REVIEW
Respiratory function and mechanics in pinnipeds and cetaceans
Andreas Fahlman1,2,*, Michael J. Moore3and Daniel Garcia-Parraga1,4ABSTRACT
In this Review, we focus on the functional properties of the respiratory system of pinnipeds and cetaceans, and briefly summarize the underlying anatomy; in doing so, we provide an overview of what is currently known about their respiratory physiology and mechanics. While exposure to high pressure is a common challenge among breath-hold divers, there is a large variation in respiratory anatomy, function and capacity between species–how are these traits adapted to allow the animals to withstand the physiological challenges faced during dives? The ultra-deep diving feats of some marine mammals defy our current understanding of respiratory physiology and lung mechanics. These animals cope daily with lung compression, alveolar collapse, transient hyperoxia and extreme hypoxia. By improving our understanding of respiratory physiology under these conditions, we will be better able to define the physiological constraints imposed on these animals, and how these limitations may affect the survival of marine mammals in a changing environment. Many of the respiratory traits to survive exposure to an extreme environment may inspire novel treatments for a variety of respiratory problems in humans.
KEY WORDS: Compliance, Marine mammal, Lung function, Respiratory flow, Tidal volume, Residual volume, Total lung capacity, Respiratory frequency, Alveolar collapse
Introduction
In 1940, Per Scholander published his 131-page-long monograph on cardiorespiratory function in marine mammals and birds. In his treatise, he summarized the respiratory and cardiovascular traits required by marine mammals to manage life in an extreme environment and cope daily with challenges such as alveolar collapse (atelectasis; see Glossary), alveolar recruitment (see Glossary), transient hyperoxia, extreme hypoxia and decompression sickness (DCS; see Glossary). In this Review, we focus on the link between form and function in the respiratory systems of diving marine mammals, but emphasize studies that have attempted to understand lung function and mechanics in pinnipeds and cetaceans (the species where the majority of work has been done). Scholander’s model of lung/alveolar collapse (see below) is of particular interest to this Review; this model provides a mechanism for how marine mammals avoid lung squeeze (see Glossary), limit their uptake of N2, avoid inert gas narcosis (see
Glossary) and DCS, and are able to generate high respiratory flows that are sustained over the entire vital capacity (VC; see Glossary).
There are several reviews that describe anatomical features of diving marine mammals (e.g. Piscitelli et al., 2013), but these reviews focus on the structural properties of excised tissues, which may not always reflect the functional properties of live animals (Kooyman, 1973; Ponganis, 2015). For example, compliance estimates of excised tissues do not account for the influence of surrounding structures that encase the respiratory system (Cozzi et al., 2005; Fahlman et al., 2011, 2014; Moore et al., 2014). Likewise, pulmonary volume changes during compression of entire dead specimens (Moore et al., 2011) cannot account for the potential effects of blood engorgement of the tracheal mucosa in cetaceans (Leith, 1976; Cozzi et al., 2005; Davenport et al., 2013). Thus, the functional properties of the whole living animal cannot be determined from deceased specimens. Here, we discuss recent advances in our understanding of pulmonary mechanics and lung function that we believe provide a theoretical framework that can merge past and future studies to enhance our knowledge of the traits that allow deep diving.
Scholander’s legacy, the model of lung/alveolar collapse Scholander (1940) argued that the compliances of the respiratory system, with a flexible thorax, would allow the elastic and highly compliant alveoli to compress and push the air into the more rigid (far less compliant) conducting airways. As the alveoli compress and collapse, the gas diffusion rate would decrease and cause a pulmonary shunt that increases with depth until the alveoli fully collapse and gas exchange ceases (Fig. 1A,D). Pulmonary shunt represents the amount of blood bypassing the lung and not participating in gas exchange, and it varies between 0% and 100%, where 0% represents a fully inflated lung with perfect gas exchange, and 100% represents termination of gas exchange. Scholander assumed that the trachea behaved like an idealized non-compressible pipe connected to a very compliant lung/alveolar space (balloon-pipe model; Fig. 1A; Bostrom et al., 2008), allowing the alveolar collapse depth to be estimated from Boyle’s law (Scholander, 1940; Bostrom et al., 2008). This has been an important assumption used to understand diving physiology and how marine mammals avoid diving-related problems, such as DCS and N2narcosis, by reducing N2 uptake and blood and tissue N2
tension. However, many aspects of marine mammal respiratory physiology are still not well understood; therefore, in this Review, we summarize past and recent studies with the aim of providing some generalizations about the different traits that have evolved to allow marine mammals to manage a life in the ocean.
Static respiratory variables
Static indices of respiratory capacity are those that do not change between breaths. Data on these variables exist for a limited number of marine mammal species and include information on the total lung capacity (TLC) and minimum air volume (MAV; see Glossary) of the relaxed lung, as discussed below (Kooyman, 1973; Kooyman and Sinnett, 1979; Piscitelli et al., 2010; Fahlman et al., 2011).
1Fundación Oceanográfic de la Comunidad Valenciana, Gran Vıa Marques deĺ Turia 19, Valencia 46005, Spain.2Department of Life Sciences, Texas A&M University-Corpus Christi, 6300 Ocean Drive, Corpus Christi, TX 78412, USA. 3Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA.4Oceanográfic-Avanqua, Ciudad de las Artes y las Ciencias, Valencia 46013, Spain.
*Author for correspondence (afahlman@oceanografic.org)
A.F., 0000-0002-8675-6479
Journal
of
Experimental
TLC or lung size
Overall, maximal lung volumes, or TLC (generally defined as the volume of air in the lung when the transpulmonary pressure is 30 cmH2O, where 1 cmH2O≈98 Pa), of diving mammals are in the
general range of those of terrestrial mammals (Kooyman, 1973; Fahlman et al., 2011; Piscitelli et al., 2013; Ponganis, 2015). Exceptions are the smaller lungs of deep-diving cetaceans and the enlarged lungs of shallow-diving species, e.g. sea otters (Scholander, 1940; Kooyman, 1973; Leith, 1989; Piscitelli et al., 2013; Ponganis, 2015). While the classification of deep and shallow divers is not well defined, and changes as we find out more about the life history of different species, one study defined a shallow diver to be a species where most dives are shallower than 100 m, e.g. bottlenose dolphin (Tursiops truncatus) and harbor porpoise (Phocoena phocoena) (Piscitelli et al., 2010).
Kooyman (1973) compiled the available values for TLC in pinnipeds and cetaceans of different body mass (Mb), from harbor
seal (Phoca vitulina, Mb≈15 kg) to fin whale (Balaenoptera physalus, Mb≈44 tonne), allowing him to derive an equation to
estimate TLC (TLCest, in liters) from a known value ofMb (kg):
TLCest=0.135Mb0.92. However, a later study suggested that the
relationship betweenMb, lung size (and possibly TLC) and muscle
myoglobin concentration differed between a number of deep- (e.g. pygmyKogia breviceps, and dwarf sperm whalesKogia sima) and
Glossary
Alveolar recruitment
The point when collapsed alveoli open up and gas exchange resumes. Atelectasis
Alveolar collapse, resulting in cessation of gas exchange. Collateral ventilation
Ventilatory flow through the lung parenchyma through alternative flow pathways, such as pores of Kohn.
Dead space
The volume of air in the respiratory system not participating in gas exchange, e.g. air in the trachea.
Decompression sickness
Also called the‘bends’or caisson disease; a collection of symptoms observed following a reduction in ambient pressure, which causes bubbles to form in the blood and tissues. In humans, symptoms include dizziness, numbness, fatigue and, in more severe cases, paralysis, problems breathing and death.
Inert gas narcosis
Caused by the anesthetic effect of lipid-soluble gases at high pressure. In air-breathing divers the symptoms may ultimately lead to loss of consciousness as pressure increases.
Lung squeeze
Pulmonary edema caused by intrathoracic pressures that are lower than environmental pressures during breath-hold diving.
Maximal/forced breath
Often used in human lung-function testing to assess the maximal capacity of lung function such as VC, PEF, PIF and airway obstruction. The individual is asked to expire maximally, followed by an inspiration. Minimum air volume
The volume of air left in the relaxed lung. Respiratory frequency
The number of breaths per unit time. Rete
A network of arteries or veins Tidal volume
The volume of air exhaled or inhaled during a normal breath. Vital capacity
The maximal volume of air that can be exchanged in one breath. In marine mammals, VC is close to TLC.
Balloon–pipe model
Rigid but compressible trachea Increasing pressure
Trachea
Alveoli
A
B
C
D
1 cm
4
3
2
1
V
olume (I)
0
0
1 2 3 4 5 6 7 8 9 10 11 12
Pressure (ATA)
Alveolar collapse Compliant trachea Rigid trachea
10 20 30 40 50
Relative dif
fusion rate (
P
⫻
A
)
0 20
Depth (m)
40 60 80 100
AI 252 m VA=0 ml, VDS=420 ml
120
VA
VDS
VA
[image:2.612.310.560.57.514.2]VDS
Fig. 1. The effect of pressure on lung volume and diffusion rate.(A) Graph showing how the compression of the respiratory system is affected when the compliance of the upper and lower airways are accounted for. The figure assumes a Weddell seal with a diving lung volume of 11 liters, an alveolar volume (VA, solid lines) of 10 liters and a dead space volume (VDS, broken lines) of 1 liter. The black lines represent the volume of the respiratory system in relation to depth for Scholander’s original balloon-pipe model, with a stiff dead space that does not compress, and the red lines represent the volume of the lung based on the lung compression model presented in Bostrom et al. (2008). The schematic below panel A provides a qualitative explanation of the two models. The balloon-pipe model, where the conducting airways do not compress, is shown in black, and the model where the airways begin to compress at a depth determined by the specific compliances of the upper and lower airways (in this case 30 m) is shown in red. (B,C) Radiographs of the trachea of a Weddell seal submerged at 1 ATA (atmospheres absolute), the surface (B) and during a dive to 31.6 ATA (C). The arrowheads show the tracheal margins. The circular object is an electrocardiogram (ECG) electrode. Reproduced with permission from Bostrom et al. (2008). (D) A graph showing the effect of alveolar compression on the diffusion rate [ pressure (P)×surface area (A), assuming that the alveolar membrane thickness is not affected], assuming Scholander’s original balloon-pipe model (black line) or the lung compression model presented in A (red line).
Journal
of
Experimental
shallow-diving (bottlenose dolphin and harbor porpoise) cetaceans investigated (Piscitelli et al., 2010). The deep-diving species with smaller lungs also had a higher myoglobin concentration whereas species that were assumed to be shallow divers had larger lungs. It was suggested that the larger lungs in shallow-diving species would help to increase the amount of available O2during short shallow
dives. In the deep-diving species, the available O2 is instead
increased through higher muscle myoglobin concentration, as a greater diving lung volume would increase the amount of N2taken
up during dives, thus increasing the risk of DCS (Piscitelli et al., 2010).
Whether these differences are evolutionary adaptations or traits derived from anatomical and physiological plasticity is unclear. There is evidence that muscle myoglobin concentration changes as juveniles increase their diving capacity throughout ontogeny (Noren and Williams, 2000; Noren et al., 2001), and that diving capacity alters hematology (Duffield et al., 1983; Ridgway and Harrison, 1986). In addition, studies have indicated that lung conditioning, through repeated chest compressions, alters the mechanical properties of the lung (Johansson and Schagatay, 2012; Fahlman et al., 2014). Consequently, there may be considerable physiological plasticity to alter the O2 stores so that animals may vary muscle
myoglobin concentration, pulmonary size and mechanical properties of the lungs, depending on their life history. This may explain the large intra-species differences in diving capability between inshore and pelagic bottlenose dolphins (Mate et al., 1995; Klatsky et al., 2007). Comparing intraspecific differences in respiratory function and myoglobin concentrations in these populations would help to clarify this issue.
Functional residual capacity, residual volume and MAV
The functional residual capacity (FRC) and residual volumes (RV) are, respectively, the amounts of air that remain in the lung following a passive and maximal exhalation. In the human lung, FRC and RV are approximately 40% and 22% of TLC, respectively (Berend et al., 1980; Crapo et al., 1981). At relaxed FRC, the inward recoil of the lung equals the outward recoil of the chest so that the forces balance, and at RV, the inward recoil of the lung is lower than the outward recoil of the chest. This outward recoil helps to retain a volume of air in the lung, thereby preventing alveolar closure and atelectasis. In the marine mammals tested, mainly pinnipeds, the chest does not resist compression, i.e. it has very high compliance (Fig. 2) (Leith, 1976; Fahlman et al., 2014). In these species, relaxed FRC is close to or equal to RV. The excised lung of a terrestrial mammal retains a certain amount of air whereas the pulmonary architecture in marine mammals allows for near-complete alveolar emptying (Denison et al., 1971; Kooyman and Sinnett, 1979; Piscitelli et al., 2010; Fahlman et al., 2011). Consequently, the MAV that remains in the relaxed excised lung is similar to FRC or RV in the pinnipeds (Kooyman and Sinnett, 1979) (see the ‘Chest compliance’section). In excised lungs from a number of species of cetaceans and pinnipeds, the mean MAV is 7% (range 0–17%) of TLC (Kooyman and Sinnett, 1979; Fahlman et al., 2011), which is close to the measured FRC in a live pilot whale (Globicephala scammoni) and California sea lion (Zalophus californianus, 12–19% of TLC) (Olsen et al., 1969; Kerem et al., 1975). Consequently, residual air in the lungs of marine mammals following a maximal exhalation is minimal, and the maximal volume that can be exchanged during a breath, the VC, would be close to TLC. This allows marine mammals to exchange almost the entire lung volume in a single breath, which minimizes dead space ventilation (see Glossary) and is an efficient ventilatory strategy. In
addition, the small volume of air that remains at MAV reduces the risk of barotrauma during breath-hold diving.
Dynamic respiratory variables
Dynamic respiratory variables are those that may change between breaths and, at least in some sense, are under voluntary control. This includes variables such as respiratory frequency (fR; see Glossary),
tidal volume (VT; see Glossary) and VC. There are limited data on
dynamic respiratory variables for pinnipeds and cetaceans, and there is considerable variability among marine mammal species (Ponganis, 2011, 2015). However, there are some general trends that can be described. For example, compared with similarly sized terrestrial mammals on land (see table 1 in Stahl, 1967), fR is
significantly lower and VT is higher in resting cetaceans and
pinnipeds when in water or breathing at the water surface, and for pinnipeds on land (Table 1).
Respiratory frequency
Data from 29 species of semi- and fully aquatic marine mammals have allowed fRto be determined as:fR=33Mb−0.42(Mortola and
Limoges, 2006). The allometric mass-exponent is significantly different from that calculated for terrestrial mammals (−0.26, Stahl, 1967); thus, for similarly sized animals,fRis significantly lower in
an aquatic mammal as compared with a terrestrial one. In addition, the terrestrial breathing strategy in adult land mammals involves a brief expiratory pause whereas the aquatic breathing strategy in marine mammals involves an inspiratory pause, which often lasts for seconds to minutes (Scholander, 1940; Spencer et al., 1967; Olsen et al., 1969; Kooyman et al., 1971; Kooyman, 1973; Kerem et al., 1975; Mortola and Lanthier, 1989; Reed et al., 1994; Mortola and Limoges, 2006; Fahlman et al., 2015b; Fahlman and Madigan, 2016). In pinnipeds, this breathing strategy persists on land (Mortola and Lanthier, 1989; Mortola and Limoges, 2006; Fahlman and Madigan, 2016). Interestingly, humans change their ventilation pattern to the aquatic form, with a respiratory pause on inspiration, when in water (Kooyman, 1973). It was hypothesized that the
120
100
80
V
olume (%
TLC
est
)
60
40
20
0
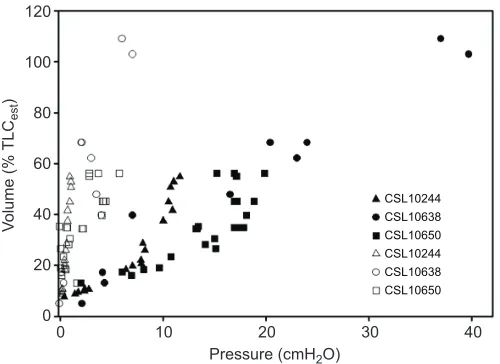
0 10 20
CSL10244
CSL10638
CSL10650 CSL10244
CSL10638
CSL10650
30 40
[image:3.612.313.563.57.239.2]Pressure (cmH2O)
Fig. 2. The pressure–volume relationship (compliance) for the lung and chest for three individual California sea lions (Zalophus californianus). Values for the lung are represented by closed symbols, and those for the chest are represented by open symbols. Each of the different shapes represents a different individual sea lion. Figure modified from Fahlman et al. (2014), with permission. TLCestis estimated lung capacity (Kooyman, 1973). The much higher compliance of the chest indicates that the chest does not resist compression, which minimizes the risk of lung squeeze.
Journal
of
Experimental
aquatic breathing strategy evolved to aid buoyancy (Mortola and Limoges, 2006). An alternative hypothesis was presented in a later paper, where Mortola and Sequin (2009) suggested that the aquatic respiration pattern in marine mammals may help maintain the arterial partial pressure of CO2 (PaCO2) at levels similar to that of land
mammals despite a much lowerfR, not entirely matched by a higher VT. Results in the walrus (Odobenus rosmarus) and California sea
lion, bottlenose dolphin, killer whale (Orcinus orca) or beluga whale (Delphinapterus leucas) suggest thatPaCO2during calm breathing is
within a range similar to that of humans (32–42 mmHg, Mortola and Sequin, 2009). Others have reported that the normalPaCO2may be
slightly higher, around 46–68 mmHg in bottlenose dolphins (McCormick, 1969; Fahlman et al., 2015b).
Respiratory duration
There is considerable variability in the duration of the expiratory and inspiratory phases of breathing in marine mammals (Table 1). One reason could be that the measurements have been performed in animals under different conditions, such as during rest or following exercise or diving. In the resting beluga whale (Epple, 2016), and in both the Atlantic and Pacific bottlenose dolphin (Kooyman and Cornell, 1981; Fahlman et al., 2015b), the duration of the expiratory phase is shorter than the inspiratory phase for normal and forced (maximal) breaths (see Glossary; Table 1). In California sea lions following recovery from diving, the expiratory phase is shorter than the inspiratory phase (Table 1) (see fig. 2 in Kerem et al., 1975). With increasingVT, the duration of the expiratory phase decreases
while that of the inspiratory phase increases (Kerem et al., 1975). In Patagonia sea lions (southern sea lion,Otaria flavescens), resting
while laying down, the expiratory duration is significantly longer than the inspiratory duration (Fahlman and Madigan, 2016). In the gray whale (Eschrichtius robustus), expiratory durations range greatly (Table 1) (Wahrenbrock et al., 1974; Kooyman et al., 1975; Sumich, 2001). Thus, there appears to be considerable variability in breath durations, and animals appear to alter these depending on respiratory efforts, possibly as flow rates reach the upper physiological limit (Kerem et al., 1975; Epple, 2016).
VC andVT
Marine mammals, and in particular cetaceans, are able to generate high expiratory flow (Table 1), and have VCs that are close to TLC. However,VT for most normal breaths, even following diving or
exercise, is well below VC (Fig. 3) (Irving et al., 1941; Olsen et al., 1969; Kooyman and Cornell, 1981; Reed et al., 2000; Fahlman et al., 2015b, 2016). It is reasonable to assume that animals may increase bothVTandfRwhen they return from a long dive, or during
intense swimming efforts at the surface, as this would maximize gas exchange and reduce time to recovery. Several studies have shown that there is a correlation between dive duration, dive depth and the
fRfollowing a dive (Würsig et al., 1984, 1986; Dolphin, 1987b).
Thus, the respiratory effort, or minute ventilation (the volume of air inhaled/exhaled per minute estimated as the product ofVTandfR), is
likely to vary with activity as in terrestrial mammals (Williams and Noren, 2009; Fahlman et al., 2016).
Some studies have usedfRto estimate field metabolic rate in
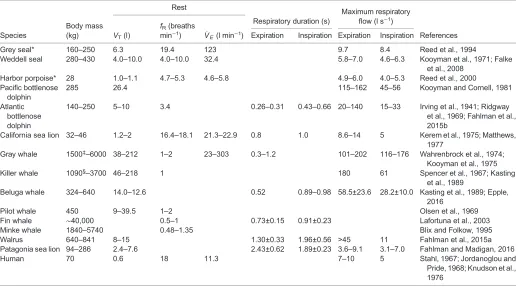
[image:4.612.48.566.79.366.2]free-ranging large whales where standard methods, like respirometry, are not logistically feasible (Sumich, 1983; Dolphin, 1987a; Armstrong and Siegfried, 1991; Folkow and Blix, 1992; Blix and Folkow, Table 1. Tidal volume (VT), breathing frequency (fR), minute ventilation (V_E) and maximum expiratory and inspiratory flows during rest in a number
of marine mammal species
Rest
Species
Body mass (kg) VT(l)
fR(breaths min−1) V_
E(l min−1)
Respiratory duration (s)
Maximum respiratory flow (l s−1)
References Expiration Inspiration Expiration Inspiration
Grey seal* 160–250 6.3 19.4 123 9.7 8.4 Reed et al., 1994
Weddell seal 280–430 4.0–10.0 4.0–10.0 32.4 5.8–7.0 4.6–6.3 Kooyman et al., 1971; Falke et al., 2008
Harbor porpoise* 28 1.0–1.1 4.7–5.3 4.6–5.8 4.9–6.0 4.0–5.3 Reed et al., 2000 Pacific bottlenose
dolphin
285 26.4 115–162 45–56 Kooyman and Cornell, 1981
Atlantic bottlenose dolphin
140–250 5–10 3.4 0.26–0.31 0.43–0.66 20–140 15–33 Irving et al., 1941; Ridgway et al., 1969; Fahlman et al., 2015b
California sea lion 32–46 1.2–2 16.4–18.1 21.3–22.9 0.8 1.0 8.6–14 5 Kerem et al., 1975; Matthews, 1977
Gray whale 1500‡–6000 38–212 1–2 23–303 0.3–1.2 101–202 116–176 Wahrenbrock et al., 1974; Kooyman et al., 1975
Killer whale 1090§–3700 46–218 1 180 61 Spencer et al., 1967; Kasting
et al., 1989
Beluga whale 324–640 14.0–12.6 0.52 0.89–0.98 58.5±23.6 28.2±10.0 Kasting et al., 1989; Epple, 2016
Pilot whale 450 9–39.5 1–2 Olsen et al., 1969
Fin whale ∼40,000 0.5–1 0.73±0.15 0.91±0.23 Lafortuna et al., 2003
Minke whale 1840–5740 0.48–1.35 Blix and Folkow, 1995
Walrus 640–841 8–15 1.30±0.33 1.96±0.56 >45 11 Fahlman et al., 2015a
Patagonia sea lion 94–286 2.4–7.6 2.43±0.62 1.89±0.23 3.6–9.1 3.1–7.0 Fahlman and Madigan, 2016
Human 70 0.6 18 11.3 7–10 5 Stahl, 1967; Jordanoglou and
Pride, 1968; Knudson et al., 1976
The range of maximal flows between different individuals is given when available. *Data collected during surface periods between breath holds/apneas.
‡Body mass of wild animals was estimated from length [4.8–5.8 m estimated to be approximately 1500–2300 kg (Krogh, 1929)].
§Beached female.
Journal
of
Experimental
1995; Rodríguez de la Gala-Hernández et al., 2008; Christiansen et al., 2014). The volume of O2taken up per breath is the product of
theVTand the difference in inhaled and exhaled O2concentration,
i.e. the O2 exchange ratio. These models assume that the O2
exchange ratio andVTremain constant during the surface interval.
However, both change during recovery from exercise and diving, and accounting for these dynamic changes in physiology improves the estimated metabolic rate (Ridgway et al., 1969; Reed et al., 1994, 2000; Miedler et al., 2015; Fahlman et al., 2016). Thus, an improved knowledge of respiratory physiology may be useful to improve estimates of field metabolic rate using this method.
Diving lung volume
The diving lung volume is the volume of air that an animal brings with it during submersion; it can be adjusted behaviorally at the beginning of the dive or by exhaling while submerged. The structural properties of the respiratory system and the ratio between alveolar and dead space volume, and therefore the diving lung volume, affect the alveolar collapse depth (Scholander, 1940; Bostrom et al., 2008). Thus, behavioral adjustment of the diving lung volume may be important to adjust the O2stores or to minimize
N2uptake and the risk of gas emboli (Hooker et al., 2005; Fahlman
et al., 2009; McDonald and Ponganis, 2012). The fact that each animal has the ability to behaviorally alter the alveolar collapse depth makes this a complicated variable to predict.
There appears to be considerable variability in the diving lung volume within and between species or even within different dives of the same individual. Our current assumption is that most seals exhale before diving whereas sea lions and cetaceans dive on inhalation (Snyder, 1983; Ridgway, 1986; Ridgway and Harrison, 1986; Kooyman, 1989). Scholander (1940) reported that gray seals
(Halichoerus grypus) exhale before diving and inhale when they return from a dive. In the Weddell seal (Leptonychotes weddelli) dives begin and end with an expiration, which indicates that they do not dive on RV (Kooyman et al., 1972). In a forced diving experiment on seals in a pressure chamber, the measured diving lung volume varied between 20% and 60% of TLC and increased for longer, but not necessarily deeper, dives (Kooyman et al., 1972). Similarly, California sea lions diving in a water-filled chamber exhaled upon surfacing (Kerem et al., 1975). Freely diving Antarctic fur seals exhale during the ascent. This is possibly a behavioral method to prevent recruitment of collapsed alveoli, thereby preventing shallow water black-out (Hooker et al., 2005), which is caused by expansion of the alveolar volume, causing a significant drop in lung O2 partial pressure and a reversal of O2
diffusion from the pulmonary capillary into the lung. This rapidly reduces the arterial O2tension and results in cerebral hypoxia and
unconsciousness. Hooker and colleagues argued that by reducing the diving lung volume, the alveolar collapse and recruitment depth would become shallower, thereby preventing the O2reversal. In the
California sea lion, the estimated alveolar collapse depth increases with dive depth, suggesting that the diving lung volume increases with depth (McDonald and Ponganis, 2012).
In cetaceans, breath-by-breath analysis and observations in dolphins (Ridgway, 1986; Fahlman et al., 2015b), beluga whale (Epple et al., 2015), harbor porpoise (Reed et al., 2000), gray whale (Sumich, 2001) and pilot whale (Olsen et al., 1969) suggest that the majority of breaths begin with exhalation, followed by inspiration and a respiratory pause (see the‘Respiratory frequency’section). This is also consistent before and after a bout of exercise or a breath-hold (Fahlman et al., 2016). These studies are often performed on restrained animals or those under human care, and thus may not entirely reflect the behavior in free-ranging whales. However, measuring the diving lung volume in free-ranging animals is logistically challenging, and few studies have been attempted to do this. Technological advances may help us to understand the behavioral strategies of various species. For example, one study used a digital acoustic recording tag (Dtag) to estimate the diving lung volume based on the acceleration and gliding patterns in the sperm whale (Miller et al., 2004). Other studies have recorded the respiratory pattern using data recorders and microphones, and these may shed some light on both respiratory patterns and effort in wild animals (Blix and Folkow, 1995; Sumich and May, 2009; van der Hoop et al., 2014).
Respiratory mechanics: flow and compliance
In addition to anatomical descriptions of the thorax of some marine mammal species (Piscitelli et al., 2010), limited work has investigated the functional and mechanical properties of the respiratory system in live animals (Scholander, 1940; Olsen et al., 1969; Kooyman et al., 1971, 1973, 1975; Kerem et al., 1975; Leith, 1976; Kooyman and Cornell, 1981; Kooyman and Sinnett, 1982; Kasting et al., 1989; Leith, 1989; Reed et al., 1994, 2000; Fahlman et al., 2014, 2015b; Fahlman and Madigan, 2016). For example, it has been suggested that the diaphragm and intercostal muscles are important to generate high respiratory flow and rapidfR(Ridgway,
1972; Dearolf, 2003; Cotten et al., 2008). However, few studies have compared the functional properties within and between species. The available studies have detailed the functional properties of the different parts of the respiratory system from excised tissues, estimated the effect of pressure on lung volume or whole cadavers, or estimated lung and chest compliance in anesthetized and awake voluntarily participating animals (Denison
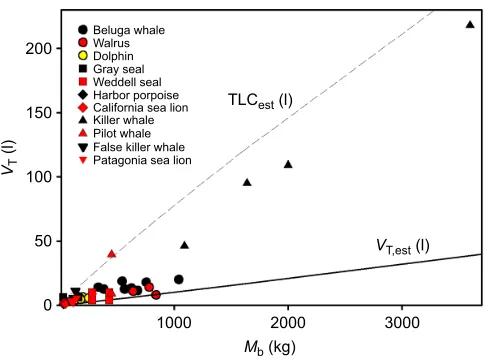
0 50
1000 2000 3000
Beluga whale Walrus Dolphin Gray seal Weddell seal
Killer whale California sea lion Harbor porpoise
Pilot whale False killer whale Patagonia sea lion
TLCest (l)
VT,est (l)
Mb (kg)
100
VT
(l)
[image:5.612.53.296.56.234.2]150 200
Fig. 3. The relationship between measured resting tidal volume (VT) and body mass (Mb) in a number of marine mammal species.Different marine mammals are represented by colored symbols. For comparison, the relationship between estimatedVT(VT,est) andMbis shown for terrestrial animals (solid line, Stahl, 1967). The relationship between estimated total lung capacity (TLCest; broken line, Kooyman, 1973) andMbfor marine mammals reveals that the volume of most breaths of marine mammals is not close to the vital capacity of the animal. References: bottlenose dolphin (Fahlman et al., 2015b), gray seal (Reed et al., 1994), Weddell seal (Kooyman et al., 1971), harbor porpoise (Reed et al., 2000), California sea lion (Kerem et al., 1975; Matthews, 1977), pilot whale (Olsen et al., 1969), killer whale (Spencer et al., 1967; Kasting et al., 1989), beluga whale (Kasting et al., 1989; Epple et al., 2015), walrus (Fahlman et al., 2015a), Patagonia sea lion (Fahlman and Madigan, 2016) and false killer whale (M. Piscitelli, Y. Molgat, P. Dominelli, M. Haulena and A.F., unpublished observation).
Journal
of
Experimental
et al., 1971; Denison and Kooyman, 1973; Kooyman, 1973; Tarasoff and Kooyman, 1973; Leith, 1976; Kooyman and Sinnett, 1979; Kooyman and Cornell, 1981; Fahlman et al., 2011, 2014, 2015b; Moore et al., 2011, 2014). Studies using trained marine mammals that voluntarily participate have been used to define flow– volume characteristics (Olsen et al., 1969; Kooyman and Cornell, 1981; Fahlman et al., 2015b; Fahlman and Madigan, 2016). These data provide mechanistic information about flow limitations, and similar methods are used in human medicine to diagnose a variety of pulmonary disorders (Clausen, 1982). Thus, assessment of lung function may be a useful way to diagnose respiratory health in marine mammals. If successful, lung function studies on wild marine mammals may be a useful method to assess respiratory health in different populations.
Flow–volume limitations
In humans, the flow during forced exhalation is effort independent, and maximal flow occurs at high lung volume and then rapidly declines as lung volume decreases (Hyatt et al., 1958). This flow limitation is caused by increasing flow resistance as the distal airways compress and close during maximal respiratory efforts (Mead, 1961). Consequently, in the human lung, greater expiratory effort does not increase the expiratory flow as the lung volume decreases below
∼80% of VC (Fig. 4) (Kooyman and Cornell, 1981; Fahlman et al., 2015b). By contrast, flow–volume curves from the excised lungs of fin whale, sei whale (Balaenoptera borealis), harbor porpoise and California sea lion, and from maximal respiratory efforts in voluntarily participating Atlantic and Pacific bottlenose dolphins and California sea lions have shown that expiratory flow is effort dependent, and maximal flow persists at all lung volumes (Fig. 4) (Leith et al., 1972; Kerem et al., 1975; Matthews, 1977; Kooyman and Sinnett, 1979; Kooyman and Cornell, 1981; Fahlman et al., 2015b). Consequently, in cetaceans and pinnipeds, it appears that the expiratory flow is not limited by the conducting airways and lung volume as in terrestrial mammals (Fig. 4).
It has been suggested that short, rapid breaths are useful to minimize the time spent breathing at the surface, especially in species that breathe while traveling (e.g. porpoising) or during a surface interval in a dive bout. Thus, it appears that the respiratory anatomy in cetaceans and sea lions allows very high, and almost
constant, flow over most of the VC (Olsen et al., 1969; Kerem et al., 1975; Kooyman et al., 1975; Kooyman and Sinnett, 1979; Fahlman et al., 2015b). In addition, this anatomy allows the lungs to almost completely empty during maximal respiratory efforts or during compression (Denison et al., 1971). Currently, we are not aware of any data in the seal, but given the divergent anatomy and lifestyle between seals, sea lions and cetaceans, one may hypothesize that the exhalations of a seal are more effort independent, and that they are not able to generate similarly high respiratory flow rates as compared with sea lions and cetaceans.
The peak/maximal respiratory flow is seen during expiration, and both cetaceans and pinnipeds (Table 1) have expiratory flows that exceed those of terrestrial mammals (see table 1 in Stahl, 1967). When expressed as a proportion of TLCestper second (TLCests−1),
only the cetaceans (gray whale, bottlenose dolphins, harbor porpoise, beluga whale and killer whale) and the California sea lion have respiratory flow exceeding that seen in humans of about 2 TLCests−1(Table 1) (Kerem et al., 1975; Kooyman et al., 1975;
Kooyman and Sinnett, 1979; Kooyman and Cornell, 1981; Fahlman et al., 2015b; Epple, 2016). In both odontocetes and otariids, expiratory flow is effort dependent over most of the VC, and is not limited by lung volume as is seen in terrestrial mammals (Kerem et al., 1975; Matthews, 1977; Kooyman and Sinnett, 1979; Kooyman and Cornell, 1981; Fahlman et al., 2015b). At least in cetaceans, normal exhalations appear to be mainly passive and driven by the elastic recoil of the chest (Fig. 5A) (Olsen et al., 1969; Fahlman et al., 2015b), typically generating flow rates of 20–40 l s−1. It has been suggested that the lack of a central tendon
in the cetacean diaphragm facilitates emptying (Olsen et al., 1969). By contrast, inspiration and maximal expiratory efforts are active, and exhalations exceeding 160 l s−1have been reported in resting
bottlenose dolphins (Fig. 5C) (Kooyman and Cornell, 1981; Fahlman et al., 2015b). During maximal efforts, the diaphragm and intercostal muscles provide active muscle force to increase the flow generated by the passive recoil of the chest (Ridgway, 1972; Dearolf, 2003; Cotten et al., 2008). It is likely that these flow rates are higher in actively swimming dolphins as the respiratory and locomotor muscles seem to be coupled (Cotten et al., 2008). Thus, evolutionary forces may have engineered a respiratory system with reinforced airways that allow sustained flow rates over most of the VC.
In humans, the ratio between peak expiratory flow (PEF) and peak inspiratory flow (PIF) typically is between 1.2 and 1.4 in healthy subjects (Jordanoglou and Pride, 1968). In the bottlenose dolphin (Kooyman and Cornell, 1981; Fahlman et al., 2015b) and beluga whale (Epple, 2016) the ratio is between 2 and 3 during maximal efforts. These results may indicate physiological limitations during active inspiration that restrict the maximal inspiratory flow rates. Alternatively, these results may be an artefact of working with trained animals, where training maximizes expiration but not inspiration. During normal respiration, the PEF/PIF ratio is between 1 and 1.5 (Fahlman et al., 2015b; Epple, 2016).
Chest compliance
Terrestrial mammals have stiff chest walls, resulting in a relatively large FRC, which prevents atelectasis when the airway is open and the respiratory muscles are relaxed (West, 2012). When terrestrial mammals breath-hold dive, the terrestrial thoracic phenotype resists compression as external hydrostatic pressure increases, which causes negative pressures to develop inside the chest (i.e. lung squeeze) (Lundgren and Miller, 1999). In humans, these negative Forced breath 1
140
120
100
80
60
40
20
0
–20
–40
Forced breath 2
0 5
Volume (l)
Flow rate (l s
–1
)
[image:6.612.58.294.517.692.2]10 15 20 Expiration
Fig. 4. Flow–volume curves for two forced breaths from a bottlenose dolphin.The marked absence of changes in flow with variation in lung volume indicates that flow is effort dependent. Expiratory flow is positive and exhalations
have positive volume (modified from Fahlman et al., 2015b, with permission).
Journal
of
Experimental
pressures cause blood to be drawn into the thoracic cavity (thoracic blood pooling) –this reduces the gas space volume and helps to reduce the pressure difference (Craig, 1968; Schaefer et al., 1968; Leith, 1989). In human breath-hold divers, pulmonary edema and hemorrhage are common; in the case of extreme negative intrathoracic pressures, cardiac arrhythmias and rupture of the vena cava have been reported (Scholander et al., 1962; Leith, 1989;
Hansel et al., 2008; Lindholm et al., 2008; Linér and Andersson, 2008; Lindholm and Lundgren, 2009).
In anesthetized pinnipeds, it seems that the chest is highly compliant (Fig. 2) (Leith, 1976; Fahlman et al., 2014). As discussed above, in species with high chest compliance, FRC and RV are almost equal, which supports Scholander’s hypothesis that the structural properties of the respiratory system allow the alveoli to compress to the limit of collapse without the risk of lung squeeze (Scholander, 1940; Kooyman, 1973; Kooyman and Sinnett, 1979; Leith, 1989; Fahlman et al., 2014).
To our knowledge, no data exist on the mechanical properties of the cetacean chest wall in live animals. In intact carcasses, the odontocete thorax appears to be stiffer than that of the pinnipeds (A.F. and M.J. M., unpublished observation), and it recoils inward to low volumes and is able to compress when exposed to pressure (Ridgway et al., 1969; Moore et al., 2011; Fahlman et al., 2015b). In the seminal work by Ridgway et al. (1969), it was shown that the chest of the dolphin compresses and changes shape during diving. Compression of the chest was observed at depths as shallow as 10 m, and the classic photograph of the trained bottlenose dolphin Tuffy at 300 m shows extensive thoracic compression behind the pectoral flippers (Ridgway et al., 1969). These results were confirmed in deceased cetacean specimens compressed in a hyperbaric chamber and imaged using computed tomography at varying pressures (see fig. 2 in Moore et al., 2011). Thus, the structural properties of the cetacean thorax may allow pressure to compress the chest and lung to very low volumes, thereby preventing pulmonary barotrauma (lung squeeze). We propose that this greater inward recoil in the cetacean might help to produce the high passive expiratory flow reported in odontocetes (Fig. 5A,B) (Olsen et al., 1969; Kooyman and Cornell, 1981; Fahlman et al., 2015b). Future studies are needed to confirm these observations of chest compliance.
Several species of cetaceans have complex thoracic arterial and venous retes (see Glossary). An arterial rete has limited ability to expand, and its tortuosity and interconnections may trap bubbles or emboli and guarantee alternative flow pathways (collateral circulation) to prevent neural emboli and possible trauma (Vogl and Fisher, 1982; Blix et al., 2013). By contrast, the venous rete, being far more distensible, can engorge with blood and reduce the volume of gas-filled spaces, thereby protecting against lung squeeze [similar to the thoracic blood pooling reported in human breath-hold divers (Harrison and Tomlinson, 1956; Murdaugh et al., 1962; Craig, 1968; Hui, 1975; Ridgway et al., 1984)]. It has also been suggested that venous retes may help regulate pressure, flow or pulse, and affect blood composition (Hui, 1975). The phocid seal has an unusually large vena cava, which may fill with blood and expand and serve to protect against pressure-related injuries.
A highly compliant chest under elastic recoil, with a FRC with minimal volume may result in atelectasis if the airway remains open and the respiratory muscles are relaxed. Closing the upper airway (blow-hole or nares) is a simple solution that helps to prevent alveolar collapse in marine mammals when they are not holding their breath underwater. Thus, the aquatic breathing strategy–with an inspiratory pause between breaths, afRthat is lower than that of
their terrestrial relatives and a mass-specificVTthat is up to three
times greater than that of terrestrial mammals (Spencer et al., 1967; Olsen et al., 1969; Kooyman et al., 1971; Kooyman, 1973; Mortola and Lanthier, 1989; Reed et al., 2000; Mortola and Limoges, 2006; Mortola and Sequin, 2009; Epple et al., 2015; Fahlman et al., 2015b)–may be a compromise to prevent alveolar atelectasis while maintaining an alveolar minute ventilation rate that is similar to that
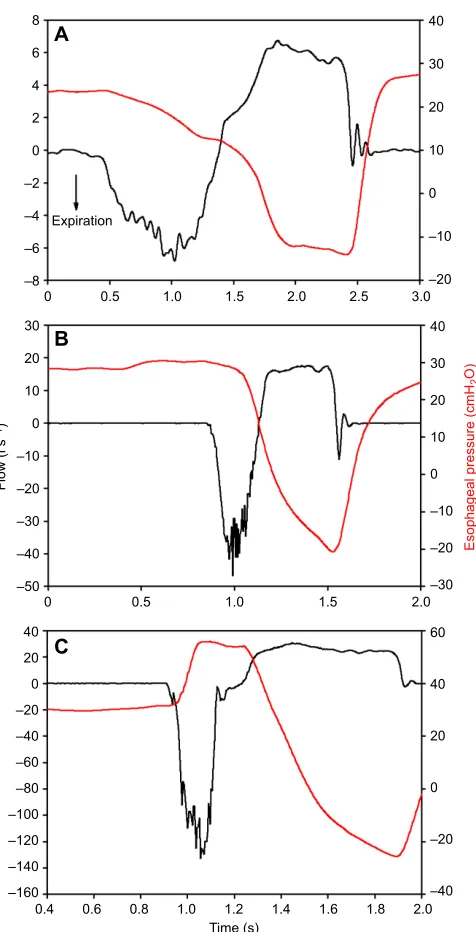
8
A
B
C
40
30
20
10
0
–10
–20 6
4
2
0
–2
Expiration –4
–6
–8
0 0.5 1.0 1.5 2.0 2.5 3.0
30 40
30
20
10
0
–10
–30 –20 20
10
0
–10
–20
–30
–40
–50
0 0.5 1.0 1.5
Flow (l s
–1
)
Esophageal pressure (cmH
2
O)
2.0
40
20
0
–20
–40
–60
–80
–100
–120
–140
–160
60
40
20
0
–40 –20
0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
[image:7.612.56.294.52.518.2]Time (s)
Fig. 5. Respiratory flow and esophageal pressure during normal and forced breaths in a bottlenose dolphin (Tursiops truncatus).The graphs show normal (A,B) and forced (C) breaths, where expiratory flow is negative. Esophageal pressure is shown in red and respiratory flow is in black. During passive exhalation (A), the elastic recoil of the thorax provides the driving force for the emptying of the lungs and the esophageal pressure decreases slightly. In B, the exhalation is in part active, and the esophageal pressures increase slightly before the exhalation and remains more or less constant until the end of the exhalation phase. (C) Maximal exhalations are marked by a large increase in esophageal pressure during the exhalation, indicating an active component to help generate the extreme flow rates seen in cetaceans. Figure modified from Fahlman et al. (2015b), with permission.
Journal
of
Experimental
of land mammals. In newborn human infants, with a highly compliant chest wall, the aquatic respiratory pattern is sometimes present for a few hours following birth (Fisher et al., 1982). Thus, the flexible chest wall may be useful to prevent pulmonary barotrauma but also reflects the need for an aquatic breathing strategy with an inspiratory apnea that helps to prevent atelectasis and improve gas exchange during the respiratory pause between breaths (Leith, 1989; Mortola and Lanthier, 1989).
Airway compliance
While physiological function is at least limited by structural properties, it is not always easy to ascertain function from form. The reinforced conducting airway of marine mammals is a good example. Marine mammals are reported to have reinforced airways (Kooyman and Sinnett, 1982), and there appears to be significant variability between orders and species (Wislocki, 1929, 1942; Bélanger, 1940; Wislocki and Belanger, 1940; Goudappel and Slijper, 1958; Denison and Kooyman, 1973; Henk and Haldiman, 1990; Wessels and Chase, 1998; Ninomiya et al., 2005; Bagnoli et al., 2011). In the sea lion and cetaceans, the cartilaginous reinforcement extends down to the entrance of the alveoli or alveolar sac – there are no respiratory bronchioles – whereas in the seal the last few millimeters of the conducting airway are reinforced with muscle and appear to be much more compliant (Tarasoff and Kooyman, 1973; Cozzi et al., 2005; Bagnoli et al., 2011; Moore et al., 2014). As discussed above, Scholander (1940) proposed that the cartilaginous reinforcement prevents the compression of the airway, facilitating alveolar collapse and cessation of gas exchange, and preventing excessive N2 uptake
(Fig. 1A,D).
It was hypothesized that if reinforced airways are crucial for alveolar collapse, the anatomical differences between the harbor seal and California sea lion would result in differences in alveolar collapse depth. However, these differences in the terminal airways did not result in a marked difference in the pressure-related pulmonary shunt during forced dives in a pressure chamber (Kooyman and Sinnett, 1982). One explanation may be the significant variation in compliance estimates of the upper airways between species (Bagnoli et al., 2011; Davenport et al., 2013; Moore et al., 2014). A comparative study showed that deep-diving pinnipeds have a more compliant trachea as compared with more shallow-diving species whereas deep-diving cetaceans have a stiffer trachea than shallow-diving cetaceans (Moore et al., 2014).
Theoretical work suggests that the structural properties of the various components of the respiratory system may significantly alter the response to pressure (Fig. 1A,D) (Bostrom et al., 2008; Fahlman et al., 2009). Studies on forced diving seals have shown that the trachea does compress during diving (Fig. 1B,C) (Kooyman et al., 1970). In addition, both theoretical work and studies on cadavers and live animals agree that the alveolar collapse depth when gas exchange ceases is probably significantly deeper (Kooyman and Sinnett, 1982; Bostrom et al., 2008; Fahlman et al., 2009; Moore et al., 2011; McDonald and Ponganis, 2012) than suggested from studies that estimate the alveolar collapse depth based on blood N2
tension (Kooyman et al., 1972; Falke et al., 1985) or muscle N2
tension (Ridgway and Howard, 1979), even when the animals exhale prior to the dive (Kooyman and Sinnett, 1982). The effect of pressure on the respiratory system is complex and there is currently limited information available.
While tracheal stiffness may be important for diving ability, submucosal vascular structures in the conducting airways have been reported in both cetaceans (striped dolphin,Stenella coeruleoalba;
bottlenose dolphin; Baird’s beaked whale, Berardius bairdii; pygmy sperm whale; sperm whale,Physeter macrocephalus) and phocids (ringed seal,Phoca hispida; Weddell seal; crabeater seal,
Lobodon carcinophagus) (Welsch and Drescher, 1982; Cozzi et al., 2005; Ninomiya et al., 2005; Smodlaka et al., 2006; Bagnoli et al., 2011; Costidis and Rommel, 2012; Davenport et al., 2013; Moore et al., 2014). This plexus consists primarily of large veins and some arterioles, which may engorge and fill the tracheal lumen with blood, thus reducing the internal volume of the airway, preventing extreme intraluminal negative pressures and minimizing deformity of the tracheal wall. With increasing pressure, tracheal compression may eventually result in negative pressures, which fills the veins and alters the effective tracheal compliance. This will affect the observed relationship between pressure and volume, and will alter the results based on measured structural properties on excised tracheal sections (Moore et al., 2014). Consequently, the effects of pressure on the respiratory system are complex and may also be affected by blood-flow regulation to areas that experience intrathoracic pressures that are below the environmental pressures. In addition, rigid air spaces like cranial sinuses (which do not exist in pinnipeds) and middle ear cavities are also lined with venous plexuses, which would engorge at depth to prevent barotrauma (Odend’hal and Poulter, 1966; Leith, 1989; Costidis and Rommel, 2012; Ponganis, 2015).
These results indicate that different species may have alternative traits or behaviors that minimize diving-related issues. However, they are based on post-mortem specimens and only account for the functional properties of the tissues. Live animals may have alternative strategies to alter compliance through engorgement of blood vessels. In addition, rigid airways may also have other benefits. For example, the increased airway stiffness in sea lions and cetaceans helps to explain how maximal flow can be maintained over the entire lung capacity (see the ‘Flow–volume limitations’ section), which allows the surface interval to be short while porpoising (Kooyman and Sinnett, 1979; Kooyman and Cornell, 1981; Fahlman et al., 2015b). In cetaceans, a functional air volume is required for sound production, and for an adult Cuvier’s beaked (Ziphius cavirostris) whale diving to 3000 m this air volume would be approximately 240 ml (Scholander, 1940; Kooyman, 1973; Schorr et al., 2014). Future studies using medical imaging of live diving specimens may allow us to clarify the importance of the structural properties of the upper airways for diving ability (Leith, 1989).
Lung compliance and collateral ventilation
In excised lungs, the pressure–volume relationship during inflation was similar between otariids, phocids and odontocetes (Fahlman et al., 2011). By contrast, during expiration, the pressure–volume curves diverge: for recoil pressures from 30 cmH2O to 10 cmH2O,
the reduction in volume was lower in the phocid than the odontocete (Denison et al., 1971; Kooyman and Sinnett, 1979; Fahlman et al., 2011). At lower volumes, the odontocete lung appears to be less compliant; these results suggest that the alveolar collapse depth is greater in odontocetes than in phocids (see fig. 4 in Fahlman et al., 2011). This is in agreement with results on cadavers imaged at pressure, for which the alveolar collapse depth was greater for cetaceans than for pinnipeds when the diving lung volume was maximal (Moore et al., 2011).
In studies on live anesthetized animals, inflation volumes are generally much lower to prevent alveolar rupture from overexpansion. As the pulmonary pressure–volume relationship is not linear, and as the method of analysis differs, comparisons
Journal
of
Experimental
between studies on excised tissues and those on live animals are difficult. The previously published data have been reanalysed below to allow a direct comparison. Because lung compliance (CL) varies
withMb (Stahl, 1967), we computed‘specific compliance’(sCL,
cmH2O, measured CL was divided by the estimated MAV)
(Fahlman et al., 2014), in order to provide an index that is independent of animal size (Table 2).
The sCL of excised lungs estimated using the equation by
Venegas et al. (1998) was greater in the phocid than the odontocete. Using the same equation to reanalyse curves of the excised lungs from the California sea lion (see table 2 in Fahlman et al., 2011) suggests that the functional properties of the sea lion lung may be similar to those of phocid lungs during inflation and odontocete lungs during deflation. However, this conclusion should be viewed with caution given the large variation in these estimates owing to the limited numbers of samples.
The sCL from live anesthetized seals and sea lions, and awake
bottlenose dolphin, Patagonia sea lion, walrus and pilot whale are similar to the results from excised lungs (Table 2). The sCL
estimates from marine mammals collected so far, with the exception of the pilot whale, are greater than the mean value in humans. Interestingly, the sCL in anesthetized pinnipeds is significantly
higher in animals from the wild that were confirmed to be free of respiratory disease (Table 2, California sea lion, harbor seal, northern fur seal) compared with those managed under human care (Steller sea lion) (Fahlman et al., 2014). These results may have been caused by the difference in age between the two groups, but may also indicate that lung conditioning and repeated diving alters lung function. Interestingly, humans undergoing divers lung training have been shown to increase VC (Johansson and Schagatay, 2012), possibly suggesting that there is considerable plasticity in mammalian lung function (Butler et al., 2012). Consequently, differences in life history, rather than evolutionary divergence, may explain the results presented by Piscitelli et al. (2010) on differences in lung size between deep- and shallow-diving species.
Taken together, these differences are interesting and may indicate biochemical differences in the lung surfactants between species (Spragg et al., 2004; Miller et al., 2005, 2006a,b; Gutierrez et al., 2015), an active role of bronchial myoelastic sphincters (Kooyman, 1973; Kooyman and Sinnett, 1979; Ninomiya et al., 2005; Piscitelli et al., 2013) and/or variation in lung architecture such as collateral ventilation (see Glossary; Fahlman et al., 2011). During preliminary experiments in the excised lungs of a harbor seal, a white-sided
dolphin and a pilot whale, we noted the possibility of collateral ventilation in the cetaceans but not in the seal (A.F and M.J.M., unpublished observation). We used the Chartis system (https:// pulmonx.com/ous/products/chartis-system/) to quantify collateral ventilation in an anesthetized bottlenose dolphin (A.F., D.G.-P. and E. Cases, unpublished observation), but the level of collateral ventilation was above anything measured in humans, and the system was unable to make an accurate estimate. We propose that indirect secondary pathways (e.g. pores of Kohn) open as the transpulmonary pressure increases to allow air to be shunted through connections between the alveoli or bronchi (Macklem, 1978; Cetti et al., 2006), and this may help prevent elevated transpulmonary pressures and facilitate the recruitment of collapsed alveoli (Namati et al., 2008).
Gas exchange during diving
In the lung, gas diffusion occurs between the gas-filled alveolar space and the pulmonary capillaries through the alveolar membrane. Fick’s law of diffusion states that the rate of diffusion increases with increasing alveolar partial pressure, increasing alveolar surface area and decreasing diffusion distance. Scholander proposed that as an animal descends, the diffusion rate would increase, reach a maximum and then decrease to zero upon alveolar collapse (Fig. 1D, rigid trachea; Scholander, 1940). The initial rise in diffusion rate is caused by an increasing alveolar–venous partial pressure gradient as the ambient pressure increases. However, alveolar compression both reduces the surface area available for diffusion and increases the diffusion distance (the alveolar membrane thickness), thereby decreasing the diffusion rate.
The pulmonary shunt was measured in harbor seals and California sea lions submerged in a pressure chamber up to 10 ATA (atmospheres absolute) (90 m; Kooyman and Sinnett, 1982). The results showed that the shunt increased with pressure but decreased with the diving lung volume, and Kooyman and Sinnett (1982) estimated that full shunt would have occurred at a depth >150 m. Recent work on free-diving California sea lions agrees, and predicts that the alveolar collapse depth is significantly deeper than 100 m, which disagrees with the much shallower alveolar collapse depth from studies in the Weddell seal (Falke et al., 1985) and bottlenose dolphin (Ridgway and Howard, 1979). Bostrom et al. (2008) suggested that a number of varying assumptions between the different studies could explain the difference in estimated alveolar collapse depth. Bostrom and colleagues combined the model of lung compression (Bostrom et al., 2008) with a model that allows the prediction of lung, blood and tissue gas contents (Fahlman et al., 2006) to provide a theoretical framework explaining how pressure affects the lungs and gas exchange (Fahlman et al., 2009). The results from this model agree with Scholander’s predictions, and suggest that the initial increase in diffusion rate followed by a decrease indicates an increasing pulmonary shunt that develops with depth (Fig. 1A,D) (Bostrom et al., 2008). Following alveolar collapse and cessation of gas exchange, the model indicates that there should be a sudden drop in arterial PN2 as venous blood
bypasses the lung without exchanging gases. Thus, upon alveolar collapse, the arterial gas tensions should reflect mixed venous gas tensions. This model provides a unified theory that extends Scholander’s alveolar collapse theory and provides an explanation for the differences between estimated alveolar collapse depths between different studies and species (Fitz-Clarke, 2007; Bostrom et al., 2008; Fahlman et al., 2009).
[image:9.612.47.300.583.716.2]These studies have shown how theoretical models may provide useful insights into complex physiological systems, where a number Table 2. Values of specific lung compliance (sCL) for a range of marine
mammal species
Species
sCL
(cmH2O) Reference
Harbor seal 1.48±0.03 Fahlman et al., 2011, 2014 Elephant seal 0.68±0.20 Fahlman et al., 2014 Steller sea lion 0.25±0.05 Fahlman et al., 2014 California sea lion 0.92±0.16 Fahlman et al., 2014 Northern fur seal 1.58 Fahlman et al., 2014 Patagonia sea lion 0.41±0.10 Fahlman and Madigan, 2016 Walrus 0.61 Fahlman et al., 2015a Bottlenose dolphin 0.31±0.04 Fahlman et al., 2015b Pilot whale 0.13 Olsen et al., 1969
Human 0.08–0.12 Stahl, 1967; Stocks and Quanjer, 1995; Galetke et al., 2007; West, 2012
sCLis computed as the measured lung compliance divided by the minimum air
volume (see the‘Lung compliance and collateral ventilation’section).
Journal
of
Experimental
of studies with seemingly varying results can be explained on the basis of a unifying theory (Scholander, 1940; Ridgway et al., 1969; Kooyman et al., 1972; Ridgway and Howard, 1979; Falke et al., 1985; McDonald and Ponganis, 2012; Hooker and Fahlman, 2015; Hodanbosi et al., 2016). However, it is important to realize that model outputs from these theoretical constructs, such as estimating blood and tissue gas distribution during diving, are limited by available information about basic respiratory physiology (e.g. minute ventilation, VT, fR, PEF, PIF, diving lung volume), the
structural properties of the various portions of the respiratory system (e.g. compliance), and the link between cardiac and respiratory function in live animals (Bostrom et al., 2008; Fahlman et al., 2009). However, an improved understanding of respiratory function in pinnipeds and cetaceans will help us to improve the accuracy of these models, which in turn will allow us to generate new hypotheses and further develop our understanding of the mechanism of the physiological limitations to diving imposed by the respiratory system in these species. For example, the theoretical lung compression model only assumes passive compression of the respiratory system to alter the pulmonary shunt. In sea lions, unlike terrestrial mammals, hypoxia causes vasodilatation of pulmonary vessels (Olson et al., 2010). This may shunt blood away from ventilated areas to collapsed hypoxic areas, increasing the functional shunt. In other words, the animals may re-route blood flow to avoid gas exchange and create an intrapulmonary shunt. Furthermore, in the zoological community it is known that cetaceans are able to control buoyancy without exhaling, possibly by modulating intrathoracic pressures. This may be a method to actively collapse some lung areas and adjust the shunt by active compression of the chest or lung, not relying solely on hydrostatic pressure. However, these potential mechanisms available to minimize complications during diving require further study.
Conclusions
In 1929, August Krogh, the Nobel laureate and grandfather of comparative physiology, first mentioned how some animals appear to have been purposefully created for certain physiological problems (Krogh, 1929). This later became known as the Krogh principle, and it states that ‘For every defined physiological problem, there is an optimally suited animal that would most efficiently yield an answer’. The respiratory physiology of marine mammals is a perfect example of that principle. For example, the highly compliant chest of seals and sea lions provide a great example of how these species prevent lung squeeze. Understanding the respiratory traits that allow marine mammals to manage life in an extreme environment and cope daily with alveolar collapse and recruitment, extreme respiratory flow, transient hyperoxia, extreme hypoxia, hyper- and hypotension, intravascular gas bubbles, lung squeeze and inert gas narcosis is vital in understanding the physiological constraints imposed on these animals, and how these limitations may affect survival.
Few studies have investigated respiratory physiology in live or awake marine mammals (Olsen et al., 1969; Ridgway et al., 1969; Kerem et al., 1975; Kooyman and Cornell, 1981; Fahlman et al., 2015b; Fahlman and Madigan, 2016). Whereas useful information can be derived from comparative studies from the molecular level to cellular, organ, systemic and whole-animal levels, probably the most valuable tool for an integrated understanding of how respiratory physiology affects diving capability is the ability to work with live trained animals voluntarily participating in research trials. Under these conditions, the ontogeny and phylogeny of respiratory function or mechanics can be investigated, and this may allow us to assess the
traits required to allow deep and prolonged diving without ensuing barotrauma or problems associated with decompression. Consequently, knowledge of the combination of structural and functional responses may be crucial to our understanding of the physiological and mechanical mechanisms that allow marine mammals to prevent potential complications associated with diving. A better understanding of the respiratory physiology of marine mammals may explain the convergent evolution of traits to prevent barotrauma, and enable alveolar collapse and recruitment. Improved understanding of respiratory physiology will also increase our understanding of how marine mammals manage gases during diving and how this improves aerobic dive durations while minimizing the risk of gas emboli and N2 narcosis. In addition,
many of the physiological solutions allowing marine mammals to avoid trauma during prolonged deep diving may be of clinical importance to humans and have potential medical applications. For example, understanding how marine mammals are able to fully collapse and recruit the alveoli without apparent trauma may have implications for people undergoing surgery where atelectasis is likely or for prematurely born children where the lung surfactants are not fully developed.
Acknowledgements
We are grateful to the many excellent colleagues, animal care specialists and students that have enabled us to complete a small portion of the studies in this review. We are also grateful for the past studies that have given us the background information, and for the many researchers and colleagues that have inspired and provided comments. Four referees provided constructive criticism, which we believe significantly improved this paper. A special thank you to Stephen Loring and James Butler for always answering an endless stream of questions, and to Gerry Kooyman for providing a copy of the original radiographs from his 1970 study to be reproduced here. We are grateful for the patience and continuous help from Charlotte Rutledge during the revisions of this review.
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.F. wrote the paper, prepared the figures and completed the analysis of previous data; M.J.M. and D.G.-P. participated in discussions on the content, provided advice and references and helped edit the paper.
Funding
Funding for this project was provided by the Office of Naval Research (ONR YIP Award no. N000141410563).
References
Armstrong, A. J. and Siegfried, W. R.(1991). Consumption of Antarctic krill by
minke whales.Antarc. Sci.3, 13-18.
Bagnoli, P., Cozzi, B., Zaffora, A., Acocella, F., Fumero, R. and Costantino, M. L. (2011). Experimental and computational biomechanical characterisation of the tracheo-bronchial tree of the Bottlenose Dolphin (Tursiops truncatus) during diving.J. Biomech.44, 1040-1045.
Bélanger, L. F.(1940). A study of the histological structure of the respiratory portion
of the lungs of aquatic mammals.Am. J. Anat.67, 437-469.
Berend, N., Skoog, C. and Thurlbeck, W. M.(1980). Lobar pressure–volume
characteristics of excised human lungs.Thorax36, 290-295.
Blix, A. S. and Folkow, L. P.(1995). Daily energy expenditure in free living minke
whales.Acta. Physiol. Scand.153, 61-66.
Blix, A. S., Walløe, L. and Messelt, E. B. (2013). On how whales avoid
decompression sickness and why they sometimes strand.J. Exp. Biol. 216, 3385-3387.
Bostrom, B. L., Fahlman, A. and Jones, D. R.(2008). Tracheal compression
delays alveolar collapse during deep diving in marine mammals.Resp. Physiol. Neurobiol.161, 298-305.
Butler, J. P., Loring, S. H., Patz, S., Tsuda, A., Yablonskiy, D. A. and Mentzer,
S. J.(2012). Evidence for adult lung growth in humans.N. Engl. J. Med.367,
244-247.
Cetti, E. J., Moore, A. J. and Geddes, D. M.(2006). Collateral ventilation.Thorax
61, 371-373.