SlJ.'\JDilUS OF SQ11f'E SUJ3STITDTED PINANE MONOTl!lRPENlUS
A th("'sis sented for the degree of Doctm:· of Philosophy in Chemistry in the University of Cante1~1)Ury1
Christchurch, Nevl Zealand.,
by
P.A,.E. Cant
:;::;.
INDEX
The T'nermal Reactions of Pinane
7 -Substituted Pinanos Nomonclatul'e DT Preparations.: Ver1Jenone Chrysanthenone Ghrysantho11ol
7-liffo thyl chry san th eno 1
Chrysanthenyl Acetate cis-Ghrysanthanol
cis-Chrysanthanyl Acetate ci_~-Chr;y· san thanone
Verbenone Cl:n:ysanthenol
7-Methylchrysanthenol Ghrysan-thenyl Acetate cis-Chrysanthanol
_£is-Ghrysanthanyl Acetate cis-Chry santhanon e
COJITCLUSIO:NS
APPENDIX I
The Pyrolysis of a -Pinene
APPENDIX II
Hydrogenation of Chrysanthonone 114
EX:PERIMEN'11AL
Pyrolysis Apparatus 118
Preparations 121
ryrolyses 137
Appendix I
154
Appendix II 135
REFEREt\ C F.:S
155
Abstract
-A number of pinane derivatives, o:xygena-l;ed at ·the ?-position, have been p;)rrolysed and the resulting product mixtures analysed. Nowhere in the extensive literature concerned 'l'li th the pyrolyses of pinanG derivatives has there been a report of the cyolobutane ring opening initially by vmy of either 1,7~ 01'
5;7-
el0avage,although there are examples of both 1
,6-
and5
96- bond cleavage. r[he present study has shovn1 that it is }')OSsible to induce cleavage of the 1/{- and5,7-
bonds by inserting suitable sub-sti tuents a·l;07.
Cleavage of the 196- and 1, 7- bonds occurs ·to a simHar extent in the p;'rrolysis of ci s-chrysanthenol (.38) (28% and 25% respectively)., whereas in that of 7--methylchry·santhenol (54)~
1,
7- cleavage becomes the more favoured mode -43%
compared to 3~; o:7fO/
.
When the hydroxyl group of cis-chrysanthenol 'vas aceJcy1ated9 the -resulting compount proved very unstuble under the pyrolysis conditions, giving a large number of products, only t\fO of which
(29% in all) l·rere present in identifiable quanti ties.
The pyrolyses of the three ?-oxygenated derivatives of
a -pinene displayed one feature in common. 1'-lhen 1,6- cleavage occurred and monocyclic products were formed, the major product in each case was formed by migration of a hydrogen from the
R
1
0~
--
R
10
..
R2
R1 H, CH3 CO
1
R
~2 H, CH3
R
1
0
+
R
10
Neither .::d.s-chrysa.nthanol (48) nor cis-~chrysanthanyl acetate
(49)
gave any cyclic :products on pyrolysis. Both compoundsrearranged to give the aldehydes citronellal and 597-dimethyl-6-·~
octenal but, in addition, cis-chrysanthanyl acetate gave the cis- (3%) ancl j;r~'2_- (28%) enol acetates of oi tronellal.
~
A cO
+
CHO
Verbenone
(5)
tn:.s p;yro1ysl~cl at 425° and. fow~ compounds no-t mentioned in previous reports v~ere identified. as 2,6 ,6-trimeth;yl-bioyolo [ 3. 2. 0] hepten·-7-one (45), 1~formyl-2, 6 ,6-.t.rimeth;y-lcycJ.o-hexa-1~3-cliene (57), and. the trienals 3,7-·dime-thyl-2.-ciS-9 4-cis-, 6-octatri enal (60) and 3, 7 -clirnethyl-2--_gj s-,4-·
t:r.'a.ns-6-octa trienal(59)
I-t 1vas also olJserved that the unsatura-ted pinene derivatives
IN'l'RODUCTION
From the time when man was first moved to improve the
quality of his surroundings, he has made use of the
sweet-smelling substances that are to be obtained from herbs and trees.
Today, in a vastly more populous and sophisticated world
enjoying greatly improved ~tandards of liv , the demand for
the perfumery chemicals used in most household products has
far outstripped the supply of the essential oils that can be
obtained from natural resources. The supply of these natural
oils is not only inadequate; it varies widely in volume and
quality, and therefore in price, from season to season - all
good reasons to encourage science and industry to look for new
and more reliable sources of perfume chemicals. Th they have
done with a good deal of success.
Many of the main components giving rise to the fragrances
of the essential oils are oxygenated
c
10 compounds belonging to
the class known as monoterpenoid. Monoterpeno were
oonsidered1 to be formed by the fusion of two isoprene units in
a "head to ta 11 orientation. Isoprene has the formula
c
5
H8
,
with the isopropenyl end bf the molecule as the11head11•
(Scheme 1)
ieoprene monoterpenoid
Many naturally occurrin~ compounds of up to 30 carbon atoms can be v wed as comprising numerous isoprene units
linked in a regular way to form an ordered sequence.
The most commonly occurring monoterpenoids are the
pinenes, a-(1) and ~-(2), which are major constituents of
wood turpentine. Turpentine is the mixture of volatile
hydrocarbons present in the wood of coniferous trees; 70?6 of
( 8 7 -1 )'
the world's current production x 10 gallon annum is
obtained as a by-product of the Kraft pulping process. ~1uch industrial research has been directed towards oxygenating the
pinenes and isomerising the derivatives by pyrolysis or
hydro-lysis to give the fragrant monocyclic and acyclic
monoter-penoids. At present 0-pinene is utilised more fully in the
fine-chemical industry, and thus more valuable than its
isomer a-pinene.
The c~mposition ot wood turpedtine varies markedly from
country to country. New Zealand, supplying as it does some
0.57.6 of the world's production, but with a potential of
6
-1>
00,000 gallon annum , has an unusually high percentage of~-pinene in its turpentine -65?0, compared with the 277{, m 1ximum
found elsewhere. a-Pinene and ~-pinene are, respectively, the
2,3- and 2,10-olefinic derivatives of the parent compound
pinane
(3).
With a structural formula of2,6,6-bicyclo-[3,1,1] heptane, the monoterpenoid nature of the pinane skeleton
is easily recognised (Fig. 1).
pinane (+)a-pinene (+)-[3-pinene
3.
The absolute configuration of (+) a-pinene and (+) ~-pinene
are as shown, with the e~erl]_-dimethyl functions above the plane of the paper.
Although (-) a-pinene was used to synthesise the
7- substituted pinanes studied in this work, the photolysis step
in which chrysanthenone (4) is obtained from verbenone (5) by
a [1,3] sigmatropic shift, also reverses the configuration.
The compounds concerned are thus 7- substituted (+) - pinanes,
As noted
b~
Teisseire et al2 the optical rotation ofchrysanthenone (4) depends upon both the irradiation time and
distillation conditions, varying between 0° and 40°.
The rhermal ~eactions of Finane Deriv~tives
Although the thermolysis of pinane derivatives was first
reported in 1841, when turpentine was heated to give oils of
7.
different volatilitiesJ, it was not until the 1950's that any
real understanding of the reaction was gained. From the early
work some of the pyrolysis products from pinane (3) itself,
and from its unsaturated analogues4'5 a-pinene (1), and
~-pinene (2), were identified, but these pyrolyses continued
to be studied up to 1970.6' 7 It was noted also that a-pinene
recovered from the pyrolysate was racemised, and this loss of
optical activity enabled Smith4 in 1927 to calculate the
activation energy for the pyrolysis of a-pinene to be
184 kJ mol-1•
The most significant result of this work has been the
commercial exploitation of the pyrolysis of both ~-pinene (2)
and pinane (3) in the production of a variety of perfumery
and flavouring materials.
Myrcene
(6)
is produced in a continuous process by4.
time. A yield of up to 85~ can be achieved in this way.
From myrcene (Scheme 2) nerol
(7),
geraniol(8),
citronellol(9)
and linaloBl (10),compounds used extensively in the perfume
industry, are synthesised by relatively simple transformations.
Citral, the oxidation product of nerol and geraniol, is widely
employed as a substitute for essential oils such as lemon grass,
and is used extensively as a perfume base in toiletries, soaps,
and disinfectants. It is utilised als0 as a precursor in the
~-pinene
OHC
+
(a)
Citral
Scheme 2
(b)
l.inalool
HCl
~ CuCl 2
Cl
Oxidise
4
-HO
HO
"--SaponifyGeraniol
l
i
AcOHCu ,,Cl...,
C L
Ct
+
I
NaOAc~
Amine!
Saponify+
HO
nerol
6.
Pinane
(3)
is also pyrolysed commercially in acontinuous process to give dihydromyrcene (11), from which
the valuable 1- menthol (12) can be produced. (Scheme
3).
OH
( 11) .Scheme ( 12)
Although commercially exploited, the pyrolysis of
pinane derivatives was but poorly understood until 1951, when
Burwe118 proposed a mechanism to explain the kinetic and product
data available for the pyrolyses of a-pinene (1) and a-pinene
(2). Earlier workers had shown that vapour or liquid phase
0 0
pyrolysis of a-pinene at temperatures of 200 - 500 resulted
initially in three simultaneous9 reactions, all first order?
racemisation of a-pinene, isomerisation to alloocimine (13), and
isomerisation to almost inactive limonene (14), The pyrolysis
. f f t d b tl d d . t . f b . . d t . . d t 1 0
1s una ec e y 1e a 1 1on o enzo1c ac1 , an 1ox1 an s,
or dipentene (d,l-limonene), implying that it cannot occur by
way of an ionic intermediate.
Burwell expanded an idea first mooted by Rice and Rice11
in 1935: that a-pinene could undergo homolytic scission of the
1,6- bond to form a biradical intermediate (1a) (Scheme
4),
one of the radicals being allylic and thus stabilised as a
resonance hybrid. Recombination of the radicals forms
d,l-o:-pinene; scission of the
3,4-
or4,5-
bonds formsocimine (15), which rearranges to give the more stable
allo~cimine (13); and intramolecular hydrogen transfe~ from
7.
d,l-limonene (14).
(d)-(1)
(1a)
(d,l)-(1)
Scheme
4
( 15) (13)
Rice and Rice11 had predicted the formation of ocimine in the reaction, and this was subsequently confirmed, but they did
not consider the stereochemical consequences of their
biradical termediate, nor all the reactions i t could undergo.
8
By a similar mechanism, Burwell was able to explain the known
pyrolysis products of ~-pinene, including the formation of limonene (14) of high optical purity (Scheme
5).
(2a) - (1)-(2)
+ +
(l)-(1L~)
(6)
8.
He also demonstrated that the activation energy of a·-pinene
was explicable in terms of the biradical intermediate. The
formation of an allylic radical from the thermolysis of
1-butene had been shown12 to involve an activation energy of
259 kJ. mol-1•
CH
=
CH - CH - CH2 2 3
L\E* - 259 kJ mol -1
CH 2
This value would be reduced to about the 171 kJ mol-1 calculated
for the pyrolysis of a-pinene if allowance is made for the bond
strain in a cyclobutane ring, some 109 kJ mol-1• 13
Burwell's diradical mechanism for the nyrolysis of
pinane derivatives remains the one which best explains the
products obtained from these reactions, although the possibility
of a concerted cycloreversion mechanism operating to give some
acyclic products has been considered.
In terms of a concerted mechanism14 the formation of
ocimine (15) from a-pinene can be envisaged as either a
[o2s + o2a] (allowed) or a [a2s + o2s] (non-allowed) process. To accommodate the allowed cycloreversion, the cyclobutane
ring must deform substantially to allow the ethylenic components
to become mutually orthogonal, as the [o2s + a2a] mechanism
requires inversion at one (or three) of the four ring carbona
for the formation of the new bonds with conservation of orbital
tl I I I 1\ l - - - f l l 1 1 \ l l l l l
A
\ l ' \ ,,-~,~,~===:::,"';, .~.·
...
*
-
~~
-..
::...
8''
II! I•-p
-..
....B
B
/
~
"' =
inversion Scheme 6Although the four-membered ring in pinane derivatives
is more puckered than cyclobutane itself by up to 20° (Fig. 2),
0
the dihedral angle is far removed from the
75
required for twoof the bonds to be orthogonal to each o~her.
\
\
+D
[o2s + o2a]
transition state
l f \
V\ ~ bond to be broken
2
cyclobutane
\
'
10.
The operation of the [o2s + process cau be~ seen
clearly in the diagram in which the incipient double bonds are
vertical and horizontal. Formation of the n-bond along the
horizontal a-bond occurs on the same face ( s.Y:.!.-) while a
n-bond is formed by orbital lobes on opposite faces (~nti) of
the vertical a-bond. It is likely that the [o2s + o2a]
cycle-reversion contribution to the pyrolysis mechanism will be small
because of the prohibitively large distortion required of the
molecule.
The [o2s + n2s] concerted 'non-allowed' reaction pathway
is so described because, where able, a molecule will react by
the lower energy [o2s + o2a] process. If, as has been observed recently15, steric factors prevent the allowed reaction,
molecular reorganisation can occur by the non-allowed route.
Huckel M.O. calculations show that for [1,3] sigmatropic shifts
(an example of a [2 + 2] process), the energy of the [n2s + o2s]
path is lower than for a diradical mechanism. Application of
this hypothesis to the [2 + 2] pinane cycloreversion awaits the
necessary calculations,
In a recent report of the thermolysis of cyclobutane
derivatives in the bicyclo[2,2,0]hexane system, an alternative
to the [a2s +a2a] process is suggested16 that conserves orbital
symmetry. Pyrolysis of all-end_~-hexamethylbicyclo[2, 2, O]hexane
0
at 153 was found to give only the all-exo·-isomer and the ring
opened erythro-3,4,5,6-tetramethylocta-2~6E,-diene (Scheme
7).
11.
The allowed [o2s + o2a] cycloreversion could account for the
observed diane, but is rejected since it would involve
unreasonable distortion of the bicyclic system. A two-step
mechanism is proposed, involving the conversion of the
C(1)-C(4) a-bond into an-bond, followed by conrotatory
open-ing of the C(2)-C(3) bond; this satisfies the ~oodward-Hoffmann
selection rules14 (Scheme
8).
The inverted bicycle- product is also accounted for
by this means. In the diagram, the two methyl groups at the
rear of the molecule have been omitted for clarity.
Ill
H
ant:i.clockwise con rotation
H
Ill
Scheme
8
Although the bicyclic isomers are in equilibrium, it is found
that the more stable all-exo- compound is favoured to·the
exclusion of the other. Concerted ring opening must be
12.
occur both clockwise and a.nticlockwise, lea<'l.ing to the formation
of the two optical isomers of the acyclic product. In th
ce 1 -!.:he1·e appears to be no steric factor to favour rotation
in one sense, and a racemic mixture should result. Unfortunately
the optical purity of the diene is not reported.16
It is unlikely, however, that this attractive alternative
to the [a2s + a2a] mechanism can be applied to the pyr s of derivatives, as models show that the constraint of the
e ring across 0(1) and C(5) s the inversion at
C(6)
C(7) necessary to form the n-bond between them.In attempts to utilise the more r ly available
isomer, ~-pinene (1), work since the mid-19401s has centred on
the pyrolysis of its oxygenated deri which are produced
by autoxidation of a-pinene.1
7
These studies have beenmade po~sible by tho advent of such techniques as gas
chroma-tography and n.m.r. spectroscopy, for the isolation and
ident cation of the pyrolysis products.
In spite of recent suggestions that the opening of the
cyclobutane ring during the pyrslysis of pinane derivatives
cou:d involve a concerted rearrangement, the mechanism which
is most widely accepted is that of Burwe11.
8
In terms·of Burwell 1s mechanism, homolytic cleavage of one of the bonds
in the cyclobutane rise to a b cal intermediate,
so that substituents which can either affect the stability of
the radical centres, or which can interact with them becaube of
a particular stereochemistry, will influence markedly the type
of products initially produced in the reaction. Some primary
reaction products undergo further intramolecular rearrangements,
13.
Pyrolysis of the pinanes involves, initially, homolytic
scission of either the 1,6- or the 5,6- bond to give a biradical
species always containing an isopropenyl radical. The relative
stability of this teridr.vc;y· radical precludes cleavage of either the 1,7- or 5,7- bonds, both of which would lead to a
primary radical at C(7). Cleavage of the 1,6- and 5,6- bonds
is affected by substituents at the C(2) and C(ll) positions
particularly, as these may stabilise an adjacent radical by
allylic or inductive delocalisation. Substituents can be placed
in order of their ability to cause bond cleavage at the
neighbouring bridgehead position.18 An examination of the
pro-duct composition from the pyrolysis of verbenene (16)19 allows
a comparison of the influence exerted on the initial cleavage
by an endocyclic and by an exocyclic double bond. Two biradical
intermediates can be envisaged for this reaction - one (16a)
stabilised by the exocyclic methylene and the other (16b) by
the endocyclic olefin (Scheme 9).
( 16)
( 16a) ( 16b)
Since all the products isolated from the pyrolysate arise
from biradical (16b), the endocyclic double bond appears to be
more effective than its exocyclic counterpart in stabilising
( 16)
(18a)
l
C~pe
Rearrangement
(18), 39?-h
Ill
(19), 14?6 Scheme 10
14.
1
H transfer
( 17)! 29%
(trace)
( 20) t 9%
The biradical can undergo a [1,5-J hydrogen transfer to give
the E-menthatriene (17), some of which rearranges and is
dehydrogenated to produce a trace of O-isopropenyltoluene.
Alternatively, the 1,6-bond can break, an intermediate
tetraene (18a) that suffers a [3,3](Cope) rearran~ement to give
a triene (18). Successive [1,5] thermal sigmatropic hydrogen
shifts account for the other two triene~, (19) and (20), found in lower yields.
From the product analyses of the pyrolyses of ~-pinene (2)
8
an d noplnone . (2 ) 20, 20a -1 , an exocyc 1' lC me· y ene can th 1 b eadjudged to induce more cleavage at the adjacent bridgehead
exclusively from 1,6- bond cleavage (p.7), nopinone (21)
on pyrolysis is observed to produce
2-methyl-2,7-octadien-4-one (22) as a result of scission of the 5,6- bond (Scheme 11).
-(21)
(22)
Lt. 5%
+
24.576
9%
·Scheme 11
This ketone (22) is, however, a minor component,
and the majority of products are derived from 1,6- bond cleavage.
Similar consideration of the pyr0lysis product compositions for
cis-verbanone (23)20, neo-isoverbanol (24)21 and nopinol ( )22'. 22a
allows a comparison.to be made of the relative ability of the
substituents carbonyl and methyl, methyl and hydroxyl, and
hydroxyl and hydrogen respectively to induce cleavage of a·bond
to the adjacent bridgehead position. The order deduced from
the foregoing observations endo- C:::C
>
exo-C=O >CH
3
>
OH >H.Substituents on C(10) have been found not to influence
the course of the pyrolysis reaction, giving products with 27,
R =
R :::
R :::
R = R ::::
R ::::
16.
+
tH
2 (o:,-pinene) 0 (myrtenal)
OH,H (myrtenol)
OAc,H (myrtenyl acetate)
CH
20H,H (nopol)
CH
2
0Ac~H (llopyl acetate)Scheme 12
Although substituents at C(3) are remote from the possible
radical centres at C(1) and C(5), interaction with the radical
is ~ften possible. Pyrolysis of cis- and trans-24 2l~a
pinocarveol (26) (27) ' produce, as well as products with
skeletons sim to those obtained from thermolysis of a-pinene,
a symmetric diene and an aldehyde (32), both of which involve
a drastic reorganisation of the carbon skeleton. The two
rearranged products arise by participation of the hydroxyl
group in a cleavage, which is initiated on the opposite side
of the ring, to produce two discrete allylic radicals (32a), (32b)
(Scheme 13). A difference in the ~eld of the aldehyde (32)
from the cis- and trans- pinocarveols is due to the difference
inter-17.
+
( 26) -} 9/~
CHO
(27) ~~ 3. 5/S
1 L~?6 (- ( 2 6 ) 4.
7?6
<- ( 27)(32)
~
+
MHO
.
'
(32b)
/
"
H--o
(32a)~~
)
(
(27a~· ~
(26a)
I!> Ill •
Ill
Ill
HO'GJ
HO
HQ,,
,,
HQ.,
'•
_.,
4--·510° 510°
(26) (27)
!
!
HO
HO
HQ.,
,,
HO·,
,,
HQ,,, .
I
+
+ +62.2%
-18.
mediates (26a) and ( ). The biradical snecies (27a),
arising from tran_~~pinocarveol (27), has an axial isopropenyl
radical and is1 therefore, less favoured energetically than
the biradical (26a) in which the isopropenyl group is
equatorial. cis-Pinocarveol (26) gives rise to 14% of aldehyde
(32)1 while trans-pinocarveol (27) produces only 4.7% when
pyrolysed at 510°.
Another trend in the products formed on pyrolysis of
pinane derivatives becomes evident if the pinane substrates are
divided into
two~pes:
25
those with an hybridised carbon, and those with sp3 hybridisation adjacent to a ring junction.Isopropenylcyclohexane derivatives are formed only in the
pyrolyses of the with an sp2 carbon adjacent to a r
junction.
Pyrolysis of nopinone (21)20 at 600° gives the following
compounds: (Scheme 14).
(21)
S.M
6.
5";6Lower b.p. compounds (??b) and five unknowns (5.576)
constitute the remainder.
19.
from 1, and from
5,6-
bond cleavage respectively (Scheme15).
Ill
3
0
Q
~
48-2~
-H
0
H
1 7 (21b)/
H
H
/
~
;Y~
\ I
cyclopentanones
staggered.
/~
almost eclipsed
1t<\
H
5
4---t
H
H
( 21a) . (21b)
20.
Bond cleavage will not occur unless the radical orbital lies
in the same plane as the bond to be broken. If this alignment
is not present, there will be a degres of orthogonality in the
developing double bond (5,7-) which may prevent its formation.
I t can be seen (Scheme 15) that the radical at C(5) is
eclipsed with the bot,d, C(1)-C(7), to be broken in biradical
(21b), and no isopropenylcyclohexane is found (Scheme 15).
In contrast, biradical (21a) gives rise to more
isopropenylcyclohexane than it does to the dienone (28).
Here the radical orbital does not easily overlap with the
breaking 5,7- bond, thus making the formation of a double bond
at C(5)-C(7) more difficult. This effect has also been noted in
the products isolated from the pyrolysis of cis- verbanone (23)20•
Cyclopentane derivatives occur frequently among the products of
pyrolysis of pinanes, and are derived from the cyclisation26
of 1,6- octadienes by an 'ene' reaction, The relative yields
of the four stereoisomeric cyclisation products are in the
order of the expected relative energies of the possible transition
states leading to them. The isomer in the highest yield arises
from the transition state in ~~ich non-bonded interactions are minimised, as shown below for linaloB127 (Scheme 16).
H
Scheme 16
:CH
~:
3
'
•, ,
..
r-21.
The other trend which pinane pyrolyses appear to follow is
the production of carbonyl compounds from those pinanols in
which a hydroxyl group is adjacent to a ring junction.28 A
1,6-octadiene (30) formed by scission of the 1,7- and
5,6-nopinol (25) bonds2,2 can cyclise to give cyclopentanols (31), in which the hydroxyl function is adjacent to the isopropenyl
group. A re!:.££-ene reaction can then occur, leading to the
14~:,~ of 4, 6-dime thylhept-6-enal ('105) which is found in the
pyrolysis of nopinol at 630° (Scheme 17).
OH
+
7
(30)
(29)
(31) (33)
( lt isomers)
II
!
Scheme 17
The four cyclopentanols (33), formed from the other dienol (29),
cannot undergo this type of rearrangement.
An example of the commercial exploitation of the pyrolyses
22.
synthesis of the a- and
a-
irones (34) (35), extremely valuable perfumery compounds found in the essential oil ofviolets. Pyrolysis of verbenol (36), produced from the
aut-oxidation of a-pinene, was achieved by introducing the chemical
dropwise at a rate of 1 ml min -1 through a
1/L~tr
i. d. stainlesssteel tube maintained at 450° - 470°. After distillation of
the pyrolysate at reduced pressure, the fractions were assayed
by g.l.c., and the pyrolysis was found to give the products as
follows (Scheme 18):
~OH
Hydrocarbons450° - Unknowns
Residue
Unchanged verb enol
Verb enol Loss
HO
HO
limonen-3-ol limonen-5-ol
+
~-
a-Scheme 18
pseudotagetones
OHCU
(37)
11%
4%
13e5% 10%
9.5%
23.
To obtain"o:.- and ~- irene, pseudocyclocitral (37) is reduced~ by a Wolff-Kischner process, to the hydrocarbon before being
condensed with formaldehyde and oxidised to give the
6-methylcyclocitrals. These are readily condensed with a ketone
to produce the perfumery materials (Scheme 19):
( 1)
(2)
Oxidation
•
Reduction
~OH
OHCD
---~'
I
...
a-pinene verb enol
~~
H
6-methylcyclogeraniols
l
oxidise~CHO
~
+
HO
a- + base
6-methylcyclocitrals
Scheme 19
pseudocyclocitral
Wolf-Kischner reduction
l
( 1) AcOH/ Ac ~;0
«a-Paraf
orma~deh;yd
saponify
n
1,3,3,4-tetramethy cyclohexene
o:.-irone
~-irene
At the time, and according to the patent,2
9
this process was more efficient than existing methods of synthesising the ironesand had the added adv~ntage that, if optically active a-pinene was used, the irone8 produced would also be optically active.
This product is commercially more valuable than the racemic irene
24.
- Substituted Pinanes
Although pinane derivatives substituted in tl1e 2,3,4
and 10 positions occur frequently in oils derived from plants,
7- substituted pinanes have seldom been observed. The first
such compound reported was chrysan then one ( 2--pinen-7-one) ( 4),
which was isolated30 by Kotake and Nonaka in 1957 from the oil
of Chrysanthemum sinense (sabin). Blanchard31 however, pointed
out that the compound which Simonsen32 in 1939 obtained from the
oil of Zieria smithii and designated 11
i~
3-caren-5,6-epoxide"
(39)
exhibited the same properties as Kotake and Nonaka's compound,
and thus must rank as the first example of a 7- substituted
pinene to be discovered. More recently, ci~.:-7-acetoxy-2-pinene (LJO)
has been isolated both from oil of 'Chrysanthemum vulgare' grown
in Finland33, and in 45% yield from the oil extract of Centipeda
cunninghamii, a small perennial plant, native to Australia34•
Chry8anthenone ( 4 ) was synthesised in 1959 by Hurst and
Whitham,35, 3
6
who subjected verbenone (5) to ultra-violetirradiation in the expectation that this system would rearrange
to give the cyclobutanone derivative. The photolysis was studied
in great detail by Erman,37' 38 who isolated a number of other
. primary and secondary products from the reaction when it was
carried out under a variety of conditions. He confirmed the
finding of Hurst and Whitham that racemisation of chrysanthenone
occurs during prolonged irradiation, and thus was led to propose
two pathways by which verbenone (5) isomerises, both of which
involve an unspecified optically active intermediate (5a)
(-)·-(5) (42)
/
Jr
slow--(-)-(4)
( 5a)
ROH
---..
R := 1'1e
R == Et
ROO
( 44)ROOC
( 43)
Scheme 20
+
The schem& shows only the primary reaction products, some
of which underwent secondary reactions.
Racemised chrysanthenone was formed by the cyclisation
of the second intermediate, ketene (42), while reaction of the first intermediate ( 5a) gave directly the optically active
product. The presenoo of the ketene (42) was demonstrated by the isolation of the methyl esters
(43, 44),
when the photolysiswas performed in methanol.
In the light of the rules proposed for photochemical
reactions by '1/oodward and
Hoffmann~
4
the formation ofchrysanthenone from verbenone can be explained by a [1,3]
sigmatropic shi~t with retention 0f configuration cf the
26.
that both the [1,3] sigmatropic shift and the ring cleavage
to give the intermediate ketene must take place from the same
excited triplet state or from thermally equilibrated excited
triplet states, From experiments utilising different triplet
sensitizers the triplet state energy was found to be at least
293
kJ mol-1and its lifetime of the order of 10-9
sec. They concluded that, in verbenone and similar compounds, intersystemcrossing to triplet states is much mor~ rapid than a [1,3]
sigmatropic shift from the excited s et state, the state
usually associated with retention of configuration.
Chrysanthenone (4) has not been pyrolysed in a carrier
gas stream1 but has been found to be unstable at temperatures
as low as 65°, This author has observed that even when stored
at 0° in a coldroom, the compound polymerised noticeably over
a period of four weeks, and it proved impossible to purify
chrysanthenone by preparative gas chromatography.2
3q 25 0
Wenkert "'heated (-)-chrysanthenone([cx]D - 36.6 )
under a nitrogen atmosphere and in a sealed tube at 250° for
20 min. Three products were obtained3
9
and identified a8(+)-2,6,6-trimethylbicyclo[3.2.0]hept-2-en-7-one (45)
([aJD25 + 1.8°) (16'::6), isopiperitenone (46) (Y6), and pipedtenone
( 47) ( 187&); the remainder ( 6y~) was presumably starting material.
A mechanism was proposed in which (-)-chrysanthenone (4) was
in equilibrium with the ketene (42), a system which could
cyclise to both the [3.2.0] bicycloheptenone (45) and the
isomeric (+)-chrysanthenone (4). Wenkert39 did not suggest a
mechanism for the production of the piperitenones in the
pyro-lysis but considered them to arise from a series of acid-induced
1,2-alkyl shifts when chrysanthenone is heated in acidic media
0~)-(4)
---!\>A,
____.,
tit
-.:---"
)(g
...--(+)-Ut)
'-'0
t
H+
(42)HO
(46)
!
( 45)
( 47) .Scheme
The incomplete racemisation of
2,6,6-trimethylbicyclo-[3.2.0]hept-2-en-7-one (45) is seen as a consequence of the rapid
isomerisation of chrysanthenone and the ketene (42), together with
.a slower, but still competitive, ionic pathway between
chrysanthenone and the isomeric cyclobutane (45).
Pyrolysis of chrysanthenone (4) in the absence of acid,
could ~roceed via a biradical intermediate (4a), which can give
isopiperitenone (46) by a [1,5] transfer of hydrogen, or can
undergo
5,7-
bond cleavage to give the ketene (42) (Scheme 22).The bicyclic product would again be produced by cyclisation of
the ketene, the
1%
optical activity probably arising from some28.
chrysanthenone
(4),
or of iso-pineritenone(46)
recovered fromthe pyrolysis are reported, but both should be racemic if the
biradical mechanism is operative.
5,7
o~c
::--.
cleavage.
~ ~
-=:--(4a) (42)
I
1,5
~ H shift
(47)
(46)
( 45)In the pyfolyses of the substituted pinanes examined
to date, the biradical intermediates f0rmed initially are
generated by cleavage of the
1,6-
or5,6-
bonds. In the Jresentwork, pinanes with substituents in the cyclohutane ring have been
pyrolysed, in order to investigate the possibility of inducing
.NOl·l 'Gl'~CLA'J.'UHE
Throughout this work, the 7-substituted-pin-2-enes will
be considered as derivatives of chrysanthenol
(38).
The name'chrysanthenol'
(38)
itself, will imply a - relationshipbetween the C(?)- hydrox;:r (!;roup and the }l~E_-dimethyl moiety on
0(6). The other isomer, which has an ~}];_ti- relationship between the~orementioned functional groups, will be referred to as
( L~1)
epichrysanthenol
(41),
following the nomenclature of Rouessac •Thus the structure cis etoxypin-2-ene
(40)
will be referredto as chrysanthenyl acetate. Similarly, the saturated
?-substituted analogues are named as derivat of
cis-chrysanthanol (Lf8), where "cis-" rtders to a - relationship
between the C(1) methyl and the C(6) - methyls. The
relationship between the hydr and the methyls is
implied by 11chrysanthanoln (F'ig. 3). ~'hus, cis
-acetoxy·~£iE;_-pinane ( 11·9) will be called cit?.-chrysanthanyl acetate, and
trans etoxy-E_Js-pimme (50), £i:E,-epichrysanthanyl acetate.
Verbenone
·---~.;;..
The is of compound relies upon the autoxidation
of a-pinene (1), a singularlJ inefficient reaction requiring that
air be bubbled through the neat starting mater for periods
of up to a week. This reaction has been studied in detail by
industrial chemists, since pinane der ves oxygenated in the
2-,
3-,
~-, and 10- positions are formed and these chemicalsfind extensive employment in perfumery • . The reaction of air
w a-pinene produces various hydroperoxides. If these are
reduced with basic sodium thiosulfate ( 1+2),
th~
main product istra~·-verbenol (51). Oxidation of the alcohol vii th sodium
dichromate in acetic acid verbenone
(5),
but the yield30.
HO
H
(38)
OH
Epiohrysanthenol (4·1)HO
H
trans-Chrysanthanol ( 106) I
H
.
\H
.
cis-Chrysanthanol (48)
HO
A cO
H
_?is-Chrysanthanyl Acetate (49)
A cO
~-Epiohrysanthanyl Acetate (50)
Fie:ure 3
Whitham<43) reacted a.-pinene (1) with lead tetra-acetate
in benzene to produce trans-2-acetoxypin-3-ene (52), which
rearranges in acetic acid to t~-verbenyl acetate (53).
Alkaline hydrolysis of the acetate and subsequent oxidation of
the resulting verbenol forms verbenone. The first two steps led
to the recovery of only 47% of the ~-verbenyl acetate.
However, it was noted that on a scale larger than the
5
g employedin this instance, the rearrangement of the
trans-2-acetoxypin-3-ene (52) in acetic acid could lead to the formation of verbtrans-2-acetoxypin-3-enene
(16), by the elimination of acetic acid.
The most promising synthetic routes to verbenone from
a.-pinene involve the use of catalysts1 and in particular chromium
trioxide, in the aerial oxidation reaction. This reaction was
reported by Badoche(44) in 1953 and reinvestigated by Klein(45)
and Schmidt in 1969. The later study showed that at least 28
compounds are produced by the oxidation, and that verbenone is
formed, under optimum conditions, in up to 30% yield. Under
other conditions the yield can be as low as
5%.
In the present work, the preparation in which the oxidation
was catalysed by chromium trioxide was used after earlier attempts,
using the two-step synthesis, had given verbenone in 16% overall
yield.
Chrlsanthenone (4)
Chrysanthenone is formed by the photochemical rearrangement
of verbenone (5). As noted earlier, much work has been carried
out on this reactiont and it has been observed that the optical
purity of chrysanthenone is dependent upon both the photolysis
conditions and the distillation conditions. Erman(37) has
was photolysed in glacial acetic acid, but this is not the
most convenient of solvents. Although resulting in significant
racemisation( 37 ), the solvent used in the present work was
pentane, from which chrysanthenone can be separated readily.
32.
The pentane solution of verbenone was purged with dry, oxygen-free
nitrogen for an hour before photolysis began. Using g.l.c.
analysis, it was found that at least 90% of the verbenone had
reacted after 6~ hours of photolysis, and
95%
after 8 hours.The ch~ysanthenone was not usually purified before being
used in the syntheses of 7-methylchrysanthenol
(54)
andchrysanth0nol (38), as it was found that large losses of the
material due to decomposition were incurred when either
spinning-band distillation or column chromatography, even on 10% deactivated
alumina, was employed as a purification technique.
Spinning-band .distillation always gave chrysanthenone which
contained from five to ten percent of impurity. When the
chrysanthenone, so isolated, was kept in a cold room (3°) for
four weeks, the ~aterial became viscous, probably due to a
s~ow polymerisation.
Attempts to purify chrysanthenone by preparative g.l.c.
also gave a material which showed signs of polymerisation.
It was found, however, that chrysanthenol (38) was stable
indefinitely at room temperature, and this was used as the
starting material in the preparations of the other 7- substituted
pinane derivatives examined in this work.
Small amounts of chrysanthenone were purified by column
chromatography on deactivated alumina and identified from infrared
and n.m.r. spectra.
A~
absorption in the infrared at 1780 cm-1is characteristic of a cyclobutanone, while ban~s typical of a
-1)
moiety
(1355
em were observed also.The n.m.r. spectrum contained a vinyl proton multiplet
h
( 65.34;
w
2
=6Hz;
C (3 )H), a four-proton multiplet at 6 2.60
comprising signals due to the·C(1) ru1d C(5) brideehead protons,and the C(4) methylene. The C(2) methyl gave rise to a multiplet
(W~
=6Hz)
at 61.70, while the C(8) and C(9) methyl signals 2partially overlapped at 61.19.
Chrysanthenol (38)
Reduction of chrysanthenone by lithium aluminium hydride
in dry ether afforded chrysanthenol in high yield, although the
course of this reaction was influenced markedly by the temperature
at which the excess reducing agent was quenched. Best results
were obtained when the reaction was carried out at -70°, and
subsequently was stirred at this temperature for two hours. If
the reaction was then brought up to room temperature before being
quenched with either water or sodium sulfate decahydrate, the
yield of chrysanthenol was variable but seldom exceeded
60%,
although no starting material remained. Analysis of the product
mixture by gol.c. showed there to be three other compounds present.
Two of these, ~- and trans-verbenol (55) (5'1) were isolated
by preparative g.l.c., but the third compound decomposed on the
column and was not identified. The cis- and trans-verbenols
were charactel"•ised by their infrared and n.m.r. spectra, which
were identical to those of authentic samples. Chrysanthenol (38)
was also isolated from the reaction mixture by preparative g.l.c.
Oxidation of a small sample, by chromium trioxide in pyridine,
gave chrysanthenone (4).
The stereochemistry of chrysanthenol at C(7) was deduced
appeared as a sharp singlet at 63.97. From Dreiding models,
the dihedral angle between the anti- C(7) proton and the C(1)
and C(5) protons is close to 90°, and no coupling of the
anti-C(7) proton would be observed. The 2-pinene derivative
containing a '1-~l'lt~- hydroxyl group (epichrysanthenol (41))
has been synthesised recently by Joulain and Rouessac(41), and
34.
they report that the syn- C(7) proton signal in the n.m.r. appears
at 62.7 as a multiplet. The yield of chrysanthenol was some
90% (g.l.c.), when the reduction of chrysanthenone (4) was quenched
0 '
at -70 by the addition of powdered sodium sulfate decahydrate,
and stirred at this temperature for at least two hours before
being worked up at room temperature.
Although the crude chrysanthenol was of adequate purity
for subsequent reaction to form chrysanthenyl acetate (40) and
cis-chrysanthanol (48), spinning-band distillation afforded the
material sufficiently pure for pyrolysis. The infrared spectrum
of chrysanthenol (38) contained bands at 790 cm-1 and 3450 cm-1
characteristic of a trisubstituted olefin and an alcoholic
function respectively. Leas distinctive were absorptions at
6 '
-16
-11 55 em and 13 0 em indicative of olefinic stretch and a
~em- dimethyl moiety respectively.
A vinyl proton multiplet in the n.m.r. spectrum(S5~22)
was assigned to the C(3) proton. Three methyl signals appeared,
h
at 61.66 (W2 ==4Hz; C(2)CH
3), at 61.56 (C(8)H3) and at 60.89 (C(9)H
3). The C(4) methylene and the two bridgehead protons at C(1) and C(5) appeared as two multiplets, each of two protons,
at 6 2.11 and 61.99 respectively. The C(7) proton gave a singlet
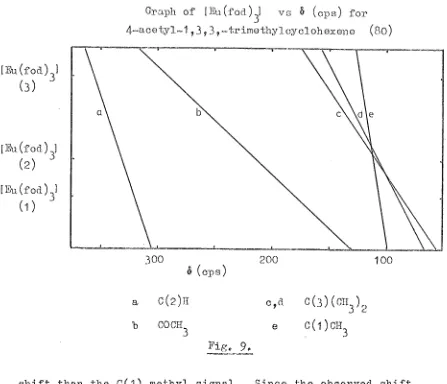
7-Methylchrysanthenol (54)
Chrysanthenone (4) was reacted with methylmagnesium iodide
in ether to obtain 7-methylchrysanthenol in 70% (g.l.c.) yield.
Purification of the alcohol was effected by chromatography on an
active alumina column, to give a colourless, low-melting,
crystalline material (m.p. 53° - 54°). The infrared spectrum
of this compound exhibited bands at 795 cm-1 and 3225 qm-1
consistent with the presence in the molecule of a trisubstituted
olefin and a hydroxyl function respectively.
The syn- relationship of the C(7) hydroxyl to the
,g~-dimethyl moiety was assigned on the following bases. First, the
Grignard reagent should attack chrysanthenone (4) from the least
hindered side, analogous to the reduction of that ketone by
35.
lithium aluminium hydride. Secondly, the positions of the methyl
signals in the n.m.r. spectrum are consistent with a C(7)0H ~~
to the C(8) methyl. 7-Methylchrysanthenol (54) shows a broad
6-proton singlet at 61.66, and two three-proton singlets at 61.27
and 00.93. One methyl contributing to the 61.66 signal will be
the vinyl C(2) methyl, and the other the C(8)H
3• Evidence for the assignment of the C(8) methyl comes from the compound
chrysanthenol (38) where the C(8)H
3 ( 61.46) is deshielded, by the hydroxyl
!El.E..-
to it, relative to the C(8) methyl of a-pinene(61.27). Both 'the C(9)H
3 and the C(7)CH3 are shielded by the 2,3- double bond, but the C(7)CH
3 will be deshielded by its
proximity to the C(7) hydroxyl. Thus, the 61.27 and 60.93 singlets
can be assigned to the C(7) and C(9) methyl groups respectively.
Multiplets at 05.25, 02.22, and 62.00 were assigned to the
C(3) vinyl proton, allylic C(4) methylene, and bridgehead
"
Acetylation of chrysanthenol (38) by reaction with .acetic
anhydride in pyridine afforded chrysanthenyl acetate (40). The
product showed bonds due to the acetate at 1735 cm-1 and 1230 cm-1
in the infrared spectrum. The vinyl 0(2) proton and the 0(7)
h
proton nppeared as a multiplet (W2 ::: 6Hz) at 05.27 and a singlet
at 04.54, resnectively in the n.m.r. Double irradiation
experiments demonstrated the broad singlet of the 0(2) methyl
at 61.68 to be coupled to the 0(3) vinyl multiplet. S ets
at 01. Lf6 and
6o.
were assigned to the 0(8) and 0(9) (JI~-)methyls respectively. Confirmation of the stereochemistry at
0(7) is gained from the deshielding of the 0(3) methyl ( 61.27)
relative to the position of the 0(8) methyl resonance for
a-pinene, by the ~yn-7-acetoxy, and from the singlet nature of
the 0(7) proton signal.
The mass spectrum of the compound did not give the expected
-1
parent ion at mass 194 g mol • The highest mass peak found was
1 g mol ..;1 , which could be accounted for by losci of ketene -1
(CH
2CO; mass 42 g mol ) from the parent ion (Scheme ).
Confirmation of the structure of chrysanthenyl acetate was
~+
HO)((J
§_chem~ 23
obtained when it was reduced, by lithium aluminium hydride
37.
The synthesis of ?-oxygenated derivatives of ~-pinane
While catalytic hydrogenation of chrysanthenone (4) gave
a mixture of cis- and l£~- pinane derivatives (Appendix
II),
ltdid not prove to be a practical synthetic route to the
series, because it would have necessitated their purification
by preparative g.l.c. Hydrogenation of chrysanthenol (38),
however, yielded only cis-chrysanthanol (1+8). Oxidation and
acetylation of the latter compound gave, respectively, ci
-chrysanthanone (56) and cis-chrysanthanyl acetate (49).
c:Ls-Chrysanthanol (48).
~==--~~---~
~is-Chrysanthanol was prepared from chrysanthenol (38) by
catalytic hydrogenation. Chrysanthenol was shaken with a suspension
of 10% Pd/C in dry ethanol under 1600 p.s.i. H
2 pressure for
eight hours. If the ratio of catalyit to substrate was less than
100 mg/1 g~ hydrogenation was incomplete, and had to be continued
after the addition of more catalyst. Following filtration through
celite, the ethanol was distilled off, leaving an oil containing
almost pure cis-chrysanthanol (48). Final purification by
preparative g.l.c. afforded the product as a white, low-melting
The infrared spectrum contained a bond due to hydroxyl
( -1)
stretch 3350 em and a doublet from gem-dimethyl stretching
modes at 1365 cm-1 and 1354 cm-1• Methyl sinE;lets in the n.m.r.
spectrum appeared at 01.48 (C(8)H
3) and 61.07 (C(9)H3). The C(?) proton gave a singlet at 63.66 and the 0(2) methyl a doublet
(J
=
7Hz) at 01.05. The syn- relationship between the C(?)OHand the C(6)(CH
3)2 was confirmed by the appearance of the C(?)H signal (singlet).
38.
basis that hydrogenation should occur from the least hindered side. If hyclrogena·tion had taken place on both faces 9 the JI'.Sll§.- pinane derivative i·Jould have been ol)served by g.J..c. as a minor product. No such minor product was found.
ciE\-Chrysawl:;hanyJ. Acetate·
(49)
"" -·-
---AcotyJ.a-tion of ili~chrysanthano:!_ (48) to form cis-ehrysanthanyl acetate was achieverl by stirring the alcohol in an acetic anh;y·dridejpyridine soJ.uUon. Column chromatography afforded tho acetate 95% (g.l. c.) pure.
AbsorptionB d1.te to the ester group appeared in the infra~
. ~1 -1 -1
red spectrum at
1745
em ,1350
em and1230
em •In the n.m.r. spectrum, the
0(7)
proton gave a singlet ato
4.23,
confirming the assigned stereochemistry at0(7)
because ·there is no apparent coupling of the0(7)
proton to the briclgehead protons. The acetoxy methyl appeared as a singlet at & 2.08 and the C(2) methyl as a doublet at & 1.06 (J ::: 6.5Rz)~ Singlets ato
1.35 and &1.07
vrere Rssignecl to the C(8) and 0(9) methyl protons respectively.The mass spectrum of the compound did not contain a peak at 196 g mo 1-1 corresponding to the parent ion, but a peak of
154 -1 was present. A loss of ketene mass
42,
in a mass g molmanner similar to that suggested for chrysanthenyJ. acetate
(40)
would account for the observed spectrum.39.
generate(l ~by the method of Rateoliff and Rod(~hur (46) and the product purified by propm•ati ve ga.s chromatography. SingletB in the n. m.r. spectrum fol' san than one &1.42 and. & 1.18 ~~ere assigned to the C(8) and 0(9)
(56) at
)
methylH respectively. The G(2) methyl appeared as a doublet at
o
1 ~ 13(.T "'
6Hz)., A broad absorption in the infr•ared npectrum at 1785 cro'-1 :i.s characteristic of a cyclobutanone. fJ'he stretching modes of the l';';.~IJ.l-dimethyl moiety gave :.t'ise to a doublet at1 1
1375 em and 1360 ern •
VeJ~s:_J22.
Although verbenone has been pyrolysed previously
(47),
th0 only products identified vTere p:iperitenone(47)
and i sopiperibenone(46), together amounting to 4Tfn of the pyrolysate. Th:iB pyrolyBis was performed in an iron pipe at 400°
In the presen·~ reinvestigation of Ulis reactimr, the pyrolysis of verbenon0 at 425° vras found to give, in addi t:i.on to the two piperitenones9 six other major products, four of which were identified from their spectra (Table
1).
Isopiperitenone(46) v1as pl~esent in 34~0 and show·ed. bands in the infrared n..t
866
om-1
and872
cm-1
1 character:i.s-tic of trisubsti tute(1 double bonds. An absorption at 1650 cm-1 indioatecl the presence ofthe conjuga·l;ed ketone function, l'Thich also exhi b:i. ted an absorption in the ultraviolet at 226 n.m. ( 1:. = 8, 345); this value is similar to that expected at 228 n.m. for a {J ~P-disubstituted conjuga,tocl enone in cyclohexane. T.he n.m.r. spectrum of the compound
contained three single-proton vinyl mul tiplets, each 1vith a
Table 1
Yields (~) of products formed in the pyrolysis
of Verbenone (5) at 425°
Low boiling point compounds
Unknown I
2, 6, 6-'t,r imethylb icyclo[3. 2.
O]hept-2-en-7-one
(45)
1-Ii'ormyl-2, 6,
6-trimethylcyclohexa-1 ,3-diene (57)
Unknown II
5%
7%
Verbenone (5) 1096
Isopiperitenone (46)
Piperitenone (47)
3, 7-Jlimethyl-2-~is-,
4-c
is-,6-octatrienal (60)
3,7-Dimethyl-2-cis-,4-trans-,6-octatrienal (59)
other compounds (10 of)
2%
3%
'+1 •
at 62.95 (J
=
?.5Hz) arose from the C(4) proton. Methyl singlets at 61.96 and 61.76 were due to the C(8)cH3 and C(1)CH3 respectively.
Piperitenone (47) was found to be present in the
pyrolysis mixture to the extent of only 2?& at lt25°. However,
when a mixture of isopiperitenone and piperitenone was pyrolysed
6 0 . -1
at 00 , with a N
2 flow rate of 30 ml m~n , g.l. c. analysis showed that the piperitenone peak had increased at the expense
of isopiperitenone. No other product was noted in this
pyro-lysate. Piperitenone was identified from its infrared, n.m.r.,
and ultraviolet spectra. Absorptions in the infrared spectrum
at 875 cm-1 and 1660 cm-1 indicated the presence of
tri-substituted olefin ru1d a conjugated ketone respectively. A
h
vinyl proton multiplet (\V2
=
3.5Hz) in the n.m.r. spectrum at 65.85, was assigned to the C(2)H, and two singlets at62.08 and 61.92 arose from the exocyclic vinyl methyl groups
at C(8). The C(1) vinyl methyl protons gave rise to a broad
singlet at 61.84.
The structure of a compound, present in the pyrolysate in
7% yield, was tentatively identified from consideratioh of its
spectra as 1-formyl-2,6,6-trimethylcyclohexa-1,3-diene (57).
6 4
-1 -1Absorptions at 1 7 em and 2730 em were respectively due
to the C=O and C-H stretching frequencies of an aldehyde group.
A
~em-dimethyl
moiety was indicated by two bands at 1367 cm-1-1
and 1390 em , while a cis-dlsubstituted double bond gave an
absorption at 680 cm-1• The n.m.r. spectrum displayed a singlet
at 69.37 and a doublet (J = 5.5Hz) at 66.59, which were
assigned to the aldehyde and C(3) vinyl protons respectively.
A vinyl
multi~let (W~
=
8Hz) at 65.93 arose from the C(4) proton.by a conjugated diene system, while a chemical shift of 05.93
corresponds to that of a proton at the end of a conjugated
diene system. A two-proton multiplet at 02.12 was assi~ned to th~
1
0(5) methylene. The two thyls gave a six-proton singlet
at 61.03 and the vinyl methyl a broad singlet at 61.90.
An absorption in the ultraviolet region (cyclohexane) at 305 n.m.
(E
=
10,265) implied that the aldehyde was conjugated, butdiffered from the calculated (cyclohexane) ~max (313 n.m.) for
a dienone with a homoannular diene component. Other structures
were considered but were eliminated on various grounds. The
two vinyl protons are mutually coupled by 5.5Hz, of the right
order for a cis- relationship, and the terminal proton is also
coupled to at least one other proton. These data, together
with the knowledge that the aldehyde is conjugated and not
coupled to an a-proton, and that is present a vinylic
methyl group, restrict the structure as is seen; (Fig.
4).
CHO
e
4
As the measured mass of the molecular ion indicated a formula
o1
c
10H1l•O' only a C(CH3)2 moiety remained to be placed into the
partial structure above. In
1-formyl-2,6,6-trimethylcyclohexa-1,3-diene (57) the gem- methyls are in a symmetrical
environment and would as is observed, give rise to a single
1-Formyl-2,6,6-trimethylcyclohexa-1,3-diene (57)
·---~·---H---~
4
H
H
HO
A bicyclic cyclobutanone,
2,6,6-trimethylbicyclo[3.2.0]hept-2-en-7-one (45) comprising 14% of the pyrolysate, was identified
from its spectra • . An absorption in the infrared spectrum at
-1
1778 em indicated the presence of a cyclobutanone moiety, while
a doublet (1365 cm-1 and 1347 cm-1)
~rose
from a gem- dimethyl8
-1group. A trisubstituted double bond gave a band at 10 em •
Two single-proton multiplets appeared in the n.m.r. spectrum at
h h
65.43
(W2
=
5Hz) and 64.07(W2
=
11Hz). The former wasassigned to the C(3) vinyl proton, and double irradiation
experiments demonstrated that it was coupled to the C(2) vinyl
methyl signal at 61.77, to a three-proton multiplet at 6 2. 55,
and to the multiplet at 64.07. Assignment of t:ne 6 4.07 signal
to the C(1) bridgehead proton followed from its positiori in the
n.m.r. spectrum,deshielded by both the carbonyl and olefinic
groups adjacent to it. Coupling was also demonstrated between
the C(1) proton and the multiplet at 62.55, which arose from the
combined C(5)H and C(4) methylene signals. Two singlets, at
44.
The data above compared well with those reported for this
. . ( 48) ( 49)
compound(45) by P.Te1sse1re and J. Beereboom •
An alternative structure, 2,7,7-trimethylbicyclo[3.2.0]
hept-2-en-6-one (58) was eliminated, because the signals
reported( 39 ) for its bridgehead protons differ considerably from
those above. In this compound(58) the C(1) proton gives a
doublet at 62.95 coupled by 8Hz to the C(5)H sextet at 63.87.
The C(5) bridgehead proton and the allylic C(4) methylene
( 62.5) are coupled by 3Hz.
Two other compounds, present in 3% and 4%, were tentatively
identified as the 2-£is-, 4-tra~..E..:. and the 2-ci~-,
4·~cis-isomers respectively of 3,7-dimethyl-2,4,6-octatrienal (59), (60).
Each compound was isola ted only rv40?0 pure, with both samples
containing the other isomer and piperitenone (47) as the major
:i.mpuri ties. The 2-·cis-, 4-cis- isomer had a slightly lower
g.l.c. retention time, and contained the larger amount of
low-boiling impurities. Aldehyde doublets in the n.m.r. spectra
appeared at 610.08 and 610.18. Although these two aldehydes
have not been report0d previously, the four isomers of
2,4,6-heptatrienal were characterised by Schiess and Wisson( 5
o>.
Chemical shifts of 69.51, 69.56, 610.11, and 010.16 wereassigned to the aldehyde proton doublets of the isomers
2-trans-, 4-trans- (61), 2-trans-, 4-cis- (62), 2-cis-,
1+-trans-(63), and 2-cis-, 4-cis- (64) 2,L~,6-heptatrienal respectively.
The authors noted that when treated with dilute acid, or during
attempted preparative gas chromatographic separation of the
isomers, all isomerised to give the most stable 2-trans-,
4-trans-compound (61). This isomer has two absorptions in the
u.v.
spectrum at 298 n.m. (~