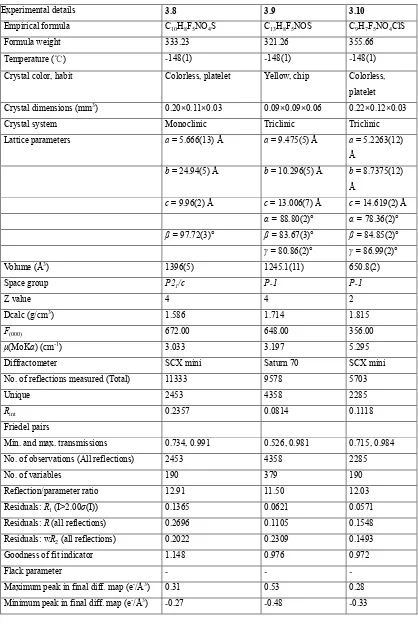
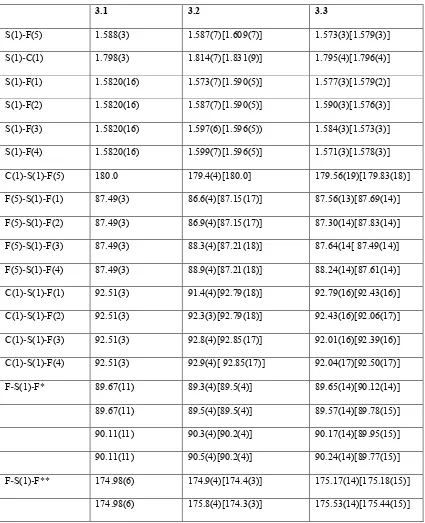
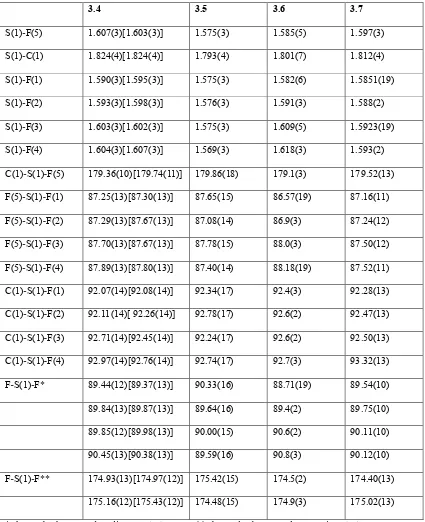
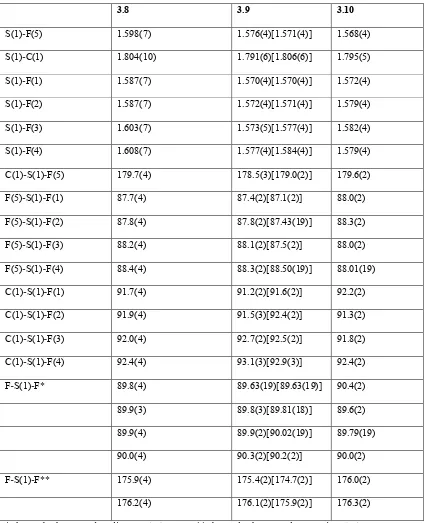
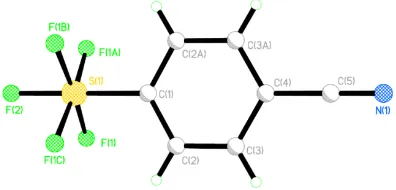
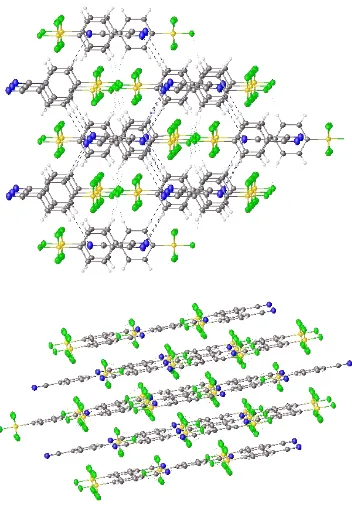
X ray crystallographic studies of sulfur/selenium heteroatom compounds
Full text
Figure
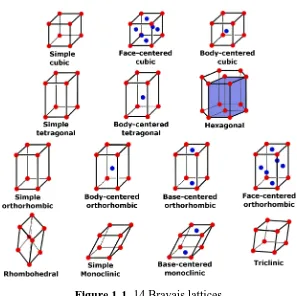
![Figure 1-3. The Unit Cell.[5]](https://thumb-us.123doks.com/thumbv2/123dok_us/8983087.394747/19.595.242.383.444.564/figure-the-unit-cell.webp)
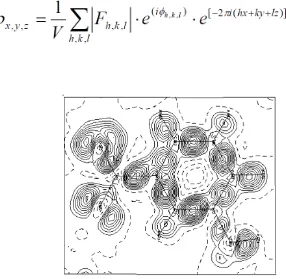
Outline
Related documents
PRV infected fish suffered from significant anaemia (as meas- ured by both parameters) compared to negative control groups at the time of IHNV exposure in the trial, which
For adipogenic, osteogenic and chondro- genic differentiation, the collected cells were cultured in the adipogenic differentiation medi- um (CAXMX-90031) and osteogenic induction
designed to study the effect therapeutic ultrasound on TMJ joint.. Fifteen patients
A presumably value-neutral statement such as "the Technology Acceptance Model (TAM) offers a good description of ICT user behaviour" is not really value-neutral. It
This study explores to twenty precepts of virtues set forth by the ancient Confucian philosopher Confucius and his students‟ writings of Analects in which
An absolute distance (actual experimental height of cropped image) of 145 mm was imputed. A calibration method was performed on the calibrated image using the “Multi Camera
The country has agonizing energy dilemma from a long period of time, as because apathy of using the indigenous coal resources properly; nonetheless according to BCMCL,
Intra- and interobserver coefficients of variation (%) for EBEF; the Simpson single-plane method of the four-chamber view; AVPDm, atrioventricular plane displacement, mean value