metal-organic papers
Acta Cryst.(2005). E61, m1039–m1041 doi:10.1107/S1600536805013760 Linfang Jiaet al. [CuCl(C
10H8N2)2]BF4CH2Cl2
m1039
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
Bis(2,2
000-bipyridine-
j
2N,N
000)chlorocopper(II)
tetrafluoroborate dichloromethane solvate
Linfang Jia,aWenfu Fu,a,b* Qi Yin,bMingming Yu,aJunfeng Zhangaand Zhanxian Lia
aTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100101, People’s Republic of China, andbCollege of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650092, People’s Republic of China
Correspondence e-mail: fuwfu@sohu.com
Key indicators
Single-crystal X-ray study
T= 293 K
Mean(C–C) = 0.012 A˚ Disorder in solvent or counterion
Rfactor = 0.074
wRfactor = 0.223
Data-to-parameter ratio = 11.5
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
The new mononuclear copper(II) complex, [CuCl(C10H8 -N2)2]BF4CH2Cl2, was prepared by a new procedure using photo-irradiation and a nitrogen atmosphere. X-ray analysis reveals a slightly distorted trigonal–bipyramidal coordination geometry of copper(II); the axial ligands and the CuIIatom deviate slightly from linearity [N—Cu—N = 175.3 (3)].
Comment
The copper(I) complex of [Cu(dmp)2] +
(dmp = 2,9-dimethyl-1,10-phenanthroline) can be photo-oxidized to [Cu(dmp)2]
2+
when irradiated by high-energy laser light in CH2Cl2solution (Horva´th & Stevenson, 1993). The same procedure was used to obtain the copper(II) complex [Cu(bipy)2Cl]BF4 (bipy is 2,20-bipyridine) (see scheme). The copper(II) complex
[Cu(bipy)2Cl]BF4 has already been prepared by a different procedure (Nagleet al., 1990); the complex was obtained using a copper(II) ion in an alcohol–water reaction system. In the complex obtained by Nagle et al. (1990), the CuII atom is coordinated by two 2,20-bipyridyl ligands and by one Cl atom
and exhibits a distorted geometry between square-based pyramidal and trigonal–bipyramidal.
In the title complex, (I), the CuIIatom is also coordinated by two 2,20-bipyridyl ligands and one Cl atom in a slightly
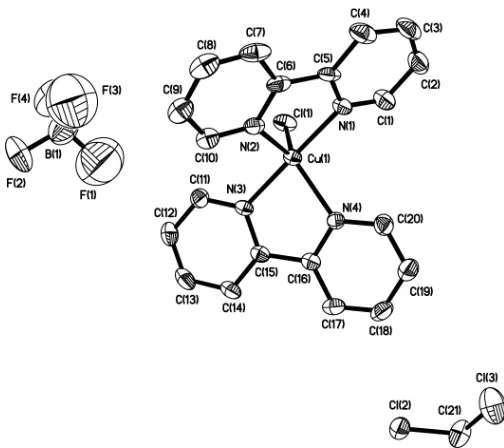
distorted trigonal–bipyramidal geometry (Fig. 1). The axial bond distances Cu—N1 and Cu—N3 are 1.995 (6) and 1.986 (5) A˚ , respectively (mean 1.991 A˚). The equatorial bond distances Cu—N2 and Cu—N4 are 2.104 (6) and 2.100 (5) A˚ , respectively (mean 2.102 A˚ ). The Cu—N distances in (I) (Table 1) are comparable with those in related compounds (Murphy, Nagle et al., 1997; Murphy, Murphy et al., 1997; O’Sullivanet al., 1999; Ma et al., 1999); differences between the title compound and the literature data are within standard deviations of chemically analogous bonds. However,
ences in molecular geometries between the complex reported by Nagleet al.(1990) and the title compound are significant; the two bonds Cu—N1 and Cu—N3 in (I) differ by 0.009 A˚ , whereas the analogous bonds reported by Nagleet al.differ by 0.023 A˚ . The difference between the two bond lengths Cu— N2 and Cu—N4 is 0.063 A˚ (Nagleet al., 1990), whereas in the title compound, the difference (0.004 A˚ ) is less than a standard deviation (0.006 A˚ ). The Cu—Cl bond distance is 2.333 A˚ in (I), which is longer than the Cu—Cl bond distance (2.285 A˚ ) reported by Nagle et al. (1990). Differences in bond angles between these two structures are even more pronounced; the trigonal–bipyramidal coordination geometry of (I) reveals nearly linear axial bonds [N1—Cu—N3 = 175.3 (3)] and the
mean value of the bond angles for equatorial ligands (with CuIIas the central atom) is 120.0. As the coordination of the
complex reported by Nagleet al.(1990) represents a transition
between square-pyramidal and trigonal–bipyramidal, the bond angles deviate significantly from the usual values for the two ideal geometries. In the crystal structure, a BF4
anion is disordered over two orientations with approximately equal populations; a dichloromethane solvent molecule is disor-dered over two positions with approximately equal popula-tions. The packing of (I) is shown in Fig. 2.
Experimental
For the synthesis of (I), a mixture of [Cu(MeCN)4]BF4(100.0 mg,
0.317 mmol) and 2,20-bipyridine (99.2 mg, 0.634 mmol) in
dichloro-methane (25 ml) was stirred for 4 h at room temperature under photoirradiation (365 nm). Green crystals suitable for X-ray diffraction analysis were obtained by recrystallization of the crude product from a dichloromethane–diethyl ether solution. The compound was obtained in 62% yield and identified as [CuCl(2,20
-bipyridine)2]BF4.CH2Cl2. Elemental analysis calculated (%) for
C21H18BCl3CuF4N4: C43.22, H 3.09, N 9.60, found (%): C 43.27,
H 3.10, N 9.55.
Crystal data
[CuCl(C10H8N2)2]BF4CH2Cl2
Mr= 583.09
Monoclinic,P21=c
a= 7.491 (3) A˚ b= 23.458 (8) A˚ c= 14.302 (5) A˚
= 104.803 (6)
V= 2429.7 (14) A˚3
Z= 4
Dx= 1.594 Mg m 3
MoKradiation Cell parameters from 820
reflections
= 2.9–25.7
= 1.28 mm1
T= 293 (2) K Block, green
0.220.200.18 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996) Tmin= 0.691,Tmax= 0.795
12 474 measured reflections
4267 independent reflections 2452 reflections withI> 2(I) Rint= 0.061
max= 25.0
h=8!8 k=12!27 l=17!17
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.074
wR(F2) = 0.223 S= 1.02 4267 reflections 372 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.1219P)2
+ 1.687P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.005
max= 1.00 e A˚ 3
[image:2.610.43.295.68.292.2]min=0.36 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
Cu1—N3 1.986 (5) Cu1—N1 1.995 (6) Cu1—N4 2.100 (5)
Cu1—N2 2.104 (6) Cu1—Cl1 2.333 (2)
N3—Cu1—N1 175.3 (3) N3—Cu1—N4 80.3 (2) N1—Cu1—N4 98.2 (2) N3—Cu1—N2 97.1 (2) N1—Cu1—N2 79.7 (2)
N4—Cu1—N2 118.5 (2) N3—Cu1—Cl1 92.10 (18) N1—Cu1—Cl1 92.44 (19) N4—Cu1—Cl1 121.16 (16) N2—Cu1—Cl1 120.30 (16)
All H atoms were initially located in a difference Fourier map. Aromatic H atoms were placed in geometrically idealized positions and constrained to C—H distances of 0.93 A˚ andUiso(H) values of
metal-organic papers
m1040
Linfang Jiaet al. [CuCl(C [image:2.610.45.294.335.512.2]10H8N2)2]BF4CH2Cl2 Acta Cryst.(2005). E61, m1039–m1041
Figure 1
View of (I), shown with 30% probability displacement ellipsoids. H atoms have been omitted for clarity. Only one disorder component is shown.
Figure 2
[image:2.610.313.565.620.700.2]1.5Ueq(C). The H atoms of the dichloromethane solvent were
constrained to an ideal geometry, with C—H distances of 0.96 A˚ and
Uiso(H) values of 1.5Ueq(C) and refined as riding.
Data collection:SMART(Bruker, 1997); cell refinement:SMART; data reduction: SAINT (Bruker, 1997); program(s) used to solve structure:SHELXS97(Sheldrick, 1997a); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997a); molecular graphics:
SHELXTL(Sheldrick, 1997b); software used to prepare material for publication:SHELXTL.
This work was supported by the Chinese Academy of
Science Hundred Talents, the Foundation (grant No.
2001E0005Z) for Key Project of Yunnan Provincial Science and Technology Commission, and The National Natural Science Foundation of China (grant Nos. 50273045 and 90210033).
References
Bruker (1997).SMARTandSAINT.Bruker AXS Inc., Madison, Wisconsin, USA.
Horva´th, O. & Stevenson, K. L. (1993).Charge Transfer Photochemistry of Coordiation Compounds, pp. 45–48. New York: Wiley–VCH.
Ma, B.-Q., Gao, S., Yi, T., Yan, C.-H. & Xu, G.-X. (1999).Inorg. Chem. Commun.3, 93–95.
Murphy, G., Murphy, C., Murphy, B. & Hathaway, B. (1997).J. Chem. Soc. Dalton Trans.pp. 2653–2660.
Murphy, G., Nagle, P., Murphy, B. & Hathaway, B. (1997).J. Chem. Soc. Dalton Trans.pp. 2645–2652.
Nagle, P., O’Sullivan, E. & Hathaway, B. J. (1990).J. Chem. Soc. Dalton Trans. pp. 3399–3406.
O’Sullivan, C., Murphy, G., Murphy, B. & Hathaway, B. (1999).J. Chem. Soc. Dalton Trans.pp. 1835–1844.
Sheldrick, G. M. (1996).SADABS.University of Go¨ttingen, Germany. Sheldrick, G. M. (1997a). SHELXL97 and SHELXS97. University of
Go¨ttingen, Germany.
Sheldrick, G. M. (1997b).SHELXTL.Bruker AXS Inc., Madison, Wisconsin, USA.
metal-organic papers
Acta Cryst.(2005). E61, m1039–m1041 Linfang Jiaet al. [CuCl(C
supporting information
sup-1
Acta Cryst. (2005). E61, m1039–m1041
supporting information
Acta Cryst. (2005). E61, m1039–m1041 [https://doi.org/10.1107/S1600536805013760]
Bis(2,2
′
-bipyridine-
κ
2N,N
′
)chlorocopper(II) tetrafluoroborate dichloromethane
solvate
Linfang Jia, Wenfu Fu, Qi Yin, Mingming Yu, Junfeng Zhang and Zhanxian Li
Bis(2,2′-bipyridine-κ2N,N′)chlorocopper(II) tetrafluoroborate dichloromethane solvate
Crystal data
[CuCl(C10H8N2)2]BF4·CH2Cl2 Mr = 583.09
Monoclinic, P21/c a = 7.491 (3) Å b = 23.458 (8) Å c = 14.302 (5) Å β = 104.803 (6)° V = 2429.7 (14) Å3 Z = 4
F(000) = 1172 Dx = 1.594 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 820 reflections θ = 2.9–25.7°
µ = 1.28 mm−1 T = 293 K Block, green
0.22 × 0.20 × 0.18 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996) Tmin = 0.691, Tmax = 0.795
12474 measured reflections 4267 independent reflections 2452 reflections with I > 2σ(I) Rint = 0.061
θmax = 25.0°, θmin = 1.7°
h = −8→8
k = −12→27
l = −17→17
Refinement Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.074 wR(F2) = 0.223 S = 1.02 4267 reflections 372 parameters 134 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.1219P)2 + 1.687P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.005 Δρmax = 1.00 e Å−3 Δρmin = −0.36 e Å−3
Special details
supporting information
sup-2
Acta Cryst. (2005). E61, m1039–m1041
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
Cu1 0.42028 (13) 0.50865 (4) 0.23960 (6) 0.0469 (3)
Cl1 0.7415 (3) 0.50140 (9) 0.28414 (13) 0.0587 (6)
N1 0.4238 (9) 0.5650 (3) 0.1356 (4) 0.0511 (16)
N2 0.2647 (8) 0.4643 (3) 0.1180 (4) 0.0445 (14)
N3 0.3949 (8) 0.4519 (2) 0.3385 (4) 0.0440 (14)
N4 0.2798 (7) 0.5586 (2) 0.3198 (4) 0.0394 (13)
C1 0.5077 (13) 0.6152 (4) 0.1517 (7) 0.068 (2)
H1 0.5662 0.6258 0.2148 0.082*
C2 0.5097 (13) 0.6520 (4) 0.0767 (8) 0.078 (3)
H2 0.5663 0.6874 0.0892 0.094*
C3 0.4275 (14) 0.6358 (4) −0.0163 (8) 0.080 (3)
H3 0.4267 0.6604 −0.0674 0.096*
C4 0.3467 (12) 0.5834 (4) −0.0337 (6) 0.068 (2)
H4 0.2937 0.5716 −0.0968 0.081*
C5 0.3442 (10) 0.5476 (3) 0.0441 (5) 0.0487 (19)
C6 0.2592 (10) 0.4913 (3) 0.0339 (5) 0.0497 (18)
C7 0.1746 (11) 0.4657 (4) −0.0542 (5) 0.064 (2)
H7 0.1706 0.4844 −0.1120 0.077*
C8 0.0977 (12) 0.4128 (4) −0.0549 (7) 0.072 (3)
H8 0.0430 0.3951 −0.1133 0.087*
C9 0.1011 (11) 0.3864 (4) 0.0294 (6) 0.065 (2)
H9 0.0461 0.3509 0.0300 0.078*
C10 0.1883 (11) 0.4133 (3) 0.1149 (6) 0.056 (2)
H10 0.1936 0.3946 0.1729 0.067*
C11 0.4573 (11) 0.3988 (3) 0.3422 (5) 0.0505 (19)
H11 0.5132 0.3862 0.2950 0.061*
C12 0.4408 (12) 0.3617 (3) 0.4152 (6) 0.061 (2)
H12 0.4829 0.3244 0.4161 0.073*
C13 0.3631 (12) 0.3804 (3) 0.4847 (6) 0.064 (2)
H13 0.3519 0.3560 0.5342 0.076*
C14 0.3001 (11) 0.4358 (3) 0.4825 (5) 0.0527 (19)
H14 0.2473 0.4491 0.5306 0.063*
C15 0.3165 (9) 0.4709 (3) 0.4082 (4) 0.0403 (16)
C16 0.2512 (9) 0.5304 (3) 0.3974 (4) 0.0394 (15)
C17 0.1667 (10) 0.5571 (3) 0.4608 (5) 0.053 (2)
H17 0.1503 0.5376 0.5147 0.063*
C18 0.1078 (12) 0.6117 (4) 0.4444 (6) 0.063 (2)
H18 0.0494 0.6297 0.4865 0.075*
C19 0.1350 (12) 0.6403 (3) 0.3655 (6) 0.063 (2)
supporting information
sup-3
Acta Cryst. (2005). E61, m1039–m1041
C20 0.2239 (11) 0.6126 (3) 0.3059 (5) 0.0546 (19)
H20 0.2461 0.6323 0.2536 0.066*
C21 0.091 (3) 0.7134 (5) 0.7242 (9) 0.087 (6) 0.570 (17)
H21A 0.2150 0.7275 0.7516 0.105* 0.570 (17)
H21B 0.0154 0.7237 0.7674 0.105* 0.570 (17)
Cl2 0.0959 (16) 0.6401 (3) 0.7133 (7) 0.071 (2) 0.570 (17)
Cl3 0.001 (2) 0.7441 (3) 0.6111 (5) 0.142 (4) 0.570 (17)
C21′ 0.020 (5) 0.7181 (9) 0.692 (3) 0.150 (16) 0.430 (17)
H21C 0.0381 0.7364 0.7543 0.180* 0.430 (17)
H21D −0.1100 0.7200 0.6589 0.180* 0.430 (17)
Cl2′ 0.088 (4) 0.6481 (7) 0.7076 (17) 0.138 (7) 0.430 (17)
Cl3′ 0.146 (2) 0.7540 (4) 0.6244 (9) 0.137 (5) 0.430 (17)
B1 0.3477 (15) 0.2479 (4) 0.1530 (8) 0.121 (5)
F1 0.343 (3) 0.2869 (8) 0.2193 (16) 0.303 (17) 0.564 (15)
F2 0.2664 (17) 0.1995 (5) 0.1755 (9) 0.119 (5) 0.564 (15)
F3 0.252 (4) 0.2639 (10) 0.0642 (12) 0.317 (17) 0.564 (15)
F4 0.5193 (18) 0.2309 (7) 0.1515 (15) 0.186 (8) 0.564 (15)
F1′ 0.411 (2) 0.2986 (5) 0.1954 (10) 0.109 (6) 0.436 (15)
F2′ 0.418 (4) 0.2037 (7) 0.2077 (15) 0.256 (15) 0.436 (15)
F3′ 0.1601 (17) 0.2514 (8) 0.1294 (13) 0.159 (9) 0.436 (15)
F4′ 0.400 (3) 0.2474 (7) 0.0685 (11) 0.146 (8) 0.436 (15)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Cu1 0.0661 (6) 0.0444 (5) 0.0349 (5) 0.0040 (5) 0.0214 (4) 0.0061 (4)
Cl1 0.0578 (13) 0.0786 (15) 0.0410 (10) −0.0018 (10) 0.0150 (9) 0.0076 (9)
N1 0.062 (4) 0.048 (4) 0.052 (4) 0.005 (3) 0.031 (3) 0.011 (3)
N2 0.047 (4) 0.052 (4) 0.037 (3) 0.009 (3) 0.016 (3) 0.004 (3)
N3 0.057 (4) 0.046 (3) 0.033 (3) 0.000 (3) 0.020 (3) 0.007 (2)
N4 0.047 (4) 0.039 (3) 0.032 (3) 0.000 (3) 0.010 (3) −0.001 (2)
C1 0.079 (6) 0.062 (6) 0.074 (6) −0.001 (5) 0.039 (5) 0.015 (4)
C2 0.081 (7) 0.057 (6) 0.114 (8) 0.009 (5) 0.058 (7) 0.026 (6)
C3 0.087 (7) 0.085 (7) 0.082 (7) 0.037 (6) 0.045 (6) 0.053 (6)
C4 0.061 (6) 0.089 (7) 0.058 (5) 0.020 (5) 0.024 (4) 0.029 (5)
C5 0.051 (5) 0.067 (5) 0.034 (4) 0.023 (4) 0.024 (3) 0.019 (3)
C6 0.045 (4) 0.069 (5) 0.039 (4) 0.019 (4) 0.019 (3) 0.005 (4)
C7 0.048 (5) 0.109 (8) 0.034 (4) 0.023 (5) 0.011 (4) 0.006 (4)
C8 0.054 (6) 0.094 (7) 0.066 (6) 0.006 (5) 0.012 (5) −0.023 (5)
C9 0.053 (5) 0.073 (6) 0.065 (6) −0.003 (4) 0.008 (4) −0.017 (5)
C10 0.053 (5) 0.061 (5) 0.054 (5) 0.002 (4) 0.017 (4) −0.003 (4)
C11 0.062 (5) 0.045 (4) 0.044 (4) 0.009 (4) 0.013 (4) 0.006 (3)
C12 0.072 (6) 0.040 (4) 0.068 (5) 0.004 (4) 0.010 (4) 0.010 (4)
C13 0.078 (6) 0.057 (5) 0.056 (5) −0.016 (4) 0.016 (4) 0.016 (4)
C14 0.058 (5) 0.059 (5) 0.045 (4) −0.013 (4) 0.019 (4) 0.008 (4)
C15 0.039 (4) 0.049 (4) 0.031 (4) −0.008 (3) 0.006 (3) 0.000 (3)
C16 0.037 (4) 0.048 (4) 0.031 (3) −0.010 (3) 0.005 (3) −0.004 (3)
supporting information
sup-4
Acta Cryst. (2005). E61, m1039–m1041
C18 0.068 (6) 0.064 (5) 0.063 (5) 0.006 (4) 0.029 (4) −0.017 (4)
C19 0.069 (6) 0.055 (5) 0.068 (5) 0.013 (4) 0.022 (4) −0.001 (4)
C20 0.068 (5) 0.048 (5) 0.046 (4) 0.006 (4) 0.012 (4) 0.005 (3)
C21 0.096 (10) 0.069 (9) 0.092 (10) 0.016 (8) 0.015 (8) −0.012 (7)
Cl2 0.084 (4) 0.060 (3) 0.074 (4) 0.012 (3) 0.027 (3) 0.003 (3)
Cl3 0.165 (9) 0.106 (5) 0.144 (5) 0.017 (5) 0.022 (5) 0.042 (4)
C21′ 0.153 (19) 0.142 (19) 0.157 (18) 0.008 (10) 0.042 (11) −0.006 (10)
Cl2′ 0.146 (11) 0.124 (10) 0.148 (10) −0.005 (8) 0.046 (8) 0.010 (7)
Cl3′ 0.133 (9) 0.102 (6) 0.176 (8) −0.002 (5) 0.042 (6) 0.034 (5)
B1 0.131 (9) 0.106 (9) 0.124 (9) 0.028 (8) 0.029 (8) −0.005 (7)
F1 0.315 (19) 0.297 (19) 0.307 (19) −0.006 (10) 0.097 (11) −0.053 (10)
F2 0.111 (8) 0.093 (7) 0.143 (9) −0.041 (6) 0.017 (6) 0.028 (6)
F3 0.313 (19) 0.308 (19) 0.321 (19) 0.003 (10) 0.062 (11) 0.016 (10)
F4 0.164 (11) 0.196 (12) 0.199 (12) 0.023 (8) 0.050 (9) −0.039 (9)
F1′ 0.163 (10) 0.062 (7) 0.106 (9) −0.014 (7) 0.041 (7) −0.035 (6)
F2′ 0.266 (18) 0.236 (18) 0.259 (17) 0.004 (10) 0.057 (11) 0.039 (10)
F3′ 0.147 (11) 0.183 (13) 0.154 (12) −0.023 (9) 0.053 (9) 0.024 (9)
F4′ 0.174 (12) 0.136 (11) 0.144 (11) −0.018 (9) 0.071 (9) 0.004 (8)
Geometric parameters (Å, º)
Cu1—N3 1.986 (5) C12—C13 1.348 (11)
Cu1—N1 1.995 (6) C12—H12 0.9300
Cu1—N4 2.100 (5) C13—C14 1.379 (11)
Cu1—N2 2.104 (6) C13—H13 0.9300
Cu1—Cl1 2.333 (2) C14—C15 1.376 (9)
N1—C1 1.327 (10) C14—H14 0.9300
N1—C5 1.355 (9) C15—C16 1.473 (10)
N2—C10 1.323 (9) C16—C17 1.382 (9)
N2—C6 1.351 (8) C17—C18 1.355 (11)
N3—C11 1.326 (9) C17—H17 0.9300
N3—C15 1.354 (8) C18—C19 1.373 (11)
N4—C20 1.333 (9) C18—H18 0.9300
N4—C16 1.355 (8) C19—C20 1.372 (10)
C1—C2 1.381 (11) C19—H19 0.9300
C1—H1 0.9300 C20—H20 0.9300
C2—C3 1.369 (13) C21—Cl2 1.727 (9)
C2—H2 0.9300 C21—Cl3 1.742 (10)
C3—C4 1.365 (13) C21—H21A 0.9700
C3—H3 0.9300 C21—H21B 0.9700
C4—C5 1.398 (10) C21′—Cl2′ 1.718 (11)
C4—H4 0.9300 C21′—Cl3′ 1.725 (11)
C5—C6 1.457 (11) C21′—H21C 0.9700
C6—C7 1.392 (11) C21′—H21D 0.9700
C7—C8 1.367 (13) B1—F2′ 1.323 (10)
C7—H7 0.9300 B1—F1 1.325 (10)
C8—C9 1.350 (12) B1—F3 1.343 (10)
supporting information
sup-5
Acta Cryst. (2005). E61, m1039–m1041
C9—C10 1.382 (11) B1—F3′ 1.361 (10)
C9—H9 0.9300 B1—F4′ 1.364 (10)
C10—H10 0.9300 B1—F2 1.364 (9)
C11—C12 1.388 (10) B1—F1′ 1.364 (9)
C11—H11 0.9300
N3—Cu1—N1 175.3 (3) C15—C14—C13 119.0 (7)
N3—Cu1—N4 80.3 (2) C15—C14—H14 120.5
N1—Cu1—N4 98.2 (2) C13—C14—H14 120.5
N3—Cu1—N2 97.1 (2) N3—C15—C14 120.8 (7)
N1—Cu1—N2 79.7 (2) N3—C15—C16 115.4 (5)
N4—Cu1—N2 118.5 (2) C14—C15—C16 123.7 (6)
N3—Cu1—Cl1 92.10 (18) N4—C16—C17 120.7 (7)
N1—Cu1—Cl1 92.44 (19) N4—C16—C15 115.5 (5)
N4—Cu1—Cl1 121.16 (16) C17—C16—C15 123.8 (6)
N2—Cu1—Cl1 120.30 (16) C18—C17—C16 119.9 (7)
C1—N1—C5 120.5 (7) C18—C17—H17 120.1
C1—N1—Cu1 123.6 (6) C16—C17—H17 120.1
C5—N1—Cu1 115.7 (5) C17—C18—C19 119.6 (7)
C10—N2—C6 118.6 (7) C17—C18—H18 120.2
C10—N2—Cu1 128.2 (5) C19—C18—H18 120.2
C6—N2—Cu1 112.9 (5) C20—C19—C18 118.4 (8)
C11—N3—C15 119.6 (6) C20—C19—H19 120.8
C11—N3—Cu1 124.1 (5) C18—C19—H19 120.8
C15—N3—Cu1 116.2 (4) N4—C20—C19 122.8 (7)
C20—N4—C16 118.4 (6) N4—C20—H20 118.6
C20—N4—Cu1 129.1 (5) C19—C20—H20 118.6
C16—N4—Cu1 112.4 (4) Cl2—C21—Cl3 109.9 (7)
N1—C1—C2 121.2 (9) Cl2—C21—H21A 109.7
N1—C1—H1 119.4 Cl3—C21—H21A 109.7
C2—C1—H1 119.4 Cl2—C21—H21B 109.7
C3—C2—C1 119.3 (9) Cl3—C21—H21B 109.7
C3—C2—H2 120.3 H21A—C21—H21B 108.2
C1—C2—H2 120.3 Cl2′—C21′—Cl3′ 110.8 (9)
C4—C3—C2 119.7 (8) Cl2′—C21′—H21C 109.5
C4—C3—H3 120.1 Cl3′—C21′—H21C 109.5
C2—C3—H3 120.1 Cl2′—C21′—H21D 109.5
C3—C4—C5 119.4 (9) Cl3′—C21′—H21D 109.5
C3—C4—H4 120.3 H21C—C21′—H21D 108.1
C5—C4—H4 120.3 F2′—B1—F1 101.4 (15)
N1—C5—C4 119.7 (8) F2′—B1—F3 144.5 (15)
N1—C5—C6 116.3 (6) F1—B1—F3 112.2 (11)
C4—C5—C6 124.0 (8) F2′—B1—F4 62.7 (11)
N2—C6—C7 120.5 (8) F1—B1—F4 114.5 (11)
N2—C6—C5 114.9 (6) F3—B1—F4 110.3 (11)
C7—C6—C5 124.6 (7) F2′—B1—F3′ 115.2 (11)
C8—C7—C6 119.4 (8) F1—B1—F3′ 85.5 (13)
supporting information
sup-6
Acta Cryst. (2005). E61, m1039–m1041
C6—C7—H7 120.3 F4—B1—F3′ 160.0 (12)
C9—C8—C7 119.9 (8) F2′—B1—F4′ 110.9 (11)
C9—C8—H8 120.1 F1—B1—F4′ 134.9 (14)
C7—C8—H8 120.1 F3—B1—F4′ 50.8 (11)
C8—C9—C10 118.5 (9) F4—B1—F4′ 59.5 (9)
C8—C9—H9 120.8 F3′—B1—F4′ 107.0 (10)
C10—C9—H9 120.8 F2′—B1—F2 49.1 (11)
N2—C10—C9 123.1 (8) F1—B1—F2 108.2 (11)
N2—C10—H10 118.5 F3—B1—F2 107.1 (10)
C9—C10—H10 118.5 F4—B1—F2 104.1 (9)
N3—C11—C12 121.4 (7) F3′—B1—F2 67.3 (9)
N3—C11—H11 119.3 F4′—B1—F2 116.7 (11)
C12—C11—H11 119.3 F2′—B1—F1′ 112.3 (11)
C13—C12—C11 119.2 (7) F1—B1—F1′ 31.7 (12)
C13—C12—H12 120.4 F3—B1—F1′ 102.5 (14)
C11—C12—H12 120.4 F4—B1—F1′ 92.3 (11)
C12—C13—C14 120.0 (7) F3′—B1—F1′ 106.1 (9)
C12—C13—H13 120.0 F4′—B1—F1′ 104.7 (9)
C14—C13—H13 120.0 F2—B1—F1′ 138.3 (11)
N3—Cu1—N1—C1 133 (3) C3—C4—C5—C6 178.9 (7)
N4—Cu1—N1—C1 62.0 (6) C10—N2—C6—C7 0.0 (10)
N2—Cu1—N1—C1 179.6 (7) Cu1—N2—C6—C7 174.9 (5)
Cl1—Cu1—N1—C1 −60.0 (6) C10—N2—C6—C5 179.2 (6)
N3—Cu1—N1—C5 −51 (3) Cu1—N2—C6—C5 −6.0 (7)
N4—Cu1—N1—C5 −122.2 (5) N1—C5—C6—N2 2.4 (9)
N2—Cu1—N1—C5 −4.6 (5) C4—C5—C6—N2 −177.3 (6)
Cl1—Cu1—N1—C5 115.8 (5) N1—C5—C6—C7 −178.5 (7)
N3—Cu1—N2—C10 −3.4 (6) C4—C5—C6—C7 1.8 (11)
N1—Cu1—N2—C10 180.0 (6) N2—C6—C7—C8 −0.3 (11)
N4—Cu1—N2—C10 −86.3 (6) C5—C6—C7—C8 −179.4 (7)
Cl1—Cu1—N2—C10 93.2 (6) C6—C7—C8—C9 1.2 (12)
N3—Cu1—N2—C6 −177.6 (5) C7—C8—C9—C10 −1.9 (13)
N1—Cu1—N2—C6 5.8 (5) C6—N2—C10—C9 −0.7 (11)
N4—Cu1—N2—C6 99.5 (5) Cu1—N2—C10—C9 −174.7 (6)
Cl1—Cu1—N2—C6 −81.0 (5) C8—C9—C10—N2 1.7 (12)
N1—Cu1—N3—C11 108 (3) C15—N3—C11—C12 1.3 (11)
N4—Cu1—N3—C11 179.9 (6) Cu1—N3—C11—C12 177.8 (6)
N2—Cu1—N3—C11 62.1 (6) N3—C11—C12—C13 −1.4 (12)
Cl1—Cu1—N3—C11 −58.8 (6) C11—C12—C13—C14 0.4 (13)
N1—Cu1—N3—C15 −75 (3) C12—C13—C14—C15 0.6 (12)
N4—Cu1—N3—C15 −3.5 (5) C11—N3—C15—C14 −0.3 (10)
N2—Cu1—N3—C15 −121.4 (5) Cu1—N3—C15—C14 −177.0 (5)
Cl1—Cu1—N3—C15 117.8 (5) C11—N3—C15—C16 −179.9 (6)
N3—Cu1—N4—C20 −179.4 (7) Cu1—N3—C15—C16 3.4 (7)
N1—Cu1—N4—C20 −3.9 (7) C13—C14—C15—N3 −0.7 (11)
N2—Cu1—N4—C20 −86.6 (6) C13—C14—C15—C16 178.9 (7)
supporting information
sup-7
Acta Cryst. (2005). E61, m1039–m1041
N3—Cu1—N4—C16 3.0 (4) Cu1—N4—C16—C17 177.6 (5)
N1—Cu1—N4—C16 178.6 (4) C20—N4—C16—C15 −180.0 (6)
N2—Cu1—N4—C16 95.9 (4) Cu1—N4—C16—C15 −2.2 (7)
Cl1—Cu1—N4—C16 −83.5 (4) N3—C15—C16—N4 −0.7 (8)
C5—N1—C1—C2 2.8 (12) C14—C15—C16—N4 179.8 (6)
Cu1—N1—C1—C2 178.4 (6) N3—C15—C16—C17 179.6 (6)
N1—C1—C2—C3 −1.6 (13) C14—C15—C16—C17 0.0 (11)
C1—C2—C3—C4 −0.7 (13) N4—C16—C17—C18 1.4 (11)
C2—C3—C4—C5 1.9 (13) C15—C16—C17—C18 −178.8 (7)
C1—N1—C5—C4 −1.6 (10) C16—C17—C18—C19 −0.9 (12)
Cu1—N1—C5—C4 −177.5 (5) C17—C18—C19—C20 −0.8 (13)
C1—N1—C5—C6 178.7 (7) C16—N4—C20—C19 −1.6 (11)
Cu1—N1—C5—C6 2.8 (8) Cu1—N4—C20—C19 −179.0 (6)