + 0009 db 0-007 + 0-030 ± 0003 + 0049 db 0-004 + 0-104 db 0013 — 0-030 db 0-021 — Ac-A[Cl] = A [SOJ,
etc. — O-IIO — 0-041 + 0-053 + o-oio — 0-071 + 0049 + 0-051
— 0 0 0 4
— 0-028
— 0087
Evidently the Cl distribution is affected by the presence of Na^C^. (a) In 1/2 tank water + o-io M Na2SO4 the normal A [Cl] for crabs in 1/2 tank water
(about 0-12 M) is abolished, but a difference of total concentration is produced by the accumulation of SO4" in the blood (A [SO4"] = + 0-053 M NaCl). In other words, the accumulation of Na" ions is the same whether they are accompanied by Cl' or SO4" anions. This result disagrees with the single experiment of Bethe
(1929), who found that in 1/2 sea water with added NaaSO,, + MgSO4, A [Cl] =
+ 0-126 M—i.e. here the added NagSC^ was without effect on the A [Cl] value for 1/2 sea water, (b) In the more concentrated solutions (3/4 tank water and tank water with added NaaSO,,) the relations become progressively different. Cl' tends to accumulate, but osmotic differences are smaller because A [SOJ is negative. Here the results resemble Bethe's experiment more closely.
(ii) Magnesium sulphate. Crabs from tank water and 1/2 tank water were kept in these solutions with 0-15 M added MgSO4, and [Cl] and Ac determined as
before. The animals remained for a long time in good condition and the results were far more consistent than with the NaaSO4 solutions (Table V). The symptoms
of Mg poisoning described by Bethe (1929) were present, but not serious.
The results are simpler than with Na^CX,. In both external media the A [Cl] values are almost unaffected by the added MgSO4 and they agree with the values
given by Quinton (1912), but in the more dilute solution Ac is reduced because the MgSO4 is unable to enter. Ac — A [Cl] is almost constant for 28-42 hours'
immersion. In the hypertonic solution Ac is the same as in sea water—here appa-rently SO4" can enter freely. According to Bethe, under these conditions, when
[Mg]0 = 4-75 mg. Mg/c.c, [Mg],- = 2-68 mg./c.c.; hence the SO4" entering must
be accompanied by more Na' or Ca" than Mg", and ionic regulation must still occur in absence of osmotic differences.
The data may be compared with the following (Table V) deduced from the data of Dakin and Edmonds (1931), for Heloecius cordiformis, which shows osmotic regulation both in concentrated and in dilute sea water. It is obvious (i) that in concentrated sea water substances other than Cl play a greater part in making up the total osmotic concen-tration (Ac - A [Cl] = + 0-260) than in sea water (Ac — A [Cl] = + 0-13), and (ii) that in the MgSO4 experiments the equality of total blood concentration with that occurring in the
concentrated sea water experiments is produced by maintenance of the blood MgSO4 at
about [(i-oo — 0-58) — (0-24 — o-i2)]/(i-oo — 0-58) or 1/7 of its concentration in the outside solution. Clearly the behaviour of this animal is quite different from that of Carcinus.
Table V. Effect of MgSO4 on the steady state in Heloecius cordiformis.
Freezing-point and [Cl] data calculated from Dakin and Edmonds (1931, pp. 173, 174).
External solution
Concentrated sea water
Sea water + MgSO4
Sea water
094
I-OI I-OI
097
I-OI I-OI
0-63
[Cl]t
-048 0-46 048
0-43 0-41 0-41
o-43
[Cl]0
0-94 I-OI I-OI
9
9
9
0
0
0
0
0
0
0 5 8
A[C1]
— 0 4 6
- o - S S - o - S 3 - o i s
- 0 1 7 - 0 1 7
- 0 1 5
Ac
- 0 2 4 - 0 3 1 -0-27
- 0 2 7 - 0 3 1 -0-27
— 002
A c - A [Cl] + O-22
+ O-24
+ 0-26
— O-I2 — 0-14 — O-IO
362 J. B. BATEMAN
(iii) Sodium nitrate. Carcinus remains almost indefinitely in excellent con-dition in sea water + NaNO3, and the latter substance is therefore convenient for
studying the effect of a foreign monovalent anion on Cl' distribution. The data are given in Table IV.
Evidently the presence of the added ions depresses the tendency of Cl' to accumulate: i.e. in 1/4 tank water and in 1/2 tank water with added NaNO3 the
total concentration differences, representing also the accumulation of Na', are practically those occurring in 1/2 and 3/4 tank water respectively. The distribution of Na' ions is unaffected, in this range of concentrations, by the substitution of part of the Cl' by NO3'. Electrical neutrality is kept by accumulation of Cl' to
an extent characteristic of crabs in 1/2 and 3/4 tank water respectively, and equal distribution of NO3'—not by shared accumulation of both Cl' and NO3'. At
higher concentrations this is no longer true. In presence of NO3', Na" still tends
to accumulate even at a total concentration rather greater than that of sea water, so that an osmotic difference is maintained, but the proportion of the two mono-valent anions is quite different from that in the more dilute media. NO3' shows
a definite and consistent accumulation, while A [Cl'] is negative. At the highest concentration—tank water with 0-15 M NaNO3—the blood concentration becomes
equal to that of the surroundings—i.e. roughly [Na']j = [Na"]0—but an ionic
steady state still exists in which [NO3'],- > [NO3']O and [Cl'], < [Cr]0. The results
do not vary with the time of immersion, and it is therefore improbable that they represent only temporary conditions.
Fredericq (1904), in one experiment, immersed a crab for 48 hours in sea water + O-II M NaNO3, and then determined freezing-points and [NO3']. In agreement with
the above results, A, == Ao, but [NOg'J^ -sg [NO3']0. This indicates a large accumulation
of Cl', but the analytical method used for [NO3] is open to suspicion.
It is of interest that NaNO3 should be harmless to Carcinus, while more poisonous
than Na4S04 to Fundulus, whose gills are far less permeable to ions (Loeb and Wasteneys,
)
(iv) Sodium acetate. The gill is almost impermeable to the acetate ion, but permeable to Na'. Hence in sea water + sodium acetate Na" tends to enter, but it cannot do so unless accompanied by Cl'. The final condition is [Na'],- < [Na']0,
[Cl']t- > [Cl'],,, and [CH3COO']t- < [CH3COO']O. Equilibrium is possible only if
the membrane remains impermeable to water. In practice these conditions are almost fulfilled, but the solution is toxic. In 1/2 tank water acetate the steady state complicates matters and here, in one experiment, the acetate ion seemed to be without effect; A [Cl'] was the same as in pure 1/2 tank water (Table IV).
rather poor condition, was 0-078 ± 0-004 M. In f °u r controls it was 0-148 ±
0-006 M. Unfortunately no blood potassium analyses were made, but the behaviour of potassium is apparently the same as in other processes, such as its effect on muscle (Sereni, 1925) and on nerve (Cowan, 1933), and rather different from the toxicity of cyanide.
The effect of non-electrolytes.
[image:9.532.45.454.320.576.2](i) Glucose. A preliminary experiment showed that the crab gill is almost impermeable to glucose in dilute solution. Crabs were immersed for a day in the smallest possible volume of tank water + 0-017 M glucose. Glucose in the solution, estimated by the Hagedorn-Jensen method, decreased by only 3-5 per cent. This agrees with Hemmingsen (1924-5), who found that 2-5 hours after injection of 100 mg. glucose into a crayfish less than 10 mg. are lost to the sur-roundings. In stronger solutions, Carcinus allows glucose to enter more rapidly, but death occurs long before equal distribution is attained. The results of [Cl'] and Ac measurements are given in Table VI. In six additional experiments in 3/4 tank water + 0-3 M glucose the mean weight change after 21 hours was 0-44 per cent.
Table VI. The effects of glucose, sucrose and urea on the steady state.
.External medium previous to experiment
Tank water
Tank water or 3/4 tank water
1/2 tank water
Tank water Tank water Tank water Tank water Tank water Tank water Tank water Experimental solution
Tank water + 0-30 M glucose 3/4 tank water + 0-30 M glucose 1/2 tank water +
0-30 M glucose 1/2 tank water +
o-6o M glucose 1/4 tank water +
0-30 M glucose
3/4 tank water + o-io M sucrose 1/2 tank water +
0-30 M sucrose
3/4 tank water +
0-30 M urea
1/2 tank water + 0-30 M urea
1/4 tank water + 0-30 M urea
Mean t (hours) IS 22 22 16 36 33 22 51 34 6 n 1 H 3 3 2 2 3 2 S 1 [Cl],
0 6 8 4
O-595 ± O-OI2
0-438 ± O-OO7
0 6 1 2 ± 0 0 0 9
0-482 ± OO2O 0-356 ± 0-026 0-503 ± O-O3S 0313 ± 0 - 0 3 1
0-371 [Cl]0 0674 0467 0-292 0-328 0486 0-276 0-484 0-306 0138 A[C1] + o-oio + 0-128 + 0-146 + 0-284 — 0-004 + 0080 + 0019 + 0-007 + 0-133 Ac — 0089 — 0-027 ± 0-004 + 0016 ± 0-007 — 0027 ± 0-006 + 0-015 ± 0-004 — + 0-078 ± 0-0005 + 0-077 ± O-OII + 0-119 Ac-A[Cl] = A[X] — 0-099
- O - I 5 5
— 0-130 — 0-311 — + 0-059 + 0-070 — 0-014
364 J. B. BATEMAN
same values are reached whatever the initial value—i.e. with crabs previously immersed in tank water or in 3/4 tank water—and are independent, during life, of the time of immersion. The "marked thickening of the blood," mentioned by Bethe (1930), was never observed. Scarcity of blood could always be attributed to moult age. With no water loss to the surroundings and no good reason for transfer to the tissues it is hard to see how "thickening of the blood" could occur. The results, not easily explained by any ordinary process of diffusion or osmosis, suggest a specific effect of glucose on the secretory activity of the gill. This is supported by the facts that (a) in one experiment Portunus puber, incapable of regulation, gave A [Cl'] = — o-oio after 23 hours in 3/4 tank water + 0-3 M glucose, and (b) sucrose gave quite different results. Further one may, without comment, connect the effect with the deposition, as glycogen, of injected glucose in the crayfish (Hemmingsen), the large increase in glycogen content of the hepatopancreas before the moult (Kirch, 1886), and the increase in blood osmotic pressure observed at this time (Baumberger and Olmsted, 1928).
(ii) Sucrose. With a membrane only slightly permeable to water a large molecule like sucrose should not affect the electrolyte distribution. A [Cl'] in the 1/2 tank water + sucrose is a little, less than in pure 1/2 tank water (Table VI). The solution is toxic.
(iii) Urea. Table VI. The solutions caused death in 10-50 hours. A [Cl'] is depressed and there is a tendency to increase in weight, so that urea probably causes a rise in permeability to all substances. In spite of the inhibition of Cl' regulation Ac remains positive, perhaps through presence of organic molecules produced from the tissues by solvent or peptising action of the urea. Whether the gill membrane is really inert to urea itself cannot be decided (contrast Dakin and Edmonds, 1931, p. 179).
The effect of changing pH.
The effect of external pH changes was determined by keeping crabs from 1/2 tank water in 1/2 tank water of abnormal pH. Acid media were made either by addition of HC1, following the table given by Kandler (1930), or of CO2. For
alkaline solutions NaHCO3 was added, some calcium precipitation being ignored.
(pH)o was determined by indicators.
Crabs in acid media, down to pH. 6-0, remained in excellent condition; below this they quickly died, whether the acidity was produced by HC1 or C02. For the
first few hours they were abnormally active, probably through peripheral stimula-tion. In the alkaline range, pH 9-0 caused death within 36 hours. The results of Ac measurements are given in Table VII. The [HC03'] values were calculated from
the pH and the alkaline reserve (Cooper, 1932), using the equation and tables given by Buch (1932).
The individual variation is large, but over a 100-fold change in [H"] there is little change in Ac. It decreases somewhat with increasing acidity.
Current theories of ion accumulation in plant cells (Osterhout, 1926, 1930; Brooks, 1929; Briggs, 1930) all stress the importance of metabolic production, and loss by diffu-sion, of CO2, and the resulting maintenance of a relatively acid sap, although they
disagree quantitatively. This importance of the ratio [H'],/[H°]0 seems to have been shown
in normal sea water the body fluids are relatively acid: according to Duval (1925) pHt = 7-7-7-8 when pH{ = 8-i, or [H'],/[H']0— 2-5, but there are numerous points of
difference between the accumulation phenomena in Carcinus and those observed in plant cells. The most striking is that the process in Carcinus is not accompanied by growth, presumably because water transfer cannot occur, and that it is dependent on total concentration. It is of interest therefore that in Carcinus />H relationships are not so important as with the plant cells. It is certainly probable that in the above experi-ments, with a 100-fold variation in [H']o, the ratio [H'],/[H']0 was considerably varied.
One might indeed suppose [H']; and [H']o to show less interdependence than other ions,
because of the buffering action of CaCO3 in resisting an acid medium. Kreps (1929)
has demonstrated such a mechanism in Balanus. Moreover, other crustacean tissues— e.g. the nerve of Maia (Cowan, 1933)—can survive over a wide pH0 range, and can pre-sumably maintain pHf fairly constant.
Table VII. The effect of pH change on the regulation of crabs living in 1/2 tank water.
[HCO3']0 is given in milliequivalents per litre.
Method of producing
pH change
NaHCO3, and blowing off excess CO2
— HC1 HC1 CO2 CO2
co
2n
i
6 S 4 i
i
i
(pn)o
8-6 7-8 7'S 7-45 6-8 6'75 6-S
[HCO3']0 calc.
1-28 1-89
2 0 O
2-02
2-08 2 O 8 2-O9
Ac
0150
0109 ± 0-009
0 1 S3 ± 0-008 0-130 ± 0-009
0-096 0-078
0-109
Miscellaneous experiments on Carcinus.
(i) Acclimatisation to diluted media. Since a number of related crabs can live in fresh water, acclimatisation of Carcinus itself was attempted. Other such ex-periments have been successful; in 1816 Beudant (see Fredericq, 1889) accli-matised a number of molluscs to fresh water, while Plateau (1871) described experi-ments on Asellus aquaticus. Carcinus caught at Plymouth cannot live for more than a few days in a medium isosmotic with 0-17 M NaCl and never attains a steady state; Schlieper (1929a), however, gives steady state data for crabs from Kiel Bay (<r~O'25 M) which had lived in sea water of concentration o-ioM. The result in Table VIII suggests a reason for the discrepancy: one crab, from a group of 20, survived a dilution of 1/6 tank water ( c ~ o - i ) for nearly 3 weeks, after an acclimatisation lasting 2 months.
(ii) The effect of temperature on regulation. Six crabs, of uniform size, were
kept for 3 weeks in 1/2 tank water, and then three of these transferred to 1/2 tank water in an ice-chest, aerated and surrounded with melting ice. After 4 days the blood vapour pressures of these and of the controls were determined :
366 J. B. BATEMAN
merely illustrating the complexity of the process. Clearly it does not depend on a single chemical reaction with a "normal" temperature coefficient, unless the limiting factor is a physical process which obscures the chemical one.
Table VIII. Acclimatisation of Carcinus to dilute solutions. Crabs from tank water, c = 062 M. n = number of crabs surviving.
Date
Co n
Apr.
10
046
20
Apr.
11
0-46
16
Apr.
18
0-41
9
Apr.
28
031 2
May
10
0-20
2
May
20
0-17
2
May
28
0-15
2
May
29
0 1 5 1
June 5
0 1 2 1
June
1 1
o-io
1
June
28
O I O I
June 3°
o-io
0
(iii) The weight of gill tissue in Carcinus. For the discussion (p. 367) it was desirable to know the average gill weight in Carcinus. Routine determinations were made, and the results showed that the weight of gill tissue is always about 2 per cent, of the total weight, whatever the value of the latter. Hence, if the shape of the filaments and their number in a given distance is the same at all stages of growth, the gill area must be relatively smaller in the larger crabs. Camera lucida drawings showed the shape to vary only slightly, while the number of filaments in unit distance is much greater at the distal than at the proximal end of the bar. This causes a still further reduction in the relative gill area in larger animals.
Although one would not expect diffusion across the gill membrane to limit the respira-tion of Carcinus, it is of interest that the oxygen consumprespira-tion per unit weight does decrease with increasing weight of crab (Montuori, 1913).
(iv) The osmotic properties of isolated gill tissue. It is possible to study the internal concentration changes in a tissue by determining the corresponding changes in the external medium,, if the volume of the latter is not too large. The method has already been used by A. V. Hill (1930 b; the "differential" method) to deter-mine the apparent free-water fraction in muscle, assuming that the muscle comes into osmotic equilibrium with any solution in which it is immersed, and making allowance for the production of osmotically active metabolites by a control experi-ment. If now the free-water fraction is assumed to be nearly equal to the total water—and this is probably true—then it is possible, from exactly the same data, to find the changes in internal concentration of the tissue corresponding to a given change in the external medium. This may be of great value when tissue fluids are difficult to obtain, or in the case of small animals where the body fluids cannot be studied directly (see p. 369).
The following shows more clearly the method: let m1 gm. of tissue, containing wij/gm.
water in a fluid of osmotic concentration clt be shaken, until a final redistribution of
water and electrolytes is reached, with Mx gm. of a salt solution of osmotic concentration
C, and containing MXF gm. water. The result of the equilibration will be that the internal
concentration of the tissue becomes c / and that of the outside solution C\', and if the tissue is capable of osmotic regulation these concentrations are unequal. Call the water transfer £ gm., from solution to tissue. Then, since the total amount of electrolyte present is constant, ^ + MjpCi = (mj+g) ^ + (MlF ~ g) C/,
or, if 0 = Cj — c± and TT = Cx' - clt
(n — 6) is easily measured, by vapour pressure or freezing-point determinations, and ^ S — °. (ci - ci) can be found without knowing c1. If actual concentrations are re-quired, or if g is too large to be neglected, cx must be known approximately. If allowance for metabolites is to be made, the tissue must be equilibrated before the experiment with a large volume of a solution whose concentration is assumed to be cx. Then the control
portion, of mass kmlt must be shaken with kMt gm. of solution cx for the same period as
that required for equilibration of the experimental tissue. The final solution, concen-tration (q + x), is compared with the experimental solution (Cx' + x). This gives -n.
In a single experiment the method has been applied to the isolated gill of Carcinus, since it seemed possible that secretory activity might in this way be de-tected. The gills of four crabs were cut out, each gill bar tied at the cut end, and kept in outside sea water for 2 hours. They were then gently blotted and weighed in two roughly equal parts. To one a weighed amount of sea water was added, and to the other an equal amount of 1/2 sea water, the amounts of water in the added solutions being approximately equal to the amounts of water in the tissue. They were corked up in small glass tubes and rotated for 4 hours. 9 and ir were determined, the gill tissue reweighed, and then the total water determined by drying at 1050 C. The results gave mean (q — q')/(ci ~~ Ci) = I"01- Thus c' = C"
and any secreting activity of the gills must have been destroyed by the experi-mental treatment.
II. DISCUSSION.
The following discussion is tentative, and therefore detailed arguments are omitted.
(i) Permeability changes in the gill. Margaria (1931) found for Carcinus in dilute sea water a linear concentration-time curve, instead of the logarithmic one to be expected in a simple diffusion process. This theoretical logarithmic curve can be drawn from the data given above for crabs in concentrated sea water (Table III), and then compared with Margaria's curve, assuming the passive permeability in dilute water to be the same as in concentrated sea water. The difference between the two gives a "work" curve which shows a rate of working at first linearly decreasing with time and then increasing to a final steady value. This can be analysed into (a) an initial curve decreasing to zero and probably representing only some temporary physical change hindering the dilution process (cp. Backmann, 1914), and (b) the true work process, initiated some time after immersion in the dilute solution and growing to a steady value depending on the external concentration. In Portunus puber (Margaria, 1931) the process (a) is present but the permanent regulation (b) is absent.
368 J. B. BATEMAN
and Winegarden, 1931; Bateman and Keys, 1932), and supports the supposition of a large decrease of passive permeability in diluted media. Moreover, at the calculated rate of working, the glycogen in a starving Carcinus would last for less than a week with an efficiency of 10 per cent., supposing it to be completely oxidised and used only for regulation (compare also Kirch, 1886; Lindblad, 1931; Cohnheim, 1911-12).
(iii) The mechanism of ionic regulation. Since urine and blood are isotonic (Schlieper, 1929 b; cp. Schlieperand Hermann, 1930), osmotic regulation cannot be the result of urine formation, but ionic regulation could partly be produced by selective secretion. The only available analyses are those of Bialaszewicz (1930), and calculation shows that for Maia squinado, at least, uptake of SO4" from sea
water, its excretion, and its production by protein breakdown are all roughly the same, so that the observed steady difference [SO4"]t- < [SO4"]0 could be
con-ceivably maintained by excretion. A similar argument applies also to Mg".
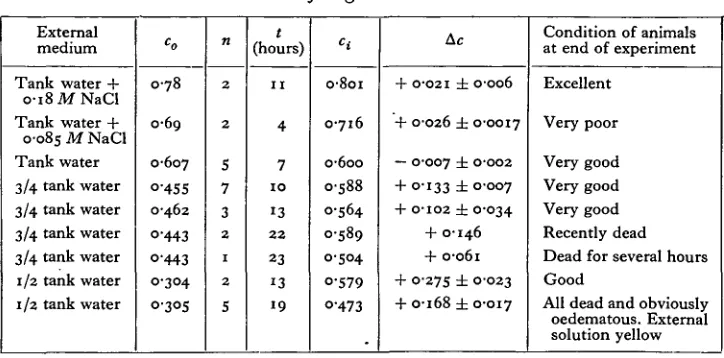
III. NOTES ON THE BLOOD CONCENTRATION OF LIGIA.
Ligia oceanica is a small isopod midway between aquatic and terrestrial forms. It lives between tide marks, apparently rarely enters the sea, but nevertheless respires branchially and needs moisture (see Hewitt, 1907; Nicholls, 1931). Plateau (1871) found that Ligia died after a few hours' immersion in fresh water; Tait (1916) made more extensive immersion experiments, finding that in fresh water the animals lost salts and became oedematous. He concluded that Ligia is derived, without an intermediate fresh-water stage, from a marine ancestor. Nicholls (1931) extends and criticises these experiments.
The present short study shows the osmotic pressure changes occurring in Ligia immersed in various dilutions of sea water.
It was possible to obtain sufficient body fluid from one animal for a vapour pressure measurement, using a small thermopile. A square of cigarette-paper (washed with dis-tilled water for 24 hours and dried at 150 C.) was placed on the thermopile surface, the
drops of fluid from a cut large antenna transferred immediately to the paper, and the thermopile set up in the usual way. Generally the second antenna gave sufficient fluid for the reverse determination; if not, a second animal was used.
(1) Animals living on moist seaweed. The vapour pressures were 0-626, 0-621, 0-618, 0-631, 0-637, 0-641, 0-641, mean 0-631 M—i.e. the concentrations were rather greater than that of sea water. The behaviour is intermediate between Carcinus, which cannot live permanently in air, and the land crabs studied by Pearse (1930-31, 1931).
like the majority of other invertebrates; the body fluids become isotonic with, or even slightly hypertonic to, the medium.
Table IX. Blood concentration of Ligia oceanica in various external solutions.
External medium
Tank water + o-i8MNaCl Tank water + 0-085 M NaCl Tank water 3/4 tank water 3/4 tank water 3/4 tank water 3/4 tank water 1/2 tank water 1/2 tank water
co
0 7 8
0-69
0-607 Q-455 0-462
O-443 O'443
0-304 0305
n
2
2
5 7 3
2
1
2
5 t
(hours)
1 1
4 7
1 0
1 3
2 2
2 3
1 3
1 9
H
o-8oi
0-716
o-6oo 0-588 0-564 0-589
0-504
0-579 O-473
Ac
+ O-O2I ± OOO6
+ O-O26 ± O-O0I7
— O-OO7 ± O-OO2
+ 0-133 ± O-OO7 + O-IO2 ± 0-034
+ 0-146 + 0-061 + 0-275 ± 0-023 + 0-168 ± 0-017
Condition of animals at end of experiment
Excellent
Very poor
Very good Very good Very good Recently dead Dead for several hours Good
All dead and obviously oedematous. External solution yellow
The survival times in the solutions used were erratic, but shorter than those recorde'd by Tait. The smallest animals, too small for vapour-pressure measure-ments, were the hardiest. The maximum survival period in sea water was 8 days.
With high osmotic resistance goes a relative insensitiveness to solutions which are physiologically unbalanced for other invertebrates; in particular, increased con-centrations of potassium which would have been fatal to Carcinus were without effect on the survival time of Ligia.
The approximate equality of concentration between the blood of Ligia and sea water may support Tait's belief in its marine ancestry; if there had been an intermediate fresh-water stage one might expect the blood concentration to re-semble that of the teleost fishes.
IV. THE EFFECT OF DILUTE SEA WATER ON THE BODY FLUIDS OF GAMMARUS LOCUSTA.
Gammarus is too small for direct study of its body fluids, but the method used for the gills of Carcinus (p. 366) can also be used for living animals if a value for the concentration of the body fluid at some known external concentration be assumed. In sea water the body fluids are probably isotonic with the external solution; in other marine invertebrates this is nearly true.
370 J. B. BATEMAN
external changes than those of Ligia, and the sensitiveness to ionic changes is correspondingly greater (Loeb, 1903 ; Schumann, 1928).
Schlieper (1931) has found the respiration of Gammarus to be nearly constant over a wide range of external concentrations, becoming significantly greater only in very dilute solutions. He supposes therefore that osmotic regulation occurs only in these very dilute solutions. If the present experiment is to be trusted, some other interpretation of the respiration measurements is necessary.
V. SUMMARY.
1. The influence of some foreign ions and non-electrolytes on the relation between the chloride concentration and vapour pressure of the blood of Cardnus and the external solution has been studied. The added substances showed inter-esting specific effects, which are discussed in the text, but no general conclusion can be drawn. It is very probable, however, that the gill membrane is almost impermeable to water, so that osmotic forces, as such, play no part in producing the observed relationships between blood and the external medium. There is no evidence for active water regulation in Cardnus.
2. The latter conclusion is supported by experiments on the effects of cyanide and hypertonic solutions. In the latter case, in spite of " osmotic " regulation being abolished, no significant water transfer occurs.
3. Miscellaneous experiments on the result of varying pH and temperature are described; in each case the effects are surprisingly small.
4. In a single experiment, the isolated gill tissue of Cardnus showed no secretory activity. The method used, based on Hill's "bound water" technique, is described in detail.
5. An analysis of the dilution-time curves for Cardnus in dilute solutions suggests that the process is accompanied by considerable changes in the properties of the gill membrane, before the beginning of the real regulatory mechanism. The same data permit the calculation of the work done in maintaining the steady state, but the result is improbably high.
6. A direct study has been made of the vapour pressure of the body fluid of Ligia oceanica living under various conditions. For short periods of immersion in dilute solutions the animals show a high degree of osmotic independence, but their resistance breaks down on prolonged immersion.
7. A method was tested for the study of internal concentration changes in animals too small for direct measurement. The results of an experiment on Gammarus locusta are discussed.
REFERENCES.
ATKINS, W. R. G. (1922). Journ. Mar. Biol. Ass. 12, 717. BACKMANN, E. L. (1914). Zentr.f. Physiol. 28, 495-7.
BATEMAN, J. B. and KEYS, A. (1932). Journ. Physiol. 75, 226-40.
BAUMBERGER, J. P. and OLMSTED, J. M. D. (1928). Physiol. Zool. 1, 531-44. BETHE, A. (1929). Pfliigers Arch. 221, 344-62.
(I93°)- Journ. Gen. Physiol. 13, 437-44.
BETHE, A. and BERGER, E. (1931). Pfliigers Arch. 227, 571-84. BIALASZEWICZ, K. (1930). Ada Biol. Exp. Warsaw, 5, 57-84.
BORSOOK, H. and WINEGARDEN, H. M. (1931). Proc. Nat. Acad. Wash. 17, 3, 13. BRIGGS, G. E. (1930). Proc. Roy. Soc. B, 107, 248-69.
BROOKS, S. C. (1929). Protoplasma, 8, 389-412.
BUCH, K. (1932). Rapports et Proces-Verb. des Reunions, Conseil perm, internal, pour Vexpl. de la mer. (In press.)
COHNHEIM, O. (1911-12). Z.f. physiol. Chetn. 76, 298. COOPER, L. H. N. (1932). Journ. Mar. Biol. Ass. 18, 201-2. COWAN, S. L. (1933). Journ. Exp. Biol. 10, 401-11.
DAKIN, W. J. and EDMONDS, E. (193I). Austr. Journ. Exp. Biol. and Med. Sci. 8, 169-87. DUVAL, M. (1924). Bull. Stat. Biol. d'Arcachon, 1-7.
(1925). Ann. de I'Inst. Oceanogr. N.S. 2, 233-407. FREDERICQ, L. (1889). La Lutte pour VExistence. Paris.
(1901). Bull. Acad. Roy. Belg. 39, 428-54. (1904). Arch, de Biol. 20, 709-30.
HEMMINGSEN, A ; M . (1924-5). Skand. Arch. 46, 51-5.
HEWITT, C. G. (1907). Liverpool Mar. Biol. Ass. Memoirs, No. 14.
HILL, A. V. (1930 a). Proc. Roy. Soc. A, 127, 9-19.
(1930 b). Proc. Roy. Soc. B, 106, 477—505. (1931). Adventures in Biophysics. Oxford.
HOBER, R. and HOBER, J. (1928). Pfliigers Arch. 219, 260-72.
HOET, J. P. and KERRIDGE, P. M. T. (1926). Proc. Roy. Soc. B, 100, 116-19. HUKUDA, K. (1932). Journ. Exp. Biol. 9, 61-68.
JAQUES, A. G. and OSTERHOUT, W. J. V. (1930). Journ. Gen. Physiol. 14, 301-14.
KANDLER, R. (1930). Int. Rev. Ges. Hydrobiol. u. Hydrog. 24, 177—224.
KIRCH, J. B. (1886). Inaug. Diss., Bonn, quoted by E. von Schonborn (1911), Zeitschr. f. Physiol. 55, 70.
KREPS, E. (1929). Pfliigers Arch. 222, 234-41.
KUMANO, M. (1929). Sci. Rep. Tohoku Imp. Univ. Ser. 4, 4, 281. LIM, R. K. S. (1917-18). Proc. Roy. Soc. Edin. 38, 14.
LINDBLAD, R. G. (1931). Studien iiber den Blutzucker bei Astacus fluviatilis. Kristianstads Buch-druckerei Gesellschaft.
LOEB, J. (1903). Pfliigers Arch. 97, 394-409.
LOEB, J. and WASTENEYS, H. (1915). Journ. Biol. Chem. 21, 223-38. MARGARIA, R. (1931). Proc. Roy. Soc. B, 107, 606-24.
MONTUORI, A. (1913). Arch. Ital. Biol. 59, 213-34.
NiCHOLLS, A. G. (1931). Journ. Mar. Biol. Ass. N.S. 17, 655-73. OSTERHOUT, W. J. V. (1926). Proc. Soc. Exp. Biol. and Med. 24, 234.
(1930). Journ. Gen. Physiol. 14, 285-300. (1931). Biol. Rev. 6, 369-411.
PANTIN, C. F. A. (1931). Biol. Rev. 6, 459-82.
PEARSE, A. S. (1930-1). Carnegie Inst. Year-Book, No. 30, 388-9. (i93i)- Journ. Elisha Mitchell Sci. Soc. 46, 161-6.
PLATEAU, F. (1871). Mem. cour. de I'Acad. roy. Belg. 36, No. 2.
QUINTON, R. (1912). L'eau de mer, milieu organique, 2me 6d., Paris, Chap. 3. RAPPLEYE, W. C. (1918). Journ. Biol. Chem. 35, 509-12.
SCHLIEPER, C. (1929 a). Z. vergl. Physiol. 9, 478-514.
(1929 b). Sitzungsber. d. Ges. z. Beforderung d. ges. Naturwiss. Marburg, 64, 143-56. (1931). Biol. Zentr. 51, 401—12.
SCHLIEPER, C. and HERMANN, F. (1930). Zool. Jahrb. 52, 624-30. SCHUMANN, F. (1928). Zool. Jahrb. 44, 623-704.