With 7 text-figures Printed in Great Britain
ACTIVE TRANSPORT BY THE CECROPIA MIDGUT
IV. SPECIFICITY OF THE TRANSPORT MECHANISM FOR POTASSIUM
BY S. NEDERGAARD* AND W. R. HARVEY
Department of Zoology, University of Massachusetts, Amherst, Massachusetts
(Received 25 September 1967)
INTRODUCTION
In the preceding paper of this series evidence was presented that the sole ionic requirement for the maintenance of the midgut potential is the presence of potassium in the solution bathing the blood-side of the isolated organ (Harvey, Haskell & Nedergaard, 1968). The conclusion was reached that the potential arises directly as a consequence of the active transport of potassium from blood-side to lumen. This conclusion depends on the demonstration that potassium is the only ion actively transported under conditions such that the mid-gut potential can be demonstrated. Harvey & Nedergaard (1964) had already shown that at least 83 % of the current produced by the midgut when the potential is short-circuited is carried by potassium. Using an improved method of short-circuiting, including high potassium con-centrations in the bathing fluids, Harvey, Haskell & Zerahn (1967) found that all of the current is carried by potassium. In the present paper the specificity of the midgut for potassium is examined in further detail. It is shown that rubidium, which so closely resembles potassium chemically, can substitute fully for potassium but that other ions such as sodium or lithium and even the chemically similar ammonium ion are unable to sustain a current. Moreover, the magnitude of the current depends on the potassium concentration in the blood-side bathing solution.
MATERIALS AND METHODS
Mature fifth-instar larvae of Hyalophora cecropia (Linnaeus) were reared in the vicinity of Amherst, Massachusetts, during the spring and summer of 1963 and 1964. During March, April and May the larvae were fed with leaves harvested from branches of willow (Salix babylonica) forced in the greenhouse. As weather conditions improved the larvae were moved outside to netted trees of this same species. Larvae which hatched after June were reared outdoors on wild black cherry trees (Prunus
serotina). The fifth-instar larvae used in the experiments reported here weighed from
6 to 18 g. with an average weight of 13 g.
Prior to dissection each larva was weighed, isolated in a small cardboard box, and immobilized by chilling at 50 C. for 0-5-1 hr. It was then placed on a cooled paraffin
block and covered with a sturdy wire screen bent to allow a i in. to i^ in. space between the screen and the paraffin. A cube of frozen carbon dioxide (approximately 3 in. x 2 in. x i in.) was placed on the screen and the assembly was covered loosely with paper tissue. In this way the larva was simultaneously chilled and narcotized by the cold carbon dioxide flowing down through the wire mesh. Ordinarily the larva was narcotized for from 5 to 10 min. or until it has regurgitated a small amount of yellow fluid and become very flaccid.
The larva was removed from the carbon dioxide but kept on the chilled paraffin block during dissection. The portion of the insect from one segment anterior to the prolegs to between the second and third prolegs was removed with scissors, and the abdominal tergites were cut longitudinally along the dorsolateral surface to expose the midgut. In order to standardize the preparation as much as possible only the middle part of the midgut was used in experiments reported in this paper. Although there is not much difference in the fine structure of the midgut cells from the anterior to the posterior part (Anderson & Harvey, 1966) there is a greater difference between the pH of midgut contents and the blood in the hind part of the midgut than in the front part. Likewise the potential difference across the isolated organ is largest in the hind part of the midgut. However, potassium is actively transported along the entire length of the organ. The peritrophic membrane containing all of the gut contents was removed by gently pulling. Small lengths of nylon twine were looped around the free ends of the gut. These ends were slipped over the ends of the glass tubing which formed the limits of the gap of the inner chamber (Fig. 1) and the twine was tightened. The chamber was lifted slightly and the ventral attachments of the midgut to the integument were severed. With practice, the dissection and cannulation could be accomplished in three minutes.
The chamber, blown from Pyrex glass, consists of an inner and outer section. Because there is no connexion between the solutions in these sections except through the tissue of the midgut mounted in the inner section, it is possible to perfuse the two sides of the midgut independently. The gap in the inner part of the chamber, where the gut tissue is placed, is 1 cm long and o-6 cm wide, which gives an area of about 1-9 cm2. To establish the same fluid level in each chamber, and thereby avoid an hydrostatic pressure gradient across the gut, the fluid volume of the inner chamber was 10-12 ml. and that of the outer chamber 60-65 ml. The two solutions were stirred and aerated by bubbles of air (A and A' in Fig. 1) which had been filtered through a Koby filter and saturated with water vapour. The temperature was kept constant during the experiments by inserting the chamber into a beaker of water whose temperature was under thermostatic control.
The short-circuiting technique of Ussing & Zerahn (1951) was adapted to the midgut system. Two silver-silver chloride electrodes (S and 5 ' in Fig. 1) were placed in the bathing solutions one on each side of the tissue. Unfortunately the conductivity of the standard bathing solution (S I) is low, because of the low concentration of ions, the osmolarity being made equal to that of larval haemolymph by using sucrose.
Fig. 1. Midgut chamber. Midgut (M); lumen-side (sparse stippling); blood-side (close stippling); inlets for aerating and stirring gas (A and A'); calomel electrodes (C and C); bridges between the bathing solutions and the calomel electrodes {E and E'); potentiometer (mV.); dry-cell battery (B); voltage divider (V); microammeter (ftA); silver-silver chloride electrodes (S and SO- (After Harvey & Nedergaard (1964). Redrawn, by permission, from
Proc. natn. Acad. Sci. 1964, 51, 758.)
As a consequence of this and of the geometry of the apparatus the current density is greater at one end of the midgut than at the other. However, if the glass bridges of the potential-measuring electrodes are placed carefully in the middle and fixed there it is reasonable to assume that something close to the actual short-circuit current is measured (see also Harvey, Haskell & Zerahn, 1967).
a mixture of alcohol and solid carbon dioxide. The thawing time of the capillaries filled with whole blood was compared with that of capillaries filled with solutions of known osmolarity. The average osmolarity of haemolymph samples taken from sixteen fifth-instar Cecropia larvae was 259 + 4-3 (standard error) m-osmoles/1. Although the osmolar activity of cell-free blood was not determined, preliminary experiments revealed that a bathing solution with a concentration at least 260 m-osmoles/1. was adequate to preserve high potentials over long periods of time. All of the solutions were brought to this osmolarity by adding sucrose.
The amounts of potassium (32 mM/1.), calcium (5 HIM/1.) and magnesium (5 mM/1.) in the standard solution were chosen from an analysis of the concentrations of these ions in haemolymph, midgut tissue, and midgut contents performed by Quatrale (1966), and from preliminary experiments designed to investigate the stability and longevity of the potential in solutions with varying concentrations of these ions. Calcium was included because of the well-known effects of the omission of this ion on membrane structure and permeability (e.g. Nakas, Higashino & Loewenstein, 1966). Because the midgut sometimes tended to contact rather strongly in solutions containing calcium but not magnesium, because magnesium is present in rather high concentrations in the midgut tissue (14 mM/1.) and in the blood (35 mM/1.), and because its concentration in the tissue changes markedly during the larval-prepupal-transformations (Quatrale, 1966), it seemed prudent to include magnesium in the bathing solution. Sodium was omitted from the standard solution because there is very little sodium in either haemolymph, midgut tissue, or midgut contents, and because its presence or absence had no effect on the maintenance of the potential.
Table 1. Composition of solutions used to bathe the isolated midgut* Name of
solution K-2
K-10 K-20
S-I K-64
K-100
K (mM/1.)
2 1 0 2 0
32
64
1 0 0
N a (mM/1.)
3° 2 2 1 2 0 0 0
Sucrose (mM/1.) 166 166 166 166
1 0 2 3°
• All solutions contain 5 mM/1. MgCl2) 5 mM/1. CaCl2 and 2 mM/1. KHCO3; the rest of the potassium
indicated in the table was included as the chloride salt. The osmolarity of all the solutions was 260 m-osmoles/1.
All of the cations were introduced into the solutions as chloride salts except that the solutions were buffered with 2 mM/1. bicarbonate at pH 8-o, which is intermediate between the value for haemolymph (63) and midgut contents (9-4) (W. R. Harvey & R. Hamelin, unpublished results). The composition of the standard solution (S I) is given in Table 1. Fisher certified reagents and glass-distilled water were used for all solutions.
counts. At this time there was still approximately 85 % of the 86Rb (half life 18-6 days) and it was then counted without the shield.
Concentrations of potassium, sodium, lithium and rubidium were measured by atomic absorption spectrophotometry.
RESULTS
(1) Sodium and lithium
Even though the active transport of potassium is completely independent of sodium in the bathing solutions (Harvey & Nedergaard, 1964) the midgut might be able to transport sodium when it is present in a high concentration. To test this possibility 32 mil/I, sodium chloride was added to the standard solution and a corresponding amount of sucrose was left out to keep the osmolarity constant (Table r). To test the effect of increasing the ionic concentration 32 raii/l. of choline chloride
500
400
300
200
100
Choline Lithium
J
CholineI I I
14.00 14.30 15.00
Time
[image:5.451.70.390.257.469.2]15.30 16.00
Fig. 2. A midgut isolated from a fifth-instar larva was bathed in the standard solution, S i , and the current was measured. At the point marked 'choline' the solutions were replaced by a variant with an additional 32 mM/1. of choline chloride and the current was depressed. When the solution with choline chloride was replaced by a variant of the standard solution containing an additional 32 mM/1. of lithium chloride, and when the choline variant was restored, no additional changes were recorded.
was added to the bathing solutions. In other systems such as the frog skin choline is an indifferent ion except in high concentrations. The short-circuit current was reversibly decreased about 25 % with added choline chloride (Fig. 2). The effect might be attributed to the increased choline, chloride, or ionic concentration (and thereby on K+ activity) or the decreased sucrose concentration. For present purposes it is sufficient to note that the replacement of the choline chloride by either sodium or lithium chloride had no further effect on the short-circuit current (Fig. 2) so that we can conclude that neither sodium nor lithium affects the current.
Following the sodium and lithium experiments samples were taken from the lumen-side and analysed by atomic absorption spectrophotometry. Quantitatively the analyses were not very accurate because the error is very high when the back-ground concentration of ion is much larger than the expected increase in concentration. However, there was invariably a net flux of potassium toward the lumen in each of seven experiments, whereas there was no net flux of either sodium or lithium (Table 2). Therefore sodium and lithium are not transported actively across the midgut tissue even when they are present in the same concentration as potassium.
Table 2. Failure of sodium or lithium to contribute to short-circuit current
of isolated Cecropia midgut
Net flux from spectro-photometry
Exp. no. 35l 3511 361 36II 70 7i 77
Current Sodium Lithium (/i-equiv./hr.) (/t-equiv./hr.) (/t-equiv./hr.)
30 10 33 26 9 26 27
o
+ 3 o — 1
o o + 4
1000
t
50016 mM/l. N H4+ 16 mM/l. K+
32 mM-K+ (S1)
1 I
10.30 11.00 11.30
T i m e
12.00 12.30 13.00
Fig. 3. A clear depression in the short-circuit current is observed when as little as half of the potassium tons in the bathing solutions are replaced by ammonium ions (arrow marked 16 mM/l. NH+i, 16 mM/l. K+).
(2) Ammonium
When all the potassium was replaced by ammonium, the short-circuit current dropped almost to zero and the recovery when the potassium was restored was rather limited. When half (16 mM/l.) of the potassium was replaced by ammonium the current dropped significantly (Fig. 3). Ammonium does not seem to substitute for potassium, the current observed in experiments with 16 mM/l. ammonium and
(3) Rubidium
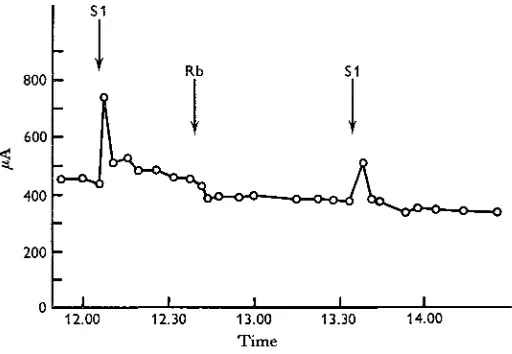
The midgut potential drops to the same extent when the concentration of rubidium on the blood side is changed from 32 mM/1. to 2 mM/1. as when the concentration of potassium is similarly changed (compare Harvey & Nedergaard (1964) and Harvey, Haskell and Nedergaard (1968)) nor is there any effect on the potential when all the potassium in the bathing solutions is replaced by rubidium. Similarly, replacement of potassium by rubidium had no effect on the short-circuit current (Fig. 4). With
800
600
400
200
12.00 12.30 13.00 Time
13.30 14.00
[image:7.451.101.357.177.354.2]Fig. 4. The short-circuit current is the same when the midgut is bathed in the standard solution containing 32 miw/l. potassium (Si), when all the potassium is replaced by 32 mM/1. rubidium (Rb on the curve), or when the potassium is restored.
Table 3. Co-operation of potassium and rubidium in carrying the short-circuit current
The agreement between total net flux and current is not good presumably due to poor short-circuiting. However, the ratio of net flux of K to Rb (column 7) agrees reasonably well with the ratio of their concentrations in the bathing solutions (column 6).
Exp. no.
K* Rb* Total K/Rb net flux net flux net flux Current concentration (/t-equiv./hr.)(/t-equiv./hr.) (/t-equiv./hr.) (/t-equiv./hr.) in solution 72
73 74 75 79 Average
11 16 18
58
14
—
6 13 22
43 9
17 29 40
101
23
16 24 31
76
11
K/Rb net flux
1-2
o-8
i'3
16
• Net flux toward the lumen-side measured by absorption spectrophotometry.
(4) Potassium
The dependence of the short-circuit current on the potassium concentration in the bathing solutions was investigated. When lowered potassium concentrations were desired sodium was substituted for potassium and when high concentrations were desired part of the sucrose was substituted by potassium so that the osmolarity remained constant (Table 1). However, in the high-potassium solutions the total ionic concentration was increased above its value in the standard solution. As described above, sodium itself has no influence on the short-circuit current in a concentration as high as 32 min/l. and therefore can be used as an inert ion.
500
400
300
200
100
—o— Decreasing [K+]
— X - Increasing [K+]
10 20 30
[K+]w
40
Fig. 5. The short-circuit current plotted as a function of the potassium concentration in the solution bathing the blood-side of the isolated midgut. T h e function is not the same during the first part of the experiment (open circles) when the potassium concentration is being lowered as during the last part (crosses) when the concentration is being raised.
on the blood-side up to at least 64 mM/1. potassium. Figure 7 also shows that the short-circuit current is not affected by the potassium concentration on the lumen-side within the concentration range studied. The blood-side potassium concentration was 32 mM/1. during the potassium concentration changes on the lumen-side.
100
so
—o— Decreasing [K+] —X— Increasing [K+]
[image:9.451.106.350.130.363.2]10 30 40
Fig. 6. The data from Fig. 5 are replotted with the current expressed as a percent of the current in 32 mM/1. potassium to correct for the normal decrease in current with time. Plotted in this way the dependency of current on blood-side potassium concentration is seen to be the same whether the potassium concentration is being raised or lowered.
Table 4. Effects of varying the blood-side potassium concentration on the short-circuit current
(The figures in square brackets at the heads of the columns are the potassium concentrations on the blood-side. The short-circuit current is expressed as a percentage of the short-circuit current measured with a potassium concentration of 32 mM/1 on the blood-side.)
mM/1. K on blood-side
Exp. no. 4 7 9 11 IS* 23 26 27 3O 31 32 33 Average [2] — — 5'S 16
- 1 3
8-8 i-4 7-2 — — — — 7-8 [10] 46 35 55 48 28 39 54 37 — — — — 45 [20] — — 81 83 55 81 88 74 — — — — 81 [32]
1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0 1 0 0
1 0 0
[64] — — — — — — — — 133 114 134 132 128 [100] — — — — — — — — 95 119 U S "3 i n
[image:9.451.71.376.479.640.2](5) Net potassium-rubidium flux and current
The ability of rubidium to replace potassium so completely suggested another way to confirm the previous findings that most of the current is carried by potassium.
42
K was used to measure the outflux and simultaneously 86Rb was used to measure the influx (or vice versa) on a single piece of midgut tissue. In seven measurements the net potassium-rubidium flux accounted for about 87 % of the short-circuit current, a value close to the results obtained from ' sandwich' experiments in which a measurement of flux toward the lumen was sandwiched between measurements of flux toward the blood-side (Harvey & Nedergaard, 1964). The short-circuiting technique was the same as that used in the sandwich experiments and limited the accuracy of the overall determination so that the agreement between short-circuit current and net flux could not be expected to be better.
150
100
50
—o— Blood-side —x— Lumen-side
10 20 30 40 50 60
[K+] (mM/1.)
Fig. 7. The short-circuit current, expressed as percentage of the current in 32 mM/1. potassium, is plotted against the potassium concentration on the blood-side of the isolated midgut (circles) and on the lumen-side (crosses) for the average of the data presented in Table 4. The current is a more or less linear function of the blood-side potassium concentration from 2 to 20 mM/1. but tends to increase less with further concentration increases.
DISCUSSION
It is more difficult to make a conclusive statement about what ions the midgut can transport without testing every known ion. The fact that most of the short-circuit current in the standard solution is carried by potassium virtually excludes the other ions present, i.e. magnesium, calcium, sodium, hydrogen, chloride and bicarbonate ions (Harvey & Nedergaard, 1964; Harvey, Haskell & Zerahn, 1967; Results 5). The failure to detect a net flux of either sodium or lithium under conditions such that a net potassium flux toward the lumen is invariably demonstrable (Table 2) shows that neither of these ions is transported even when they are present in high concen-tration in the bathing fluids. Although the ammonium ion is similar to the potassium ion the short-circuit current decreases rapidly when ammonium replaces all the potassium. Moreover, the current drops irreversibly when half of the potassium is substituted by ammonium (Fig. 3). At the pH of the bathing solutions (c. 8-o) about 95 % °f t n e added 16 mM/1. ammonium is in the ionic form.
The only ion which can substitute for potassium is rubidium. When all the potas-sium in the bathing solutions is substituted by rubidium there is no detectable change in the short-circuit current (Fig. 4). Even when the midgut is maintained for hours with rubidium replacing potassium and the tissue is washed repeatedly to remove traces of potassium which may leak from the cells the current is maintained. Potassium and rubidium contribute to the net flux across the tissue and thereby to the short-circuit current approximately in the ratio in which they are present in the bathing solutions (Table 3). Even with the technical limitations of these absorption measure-ments mentioned in the results the large net fluxes of potassium and rubidium in Table 3 contrast strongly with the negligible net fluxes of sodium and lithium in Table 2. This ability of rubidium to substitute for potassium is not surprising because in addition to their chemical and physical similarities rubidium is known to substitute for potassium in living cells such as red blood cells (Solomon, 1952). The short-circuit current and therefore the active potassium transport is dependent on the potassium concentration in the blood-side bathing solution but is completely independent of the potassium concentration on the lumen-side (Fig. 7). The values in Table 4 for 100 mM/1. K are not included in Figure 7 because the lowered current may be due to a lowered sucrose concentration, rather than the elevated potassium con-centration. Unpublished results indicate that it is not possible to maintain the short-circuit current in the absence of sucrose. The details of curves such as Fig. 7 depend very much on exactly how the measurements are made and are valid only for the conditions stated. However, a similar sort of curve showing a dependency of current on blood-side potassium concentration would be expected under most conditions.
SUMMARY
1. Although the addition of 32 min/1. choline chloride to the standard bathing solutions depresses the short-circuit current of the isolated midgut, the replacement of the added choline chloride by either sodium or lithium chloride has no further affect on the current. No net flux of either sodium or lithium is measureable by atomic absorption spectrometry. Therefore neither sodium nor lithium is actively transported across the midgut.
2. Rubidium is able to substitute fully for potassium in maintaining a short-circuit current. The net fluxes of rubidium and potassium are approximately in the same ratio as their concentrations in the solutions bathing the blood-side of the isolated midgut. Therefore the midgut is unable to distinguish between potassium and rubidium. Ammonium is unable to replace potassium in sustaining a current.
3. In double-label experiments using 42K to measure one unidirectional flux and
86Rb to measure the other, the net potassium-rubidium flux accounts for about 87 %
of the short-circuit current.
4. The short-circuit current increases with increasing potassium concentration, at least in the range from 2 to 64 CIM/I., although the exact function depends on the conditions under which the measurements are made.
5. The midgut isolated from the Cecropia silkworm appears to possess a ' potassium pump' with the simple ionic requirement that potassium be present in the solution bathing the blood-side and presumably that potassium be present in the cells.
This research was supported in part by a research grant (AI-04291) from the National Institute of Allergy and Infectious Diseases, U.S. Public Health Service, and a grant from the University of Massachusetts Research Council. We thank Dr K. Zerahn for criticizing the manuscript.
REFERENCES
ANDERSON, E. A. & HARVEY, W. R. (1966). Active transport by the Cecropia midgut. II. Fine structure of the midgut epithelium. J. Cell Biol. 31, 107-34.
GROSS, W. J. (1954). Osmotic responses in the Sipunculid Dendrostomum zostericolum. J. exp. Biol. 31,
402-23.
HARVEY, W. R., HASKELL, J. A. & NEDERGAARD, S. (1968). Active transport by the Cecropia midgut. III. Midgut potential generated directly by active K-transport. J. exp. Biol. 48, 1-12.
HARVEY, W. R., HASKELL, J. A. & ZERAHN, K. (1967). Active transport of potassium and oxygen consumption in the isolated midgut of Hyalophora cecropia. J. exp. Biol. 46, 235-48.
HARVEY, W. R. & NEDERGAARD, S. (1964). Sodium-independent active transport of potassium in the isolated midgut of the Cecropia silkworm. Proc. natn. Acad. Sci. 51, 757-65.
NAKAS, M., HIGASHINO, S. & LOEWENSTEIN, W. R. (1966). Uncoupling of an epithelial cell membrane junction by calcium-ion removal. Science 151, 89-91.
QUATRALE R. P. (1966). Cation concentration changes during development of the silkworm, Hyalophora
cecropia. Ph.D. Thesis, University of Massachusetts, Amherst, Massachusetts.
SOLOMON, A. K. (1952). The permeability of the human erythrocyte to sodium and potassium. J. gen.
Pkysiol. 36, 57-110.