© Horizon Scientific Press. Offprints from www.cimb.org
*For correspondence: paul.williams@nottingham.ac.uk
Steven Atkinson1, R. Elizabeth Sockett2, Miguel Cámara1 and Paul Williams1*
1Institute of Infection, Immunity and Inflammation, Centre
for Biomolecular Sciences, University of Nottingham, Nottingham NG7 2UH, UK
2Institute of Genetics, University of Nottingham, Queen’s
Medical Centre, Nottingham NG7 2UH, UK
Abstract
Bacterial cell-to-cell communication (‘quorum sensing’) is mediated by structurally diverse, small diffusible signal molecules which regulate gene expression as a function of cell population density. Many different Gram-negative animal, plant and fish pathogens employ N-acylhomoserine lactones (AHLs) as quorum sensing signal molecules which control diverse physiological processes including bioluminescence, swarming, antibiotic biosynthesis, plasmid conjugal transfer, biofilm development and virulence. AHL-dependent quorum sensing is highly conserved in both pathogenic and non-pathogenic members of the genus Yersinia. Yersinia pseudotuberculosis for example, produces at least eight different AHLs and possesses two homologues of the LuxI family of AHL synthases and two members of the LuxR family of AHL-dependent response regulators. In all Yersinia species so far examined, the genes coding for LuxR and LuxI homologues are characteristically arranged convergently and overlapping. In Y. pseudotuberculosis AHL-dependent quorum sensing is involved in the control of cell aggregation and swimming motility, the latter via the flagellar regulatory cascade. This is also the case for swimming and also swarming motility in Yersinia enterocolitica. However the role of AHL-dependent quorum sensing in Yersinia pestis remains to be determined.
Introduction
Until relatively recently, cell-to-cell communication was rarely considered to constitute a major mechanism for facilitating bacterial adaptation to an environmental challenge. However, it is now clear that diverse bacterial genera communicate using specific, extracellular signal molecules, which facilitate the coordination of gene expression in a multi-cellular fashion. Signalling can be linked to specific environmental or physiological conditions and is employed by bacteria to monitor their cell population density. The term quorum sensing is commonly used to describe the phenomenon whereby the accumulation of a diffusible, low molecular weight signal molecule (sometimes called an ‘autoinducer’) enables individual bacterial cells to sense when the minimum number or quorum of bacteria has been achieved for a concerted response to be initiated (Williams et al., 2000; Swift et al., 2001; Cámara et al., 2002). The term ‘autoinducer’,
implies a positive feedback or autoregulatory mechanism of action. However this is frequently not the case and therefore the term can be misleading and will be avoided here (Cámara et al., 2002). The accumulation of a diffusible signal molecule also indicates the presence of a diffusion barrier, which ensures that more molecules are produced than lost from the micro-habitat (Winzer et al., 2002b; Redfield, 2002). This could be regarded as a type of ‘compartment sensing’, where signal molecule accumulation is both the measure for the degree of compartmentalization and the means to distribute this information among the entire population. Similarly, diffusion of quorum sensing signal molecules between spatially separated bacterial sub-populations may convey information about their physiological state, their numbers, and the individual environmental conditions encountered.
At the molecular level, quorum sensing requires a synthase together with a signal transduction system for producing and responding to the signal molecule respectively. The latter usually involves a response regulator and/or sensor kinase protein (Swift et al., 2001; Cámara et al., 2002). While quorum sensing systems are ubiquitous in both Gram-negative and Gram-positive bacteria, there is considerable chemical diversity in the nature of the signal molecules involved which range from post-translationally modified peptides to quinolones, lactones and furanones (Cámara et al., 2002). In addition, siderophores, which previously were considered only in the context of iron transport, may also function in the producer organism as signal molecules capable of controlling genes unrelated to iron acquisition (Lamont et al., 2002). While there is, as yet, no clear cut evidence for a molecularly conserved quorum sensing system throughout the bacterial kingdom, the LuxS protein and the furanone generated from the ribosyl moiety of S-ribosylhomocysteine (termed AI-2 for autoinducer-2) have been suggested to fulfil such a role (Bassler, 2002). However, many bacteria (e.g. the pseudomonads) do not possess luxS and hence do not produce AI-2 (Winzer et al., 2002a). Furthermore, as LuxS is a key metabolic enzyme in the activated methyl cycle responsible for recycling S-adenosylmethionine (SAM) (Winzer et al., 2002b; Winzer et al., 2002a) phenotypes associated with mutation of luxS are often not a consequence of a defect in cell-to-cell communication but the result of the failure to recycle SAM metabolites (Winzer et al., 2002b; Winzer et al., 2002a). To date, only in Vibrio harveyi is there any direct experimental data to support the function of LuxS as a quorum sensing signal molecule synthase. Thus although Yersinia spp. possess a luxS gene and produce AI-2 (unpublished data) this does not constitute evidence for the presence of a quorum sensing system based on AI-2. Here we examine the nature and contribution of N-acylhomoserine lactone (AHL)-mediated quorum sensing to the lifestyle of Yersinia spp.
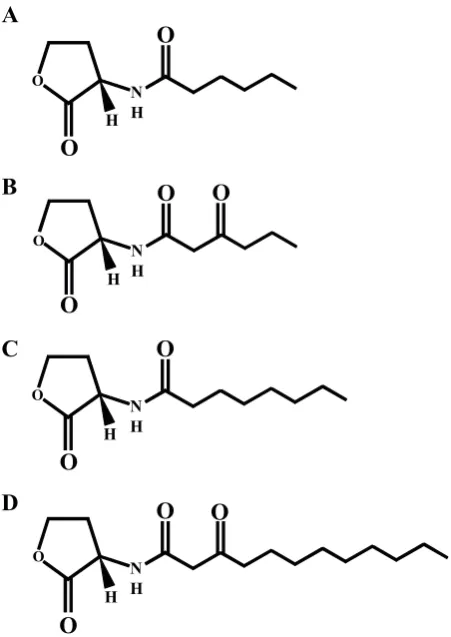
N-Acylhomoserine lactone-dependent quorum sensing The most intensively investigated family of quorum sensing signal molecules in Gram-negative bacteria are the AHLs. These are produced by many different bacterial genera including Aeromonas, Agrobacterium, Brucella, Burkholderia, Chromobacterium, Erwinia, Enterobacter, Pseudomonas, Rhizobium, Serratia, Vibrio and Yersinia although not in all strains and species of the same genus (Swift et al., 2001). AHLs consist of a homoserine lactone ring covalently linked via an amide bond to an acyl side chain (Fig. 1). To date, naturally occurring AHLs with chain lengths between 4 and 18 carbons have been identified which may be saturated or unsaturated and with or without a hydroxy-, oxo- or no substituent on the carbon at the 3 position of the N-linked acyl chain (Fig. 1). AHLs are usually synthesized by enzymes belonging to the LuxI family, which employ the appropriately charged acyl acyl-carrier protein (acyl-ACP) as the major acyl chain donor while S-adenosyl methionine (SAM) provides the homoserine lactone moiety (Jiang et al., 1998; Watson et al., 2002, Moré et al., 1996). Recent data derived from the crystal structure of EsaI from Pantoea stewartii indicates that LuxI proteins share structural similarities with eukaryotic N-acetyltransferases which employ similar fatty acid precursors as substrates (Watson et al., 2002). Once synthesized, AHLs either move across the bacterial cell envelope by simple diffusion in the case of AHLs with
a short acyl side chain, or are pumped out of the cell if the AHLs have a long, e.g. C12 acyl side chain (Pearson et al., 1999).
Apart from the AHL synthase, the second regulatory component of an AHL-dependent quorum sensing circuit is usually a member of the LuxR family of transcriptional regulators (Swift et al., 2001; Cámara et al., 2002; Fuqua et al., 1996). AHLs bind to and activate LuxR proteins such that the LuxR/AHL complex is responsible for either activating or repressing target structural genes (Swift et al., 2001; Cámara et al., 2002; Zhang et al., 2002). The
V. fischeri LuxR protein for example, consists of an N-terminal, cytoplasmic membrane-associated regulatory domain that binds AHLs and a cytoplasmic C-terminal domain that binds DNA. LuxR is considered to activate transcription as a dimer and a number of studies have identified regions within the protein required for multimerization (Choi and Greenberg, 1992a), AHL binding (Shadel et al., 1990; Slock et al., 1990; Hanzelka and Greenberg, 1995), DNA binding, transcriptional activation (Choi and Greenberg, 1991; Choi and Greenberg, 1992b) and autorepression (Choi and Greenberg, 1991). The V.
fischeri LuxR protein appears unable to associate with DNA until a conformational change has been induced via an interaction between the N-terminal AHL binding-domain and the cognate AHL molecule(s) (Choi and Greenberg, 1992b). However, among the growing family of LuxR homologues, it is now clear that there are variations between their respective mechanisms of transcriptional activation (Luo and Farrand, 1999). In addition, certain LuxR homologues function as repressors rather than activators (e.g. EsaR) (von Bodman et al., 1998). To date the structure of only one LuxR homologue has been solved, that of TraR from Agrobacterium tumefaciens (Zhang et al., 2002). These data revealed that TraR forms a dimer and confirmed that the amino-terminal domain contains the AHL-binding region. However, the cognate AHL, N-3-(oxooctanoyl)homoserine lactone (3-oxo-C8-HSL) is completely buried within the protein and it appears that the AHL associates with nascent, actively folding TraR during protein synthesis. However this ‘co-translational’ mechanism may prove to be unique to TraR and does not appear to be representative of all LuxR homologues.
The DNA sequence of the LuxR binding site has been determined and is called the lux box. This is a 20 nucleotide inverted repeat, which, in V. fischeri is situated within the intergenic region between luxR and luxI, and is required for the primary regulation of lux gene expression. This DNA element has features that are conserved with the binding sites of other LuxR-type proteins and share the consensus RNSTGYAXGATNXTRCASRT where N = A, T, C or G; R = G or A; S = C or G; Y = C or T and X = N or a gap in the sequence. (Stevens and Greenberg, 1997). In Pseudomonas aeruginosa for example, lux box-like elements have been identified in the promoter regions of many different target structural genes (Whiteley and Greenberg, 2001) and purified LuxR proteins such as TraR (from A. tumefaciens) and ExpR (from Erwinia chrysanthemi) have been shown to bind in vitro to lux box-type sequences (Zhu and Winans, 1999; Nasser et al., 1998; Reverchon et al., 1998).
N H
O
O
N H
O
O
O
N H
O
O
O
O
N H
O
O
O
O
O
H H H H
A
B
C
D
AHL-controlled multicellular behaviour in Gram-negative bacteria includes a variety of physiological processes such as bioluminescence, swarming, swimming and sliding motility, antibiotic biosynthesis, biofilm maturation, plasmid conjugal transfer and the production of virulence determinants in animal, fish and plant pathogens (for reviews see Swift et al., 1999; Swift et al., 2001; Williams., 2002).
The search for AHL-mediated quorum sensing in Yersinia
Because of their relative hydrophobicities AHLs can readily be concentrated from culture supernatants by partitioning into organic solvents such as dichloromethane or ethyl acetate. However, since they lack good chromophores, relatively high concentrations are required for detection via their ultra violet spectroscopic properties following separation by HPLC. As a consequence, a number of sensitive bioassays have been developed which facilitate detection of AHL production by bacterial colonies on plates, in microtitre plate assays, on thin layer chromatograms (TLC) and in fractions collected after HPLC-based separation (Bainton et al., 1992; Throup et al., 1995; McClean et al., 1997; Shaw et al., 1997; Winson et al., 1998). For example, Throup et al. (1995) constructed a recombinant AHL reporter plasmid which coupled luxR and the luxI promoter region from
Vibrio fischeri to the luxCDABE structural operon of Photorhabdus luminescens. When introduced into E. coli, this lux-based AHL reporter (termed pSB401) is dark but responds to the presence of exogenous AHLs by emitting light. The advantage of this AHL reporter compared with earlier versions employing luxAB alone (Bainton et al., 1992; Swift et al., 1993) is that it does not require the addition of a long chain fatty aldehyde (which is required by the luciferase) since the inclusion of the luxCDE genes provides for in situ synthesis of the aldehyde.
Using this AHL bioassay, cell free supernatants from several species of Yersinia including isolates of Yersinia enterocolitica belonging to serotypes O:3, O:8, O:9, O:10K, O:1(2a, 3), a non-typeable strain along with Yersinia pseudotuberculosis serotype III pIB1, Yersinia frederiksenii, Yersinia kristensenii, and Yersinia intermedia were screened. All of these strains induced bioluminescence in the reporter offering preliminary evidence that Yersinia spp. were AHL producers (Throup et al., 1995). Subsequently, Yersinia pestis (Swift et al., 1999) and Yersinia ruckeri (unpublished data) were also confirmed as AHL-producers using the same strategy.
AHL-mediated quorum sensing in Y. enterocolitica Although E. coli [pSB401] incorporates LuxR from
V. fischeri, the cognate AHL for which is N-(3-oxohexanoyl)homoserine lactone (3-oxo-C6-HSL), it responds, with differing sensitivities, to a variety of AHLs (Winson et al., 1998). While a positive bioassay result confirms the presence of AHLs, it does not indicate either which AHL or indeed how many different AHLs may be present in the sample. To characterize unequivocally the AHLs present in spent Y. enterocolitica culture supernatants, they were first concentrated by solvent extraction and subjected to preparative HPLC. The fractions found to
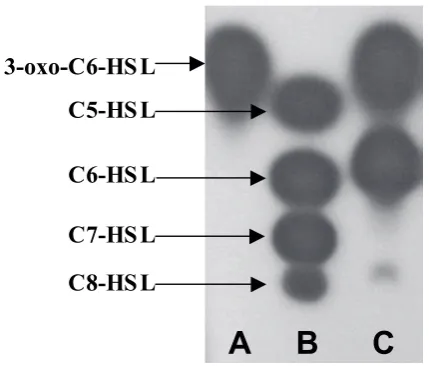
be positive in the AHL bioassay were subjected to high-resolution tandem mass spectrometry (MS-MS) which revealed the presence of two AHLs in an approximately 50:50 ratio. These AHLs were identified as 3-oxo-C6-HSL and N-hexanoyl homoserine lactone (C6-HSL) respectively (Fig. 1) (Throup et al., 1995) and are easily separated and visualized by thin layer chromatography (TLC) as purple spots on a white background using the Chromobacterium violaceum CV026 biosensor in an agar overlay (see Fig. 2 for an example of this TLC overlay originally described by McClean et al., 1997).
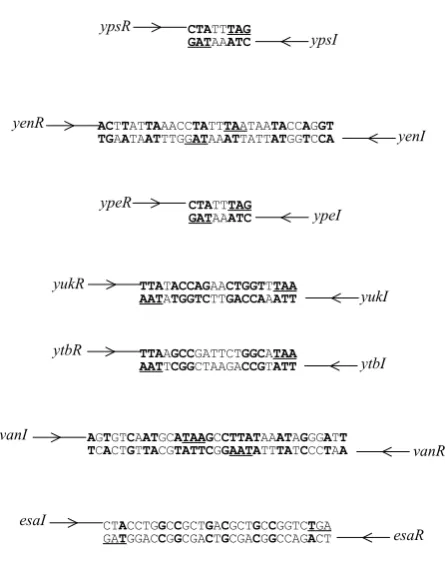
Since AHL synthesis depends on the presence of an AHL synthase, a plasmid-based in trans complementation strategy in which a Y. enterocolitica chromosomal gene library was transformed into E. coli [pSB401] was devised (Swift et al., 1993; Throup et al., 1995). This approach yielded a highly bioluminescent clone indicative of an insert carrying a gene whose product was capable of inducing the AHL reporter fusion. This clone was subsequently shown to be producing both 3-oxo-C6-HSL and C6-HSL and to contain two convergently transcribed and overlapping open reading frames (Fig. 3). The first of these genes was termed yenI, the product of which exhibited homology to the LuxI protein family while the product of the second, termed yenR belongs to the LuxR protein family. TLC in conjunction with the CV026 biosensor showed that inactivation of yenI by deletion mutagenesis abolished AHL production in Y. enterocolitica confirming the function of yenI as an AHL synthase.
In V. fischeri luxI expression is governed by a positive feedback loop as a consequence of its induction by LuxR activated by 3-oxo-C6-HSL (Meighen, 1994). However, in Y. enterocolitica, yenI is not subject to such autoinduction since inactivation of yenI did not abolish production of yenI mRNA (Throup et al., 1995). This observation was
A
B
C
3-oxo-C6-HS L
C6-HSL
C7-HSL
C8-HSL
C5-HSL
Fig. 2. TLC chromatogram of the AHLs present in cell-free supernatants of Y. pseudotuberculosis grown with shaking in LB at 22ºC and detected using the C. violaceum CV026 AHL biosensor. Lane A, 3-oxo-C6-HSL; 1.4 x 10–8 mol; lane B from top, AHL standards, N-pentanoylhomoserine
lactone (C5-HSL) (5.4 x 10–8 mol), C6-HSL (5 x 10–9 mol), C7-HSL
(4.7 x 10–7 mol) and C8-HSL (2.2 x 10–8 mol); lane C, Y. pseudotuberculosis
confirmed by examining the expression of yenI’::luxAB transcriptional fusions as chromosomal insertions in both the parent and yenI mutant respectively. In addition, the promoter region of yenI contains no matches for the lux box consensus sequence. Thus the expression of yenI is not part of an autoinducible system analogous to that of V.
fischeri but is independent of growth phase.
A preliminary analysis of potential Y. enterocolitica target genes which may be controlled by AHL-dependent quorum sensing was undertaken by comparing 2D SDS-PAGE profiles of the Y. enterocolitica strains 90/54 (serotype O:9) and 10460 (biotype 3, serotype O:1 (2a, 3); NCTC 10460) and their corresponding yenI mutants. While no obvious differences between the 10460 parent and mutant were observed, the 90/54 yenI mutant profile lacked a number of proteins that were present in the parent strain. However, as yet, none of these proteins has been identified. The major differences between 10460 and 90/54 relate to virulence since the former lacks the pYV virulence plasmid and this implies a possible role for AHL-mediated quorum sensing in controlling plasmid-borne functions. Although we were unable to demonstrate a role for AHL-mediated quorum sensing in regulating type III Yop secretion in Y. enterocolitica (Throup et al., 1995) the yenI mutant of 90/54, in contrast to the 10460 yenI mutant was unable to swim or swarm (unpublished data). Swarming motility is a flagellum-dependentbehaviour that facilitates bacterial migration over solid surfaces andis distinct from swimming motility, which occurs in fluid environments. Such multicellular behaviour has been implicated in
the formationof biofilms and in bacterial pathogenesis (Fraser and Hughes, 1999). In the Y. enterocolitica yenI mutant, swimming and swarming could both be restored by complementation with a plasmid-borne copy of yenI. Although Y. enterocolitica strains 10460 and 90/54 are not isogenic, these data do provide preliminary evidence linking quorum sensing with the virulence plasmid even though the loss of motility and swarming are both likely to involve changes in expression of the chromosomally encoded flagellar genes. In bacteria such as Serratia liquefaciens (Lindum et al., 1998), Burkholderia cepacia (Huber et al., 2001) and Pseudomonas aeruginosa (Kohler et al., 2000) the loss of swarming motility in quorum sensing mutants is associated with the loss of biosurfactant production. For mutants defective in AHL production, swarming can be restored by the provision of either the appropriate AHL signal molecule(s) or a biosurfactant. In addition, although such mutants do not swarm they remain capable of swimming motility. In the Y. enterocolitica 90/54 yenI mutant, swarming could not be restored by providing an exogenous surfactant and since the mutant also failed to swim, this suggests that AHL-mediated quorum sensing in this organism is directly linked to flagellin expression/function given that flagellar are required for both swimming and swarming motility. When analysed by SDS-PAGE the 90/54 yenI mutant fails to produce flagellin when compared to the parent and using RT-PCR the expression of flhCD, fliA and fliC was examined in the Y.enterocolitica parent and yenI mutant. These data suggested that quorum sensing regulates motility at the level of fliC expression (unpublished observations). Furthermore, given that motility is required to initiate host cell invasion by Y. enterocolitica (Young et al., 2000), it is possible that AHL-mediated quorum sensing may also turn out to play a role in this context.
AHL-mediated quorum sensing in Y. pseudotuberculosis TLC analysis of Y. pseudotuberculosis spent culture supernatants grown at (22°C) extracted with
dichloromethane using both C. violaceum CV026 (Fig. 2) and E. coli [pSB401] revealed the presence of at least three AHLs, the chemical identities of which were subsequently confirmed using HPLC and MS as 3-oxo-C6-HSL, C6-HSL and N-octanoyl-
L
-homoserine lactone (C8-HSL) (Fig. 2). However, when grown to stationary phase at 37°C inLB medium, Y. pseudotuberculosis appears to produce extremely low levels of these AHLs. Since temperature plays an important role in the regulation of virulence genes in Yersinia (Kapperud et al., 1985; Cornelis et al., 1987; Pepe and Miller, 1993; Pierson and Falkow, 1993; Cornelis and Wolf-Watz, 1997; Bleves and Cornelis, 2000), AHL production was examined in stationary phase cultures grown for 24 h at 28°C and 22°C (Yates et al.,
2002). By comparing the AHL profiles of cultures grown at the three different temperatures, AHL levels were found to be markedly dependent on growth temperature. The higher levels appeared to be greater after growth at 22°C,
substantially reduced at 28°C and almost undetectable
after growth at 37°C. Initially this suggested that either the expression or function of the corresponding AHL synthase(s) or substrate availability might be temperature dependent, implying that quorum sensing was temperature
ypsR
ytbR yenR
ypeR
yukR
TTAAGCCGATTCTGGCATAA
AATTCGGCTAAGACCGTATT
CTATTTAG GATAAATC
ACTTATTAAACCTATTTAATAATACCAGGT TGAATAATTTGGATAAATTATTATGGTCCA
CTATTTAG GATAAATC
TTATACCAGAACTGGTTTAA AATATGGTCTTGACCAAATT
ytbI ypsI
yenI
ypeI
yukI
vanI
vanR
AGTGTCAATGCATAAGCCTTATAAATAGGGATT TCACTGTTACGTATTCGGAATATTTATCCCTAA
CTACCTGGCCGCTGACGCTGCCGGTCTGA
GATGGACCGGCGACTGCGACGGCCAGACT
esaI
esaR
dependent. However, it subsequently became clear that there was a simple physico-chemical explanation. This is because the AHL ring is highly susceptible to pH-dependent lactonolysis. Ring opened compounds are unable to activate LuxR-type proteins and therefore do not activate AHL biosensors. By simply acidifying spent culture supernatants to below pH 2.0, lactonolysis can be reversed and the AHLs recovered from cultures grown at 37°C (Yates et al., 2002). In LB, which is unbuffered,
the growth of Y. pseudotuberculosis rapidly renders the pH alkaline as a consequence of the metabolism of amino acids and the release of ammonia. The rate of pH-dependent lactonolysis is further enhanced by raising the incubation temperature. Thus, these parameters must be taken into consideration for any study which seeks to quantitate AHL levels in culture supernatants. Furthermore, the AHL profile is also dependent on the structure of the AHLs present. In the context of lactonolysis, the longer the acyl side chain of an AHL then the more stable it will be towards increases in pH and temperature such as those encountered in vivo in mammalian hosts. Indeed the data obtained by Yates et al. (2002) revealed that to be functional under physiological conditions in mammalian tissue fluids, AHLs require an acyl side chain of at least 4 carbons in length. This explains why homoserine lactone itself cannot function as a quorum sensing signal molecule as it rapidly undergoes lactonolysis at pHs above 2 (Yates et al., 2002). This in turn suggests that the production of a range of AHL signal molecules with different acyl side chain lengths provides a pathogen with greater versatility in adapting to changing environments.
Y. pseudotuberculosis produces multiple short and long chain AHLs
Using the same trans-complementation strategy described by (Throup et al., 1995) i.e. the introduction of a genomic library into the AHL biosensor E. coli [pSB401], Atkinson et al (1999) cloned a luxI homologue from Y. pseudotuberculosis by screening for bioluminescent clones. This approach facilitated the identification of a LuxI homologue, YpsI and a LuxR homologue, YpsR. Deletion insertion mutations were introduced into both ypsI and ypsR and an analysis of the AHL profiles of both mutants revealed that after growth at 37°C, the mutants
produced the same three AHLs (3-oxo-C6-HSL, C6-HSL and C8-HSL) as the wild type. However, the ypsI mutant produced no 3-oxo-C6-HSL at 28°C while the ypsR
mutant produced reduced levels of 3-oxo-HSL and C6-HSL and no C8-C6-HSL could be detected (Atkinson et al., 1999). These data suggested that Y. pseudotuberculosis possessed an additional AHL synthase, the expression of which was likely therefore to be controlled by YpsR.
To locate this second putative AHL synthase, the same complementation strategy used above was employed but using genomic DNA prepared from the Y. pseudotuberculosis ypsI mutant (Atkinson et al., 1999). This approach yielded a clone containing two genes termed ytbI and ytbR which when translated, exhibited homology to YpsI and YpsR and were therefore additional members of the LuxR and LuxI protein families. When expressed in E. coli, YtbI is responsible for the production of C6-HSL and C8-HSL and a molecule we tentatively
identified as, N-heptanoylhomoserine lactone (C7-HSL) while YpsI generates 3-oxo-C6-HSL and C6-HSL (Atkinson et al., 1999 and unpublished data). These data suggest that there is a difference in the AHLs made via YtbI and YpsI in an E. coli genetic background and those made in Y. pseudotuberculosis since in the latter homologous background, YtbI can clearly produce all three major AHLs in the absence of YpsI (Atkinson et al., 1999). This finding was unexpected since the AHL profile of recombinant LuxI proteins from other bacteria expressed in E. coli has previously corresponded closely with the AHL profile in the original organism (Atkinson et al., 1999). Notwithstanding our observations with respect to pH-dependent lactonolysis, these data indicate that YtbI is able to vary the AHL profile produced in Y. pseudotuberculosis in a temperature-dependent manner and in the absence of YpsI. AHL production in Y. pseudotuberculosis is abolished when both ypsI and ytbI are mutated but not after deletion of both ypsR and ytbR indicating that, as in Y. enterocolitica, AHL production is not dependent on the presence of a functional YpsR or YtbR protein (Buckley, 2002).
Much of the analysis of AHL production in Y. pseudotuberculosis was undertaken using AHL biosensors which preferentially respond to short chain (C4-C8) AHLs (Fig. 2). Recently, we have re-investigated AHL production in Y. pseudotuberculosis using an AHL biosensor (pSB1075) based on the LuxR homologue, LasR from Pseudomonas aeruginosa, the cognate AHL for which is the long chain N-(3-oxododecanoyl)homoserine lactone (3-oxo-C12-HSL). In pSB1075, we have incorporated lasR and the lasI promoter fused to luxCDABE (Winson et al., 1998). When E. coli [pSB1075] was used as an overlay in conjunction with a TLC system optimized for the chromatography of long chain AHLs employing reverse phase RP-2 TLC plates and 45% v/v methanol in water as the mobile phase (Yates et al., 2002), dichloromethane extracts of the Y. pseudotuberculosis wild type were found to contain three long chain AHLs. These were tentatively identified as N-oxodecanoyl)homoserine lactone (3-oxo-C10-HSL), N-(3-oxododecanoyl)homoserine lactone (3-oxo-C12-HSL) and N-(3-oxotetradecanoyl) homoserine lactone (3-oxo-C14-HSL). None of these AHLs were made by the ypsI/ytbI double mutant nor by the ytbI mutant suggesting that YtbI was the synthase responsible for their synthesis. This was confirmed by demonstrating that E. coli expressing ytbI but not ypsI also produces the long chain AHLs (Buckley, 2002).
AHL-mediated quorum sensing controls motility and cell aggregation in Y. pseudotuberculosis
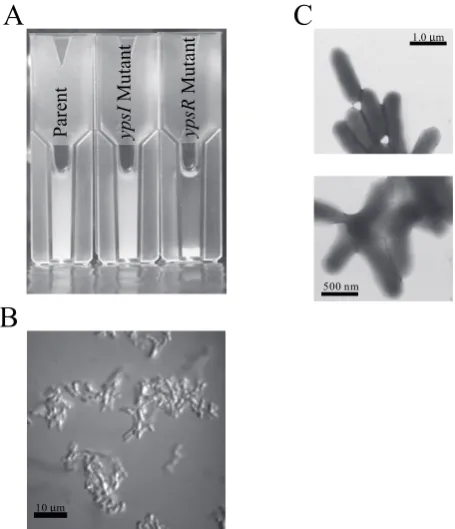
When grown in LB at 28°C or 37°C, Y. pseudotuberculosis
ypsR mutant cells aggregate, a phenomenon which does not occur at 22°C or in either the parent or ypsI
in the ypsR mutant (Atkinson et al., 1999). N-terminal sequence analysis indicated that this protein exhibited significant homology to the major flagellin structural proteins FleA, FleB and FleC of Y. enterocolitica (Kapatral and Minnich, 1995). These data implicated ypsR in the control of flagellar mediated motility. On swimming motility plate assays, both the ypsR and ypsI mutants, but not the parent strain, were motile after 24h when grown at 22°C. None of the strains was motile after 24 h or 48 h
at 37°C by phase contrast microscopy or on swarming
motility plate assays. By following the induction of motility in liquid medium over the growth curve, it is clear that at 22°C, motility is induced after 6 h in both ypsI and ypsR
mutants but delayed until 26 h in the parent (Atkinson et al., 1999). Thus cell density appears to be one important environmental cue for activating swimming motility in Y. pseudotuberculosis (Atkinson et al., 1999). Furthermore, although the ypsR/I locus is involved in the repression of motility, mutation of this locus does not overcome the temperature dependence of swimming motility.
To determine whether the ytbR/I locus is also involved in the control of flagellar-mediated motility, both ytbI and ytbR genes were inactivated. We speculated that if the ytbR/I system was not involved in the regulation of motility, the mutants would display the hypermotile phenotype characteristic of the ypsR/I mutants. However, in common with the parent strain, both ytbI and ytbR mutants were non motile even after 48 h at 22°C. While this initially implied
that the ytbR/I system was not involved in controlling motility, the corresponding double I or double R mutants i.e. the ypsI ytbI and ypsR ytbR mutants were also
non-motile. These data indicate that the ytbR/I locus may be an activator of motility while the ypsR/I locus functions to repress motility. The two quorum sensing circuits also appear to be linked since YpsR appears to be involved in the control of ytbI expression since ypsR mutants fail to produce the YtbI-generated AHL, C8-HSL during growth at 28°C.
Since ypsI/ytbI and ypsR/ytbR double mutants are non-motile while the ypsR and ypsI single mutants exhibit early motility in batch culture when compared to the wild type, it is likely that the quorum sensing circuitry controls the expression of key regulatory genes involved in the control of swimming motility. In enteric bacteria, flagellar gene transcription is arranged as a hierarchy with genes in three regulatory levels or classes which are responsible for producing and maintaining functional flagella and the chemotaxis apparatus (MacNab, 1996). FlhD and FlhC activate the class II structural genes encoding the basal body and hook proteins, along with an activator FliA (σF) and a repressor FliM (anti-σF) of class III genes.
Class III genes encode the proteins which constitute the mature flagellar filament, chemotactic sensors and motor and are transcribed from FliA-dependent promoters in a temperature dependent manner (MacNab, 1996). Although the flhDC master regulatory system was once considered to be exclusively involved in regulating the production of flagella for swimming motility(Fraser and Hughes, 1999) it is now known to play a more global role in regulating diverse physiological processes(Pearson et al., 2000; Tan et al., 1999). In Y. enterocolitica (Young et al., 1999) as well as S. liquefaciens (Givskov et al., 1997) and P. mirabilis (Fraser and Hughes, 1999), flhDC
is required for flagellin production, swimming motility and swarming motility.
An analysis of the Y. pseudotuberculosis flhDC and
fliA promoter/operator region revealed the presence of putative lux-box like elements. By employing reporter gene fusions in single and double ypsR/I and ytbR/I mutants we have established that, for example flhDC expression is repressed by YpsR and YtbR at 37°C. In addition, YtbI
appears to be involved as an activator of flhDC expression at temperatures below 28°C (unpublished data).
In a Y. enterocolitica flhDC mutant (Bleves et al., 2002) noted an increase in Yop expression and a loss of the temperature dependency usually associated with Yop expression at 37°C. Since the Y. pseudotuberculosis
quorum sensing circuitry appears to control flhDC, this implies the existence of a regulatory link between type III secretion and motility. However such a link has to be demonstrated experimentally.
Although the molecular basis behind the clumping phenotype associated with the ypsI and ypsR mutants is currently unknown this observation lead to an investigation into the relationship between biofilm formation and the quorum sensing system of Y. pseudotuberculosis using a biotic surface model. In this model Y. pseudotuberculosis produces biofilms after 24 h whereas the ypsI ytbI and ypsR ytbR double mutants are attenuated for biofilm formation (unpublished observations). The implications for these findings are far reaching given the fact that in Y.pestis biofilms have been shown to develop in the proventriculus and fore gut of the flea post infection and Fig. 4. Mutation of ypsR causes cell aggregation in Y. pseudotuberculosis.
(A) 24 h Static LB cultures of the parent ypsI and ypsR mutants following growth for 24 h shaking at 200 rpm. (B) Light and (C) electron micrographs of the ypsR mutant prepared from overnight cultures grown with shaking at 22°C.
C
500 nm
1.0µm
A
B
P
ar
en
t
yp
sI
M
ut
an
t
yp
sR
M
ut
an
t
biofilm development is associated with the expression of components of the hms locus (Jarrett et al., 2004). However, under the conditions tested and using a triple Y. pestis mutant defective for ypeI, yepI and luxS, Jarrett et al (2004) were unable to establish a link between the quorum sensing system of Y. pestis and the hms locus. The relationship between the hms locus and quorum sensing has yet to be characterized in Y. pseudotuberculosis but once tested may stimulate further investigations into the differential control of biofilm formation in both yersinia species.
The biological significance of long chain AHLs produced
by Y. pseudotuberculosis
Although only C6-HSL, 3-oxo-C6-HSL and C8-HSL were initially identified and fully chemically characterized (Atkinson et al., 1999), it is also clear, as indicated above that Y. pseudotuberculosis produces three long chain AHLs, oxo-C10-HSL, oxo-C12-HSL and 3-oxo-C14-HSL. These AHLs which are also produced via recombinant YtbI expressed in E. coli are not produced by recombinant YpsI. They are present in spent supernatants prepared from ypsI mutants but absent from spent culture supernatants prepared from ytbI mutants or ypsI ytbI double mutants, the latter of which make no detectable AHLs (Buckley, 2002). While the significance of this finding in the context of quorum sensing dependent gene regulation in Y. pseudotuberculosis is not yet clear, it is worth noting that 3-oxo-C12-HSL (which is also produced via LasI in P. aeruginosa; (Passador et al., 1993; Pearson et al., 1994) possesses potent, dose dependent, pro-inflammatory, immune modulatory and vasorelaxant properties (Telford et al., 1998; Lawrence et al., 1999; Smith et al., 2001; Chhabra et al., 2003). For example 3-oxo-C12-HSL modulates both T and B cell functions, blocks lipopolysaccharide-stimulated production of tumour necrosis factor alpha (TNF-α) by peritoneal macrophages
and mediates switching of the T-helper-cell response from the antibacterial Th-1 response (characterized by IL-12 and gamma interferon production) to a Th-2 response. In addition, 3-oxo-C12-HSL also exerts a pharmacological effect on the cardio-vascular system suggesting that host cardiovascular function may be modulated, or influenced, by bacterial quorum sensing molecules. In isolated porcine coronary arteries, 3-oxo-C12-HSL caused a concentration dependent relaxation effect on thromboxane mimetic-induced contractions (Lawrence et al., 1999) and also induces a marked bradycardia in live conscious rats (Gardiner et al., 2001). Structure activity studies have indicated that the immune modulatory properties of AHLs are optimal in compounds with 11 to 13 acyl chain carbons and a 3-oxo substituent (Chhabra et al., 2003). Short chain (4 to 8 acyl carbons) AHLs have little activity in this context. Thus, 3-oxo-C12-HSL and to a lesser extent 3-oxo-C10-HSL and 3-oxo-C14-HSL may contribute to virulence directly through manipulation of eukaryotic cells and tissues to maximize the provision of nutrients via the bloodstream while down regulating host defence mechanisms
Quorum sensing in Y. pestis and Y. ruckeri
Given the close genetic relationship between Y. pseudotuberculosis and Y. pestis, it is perhaps not surprising to find two pairs of LuxRI homologues, the genes for which have been termed ypeRI and yepRI in the latter (Isherwood, 2001; Swift et al., 1999). In addition, preliminary studies on Y. ruckeri, which is responsible for enteric red mouth disease in salmonid fish, have also revealed the presence of a luxRI pair, termed yukRI (unpublished data). Each of these Yersinia LuxR and LuxI homologues share significant homology with each other and each genetic locus is organized similarly in that the two genes are convergently transcribed and overlapping by either 8 or 20 bp in the respective host species (Fig. 3). Neither the genetic nor physiological consequences of such a gene arrangement are clear since luxR and luxI homologues in different organisms can be transcribed in tandem, divergently or convergently (Salmond et al., 1995; Swift et al., 1999)
Using AHL biosensors such as C. violaceum CV026, initial cross streak analysis of Y. pestis isolates taken from a range of environmental and geographic sources revealed that AHL production is conserved in this Yersinia species. Subsequent analysis indicated that Y. pestis, in common with Y. pseudotuberculosis produces C6-HSL, 3-oxo-C6-HSL and C8-HSL (Swift et al., 1999). Furthermore, YpeI (which is more closely related to YpsI and YenI than YtbI), directs the synthesis of C6-HSL and 3-oxo-C6-HSL suggesting that the second Y. pestis LuxI homologue, YepI is responsible for C8-HSL synthesis. Sequence analysis indicates that YepI is more closely related to YtbI than YpsI which is consistent with the nature of the respective AHLs produced. Whether YpeI in Y. pestis is also responsible for producing additional AHLs including compounds with long (C10-C14) acyl side chains is not yet known. For Y. ruckeri, preliminary TLC analysis of the recombinant YukI expressed in E. coli suggests that this fish pathogen also produces C6-HSL, 3-oxo-C6-HSL and C8-HSL (unpublished data). Indeed although analysis of the translated sequence for a given LuxI homologue provides little information on the nature of the corresponding AHL(s) produced, the presence of a threonine at position 143 in the carboxy terminal region relative to the V. fischeri LuxI protein (Watson et al., 2002) is characteristic of synthases which direct the synthesis of 3-oxo-substituted AHLs. This observation appears to hold for all six Yersinia LuxI homologues all of which are capable of 3-oxo-C6-HSL production.
of Y. pestis virulence gene expression in parent, ypeI and ypeR mutants grown at 28°C and 37°C did not reveal any
differences with respect to V-antigen, pH 6 antigen, the coagulase/fibrinolytic protein Pla or lipopolysaccharide profile (Swift et al., 1999). However in a mouse infection model an increase in time to death was observed for mice challenged with the mutant when compared to the parent. For example at a challenge dose of approximately 104
cfu the mean time to death increased from 5.4 days for mice challenged with Y. pestis GB to 6.7 days for mice challenged with the ypeR mutant implying a possible role for AHL-dependent quorum sensing in the pathogenesis of Y. pestis infections (Swift et al., 1999).
Conclusions
AHL-dependent quorum sensing is clearly highly conserved within the genus Yersinia. Together with temperature, bacterial cell population density is at least one additional environmental parameter which the organism must sense during adaptation to life in different ecological niches. In Y. pseudotuberculosis and Y. enterocolitica, motility depends on the integration of both parameters. In Y. pestis, the identity of the target structural genes regulated via AHL-dependent quorum sensing is not yet apparent and may reflect the recent gene/promoter loss or rearrangement as a consequence of its recent divergence from the Y. pseudotuberculosis infection route to the insect vector route. For all three species of human pathogenic yersiniae, genome wide microarray analysis should rapidly facilitate mapping of the quorum sensing regulons and the subsequent definition of their contribution to survival and virulence.
Acknowledgements
Work in the authors’ laboratories has been supported by grants and studentships from the Wellcome Trust, Biotechnology and Biological Sciences Research Council, UK and the Medical Research Council, UK which are gratefully acknowledged.
References
Atkinson, S., Throup, J.P., Stewart, G.S.A.B., and Williams, P. (1999). A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33, 1267–1277.
Bainton, N.J., Bycroft, B.W., Chhabra, S.R., Stead, P., Gledhill, L., Hill, P.J., Rees, C.E.D., Winson, M.K., Salmond, G.P.C., Stewart, G.S.A.B., and Williams, P. (1992). A general role for the lux autoinducer in bacterial-cell signaling – control of antibiotic biosynthesis in Erwinia. Gene 116, 87–91.
Bassler, B.L. (2002). Small talk: Cell-to-cell communication in bacteria. Cell 109, 421–424.
Bleves, S. and Cornelis, G.R. (2000). How to survive in the host: the Yersinia lesson. Microb. Infect. 2, 1451– 1460.
Bleves, S., Marenne, M.N., Detry, G., and Cornelis, G.R. (2002). Up-regulation of the Yersinia enterocolitica yop regulon by deletion of the flagellum master operon
flhDC. J. Bacteriol. 184, 3214–3223.
Buckley, C. M. F. G. (2002) Quorum Sensing in Yersinia pseudotuberculosis. Nottingham University. Thesis. Cámara, M., Williams, P., and Hardman, A. (2002).
Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect. Dis. 2, 667–676.
Chhabra, S.R., Harty, C., Hooi, D.S.W., Daykin, M., Williams, P., Telford, G., Pritchard, D.I., and Bycroft, B.W. (2003). Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-L-homoserine lactone as immune modulators. J. Med. Chem. 46, 97–104.
Choi, S.H. and Greenberg, E.P. (1992a). Genetic evidence for multimerization of LuxR, the transcriptional regulator of Vibrio fischeri luminescence. Mol. Mar. Biol. Biotechnol. 1, 408–413.
Choi, S.H. and Greenberg, E.P. (1991). The c-terminal region of the Vibrio-fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc. Natl. Acad. Sci. USA. 88, 11115–11119.
Choi, S.H. and Greenberg, E.P. (1992b). Genetic dissection of DNA-binding and luminescence gene activation by the Vibrio-fischeri LuxR protein. J. Bacteriol. 174, 4064–4069.
Cornelis, G., Vanooteghem, J.-C., and Sluiters, C. (1987). Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2, 367–379.
Cornelis, G.R. and Wolf-Watz, H. (1997). The Yersinia Yop virulon: A bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23, 861–867.
Fraser, G.M. and Hughes, C. (1999). Swarming motility. Curr. Opin. Microbiol. 2, 630–635.
Fuqua, C., Winans, S.C., and Greenberg, E.P. (1996). Census and consensus in bacterial ecosystems: The LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50, 727–751.
Gardiner, S.M., Chhabra, S.R., Harty, C., Williams, P., Pritchard, D.I., Bycroft, B.W., and Bennett, T. (2001). Haemodynamic effects of the bacterial quorum sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone, in conscious, normal and endotoxaemic rats. Br. J. Pharmacol. 133, 1047–1054.
Givskov, M., Eberl, L., and Molin, S. (1997). Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol. Lett. 148, 115–122.
Hanzelka, B.L. and Greenberg, E.P. (1995). Evidence that the N-terminal region of the Vibrio-fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177, 815–817.
Huber, B., Riedel, K., Hentzer, M., Heydorn, A., Gotschlich, A., Givskov, M., Molin, S., and Eberl, L. (2001). The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology-Sgm 147, 2517–2528.
Isherwood, K. E. (2001). Quorum sensing in Yersinia pestis. (2001). Nottingham University Thesis.
Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190, 783–792.
Jiang, Y., Cámara, M., Chhabra, S.R., Hardie, K.R., Bycroft, B.W., Lazdunski, A., Salmond, G.P.C., Stewart, G.S.A.B., and Williams, P. (1998). In vitro biosynthesis of the Pseudomonas aeruginosa quorum- sensing signal molecule N-butanoyl-L-homoserine lactone. Mol. Microbiol. 28, 193–203.
Kapatral, V. and Minnich, S.A. (1995). Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol. Microbiol. 17, 49– 56.
Kapperud, G., Namork, E., and Skarpeid, H.J. (1985). Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect. Immun. 47, 561– 566.
Kohler, T., Curty, L.K., Barja, F., Van Delden, C., and Pechere, J.C. (2000). Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182, 5990–5996. Lamont, I.L., Beare, P.A., Ochsner, U., Vasil, A.I., and
Vasil, M.L. (2002). Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 99, 7072– 7077.
Lawrence, R.N., Dunn, W.R., Bycroft, B., Cámara, M., Chhabra, S.R., Williams, P., and Wilson, V.G. (1999). The Pseudomonas aeruginosa quorum-sensing signal molecule, N- (3-oxododecanoyl)-L-homoserine lactone, inhibits porcine arterial smooth muscle contraction. Br. J. Pharmacol. 128, 845–848.
Lindum, P.W., Anthoni, U., Christophersen, C., Eberl, L., Molin, S., and Givskov, M. (1998). N-acyl-L-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J. Bacteriol. 180, 6384–6388.
Luo, Z.Q. and Farrand, S.K. (1999). Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc. Natl. Acad. Sci. USA. 96, 9009–9014.
MacNab, R.M. (1996). Escherichia coli and Salmonella typhimurium. F.C. Neidhardt, R.I. Curtiss, J.L. Ingraham, E.C.C. Lin, K.B. Low, et al., eds. (Washington DC: ASM press), pp. 123–145.
McClean, K.H., Winson, M.K., Fish, L., Taylor, A., Chhabra, S.R., Cámara, M., Daykin, M., Lamb, J.H., Swift, S., Bycroft, B.W., Stewart, G.S.A.B., and Williams, P. (1997). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology-UK 143, 3703–3711.
Meighen, E.A. (1994). Genetics of bacterial bioluminescence. Annu. Rev. Genet. 28, 117–139. Moré, M.I., Finger, L.D., Stryker, J.L., Fuqua, C., Eberhard,
A., and Winans, S.C. (1996). Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272, 1655–1658.
Nasser, W., Bouillant, M.L., Salmond, G., and Reverchon, S. (1998). Characterization of the Erwinia chrysanthemi expl-expR locus directing the synthesis of two
N-acyl-homoserine lactone signal molecules. Mol. Microbiol. 29, 1391–1405.
Parkhill, J., Wren, B.W., Thomson, N.R., Titball, R.W., Holden, M.T.G., Prentice, M.B., Sebaihia, M., James, K.D., Churcher, C., Mungall, K.L., Baker, S., Basham, D., Bentley, S.D., Brooks, K., Cerdeno-Tarraga, A.M., Chillingworth, T., Cronin, A., Davies, R.M., Davis, P., DOUGAN, G., Feltwell, T., Hamlin, N., Holroyd, S., Jagels, K., Karlyshev, A.V., Leather, S., Moule, S., Oyston, P.C.F., Quail, M., Rutherford, K., Simmonds, M., Skelton, J., Stevens, K., Whitehead, S., and Barrell, B.G. (2001). Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527. Passador, L., Cook, J.M., Gambello, M.J., Rust, L., and
Iglewski, B.H. (1993). Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260, 1127–1130.
Pearson, J.P., Feldman, M., Iglewski, B.H., and Prince, A. (2000). Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68, 4331–4334.
Pearson, J.P., Gray, K.M., Passador, L., Tucker, K.D., Eberhard, A., Iglewski, B.H., and Greenberg, E.P. (1994). Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA. 91, 197–201.
Pearson, J.P., Van Delden, C., and Iglewski, B.H. (1999). Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181, 1203–1210.
Pepe, J.C. and Miller, V.L. (1993). Yersinia enterocolitica invasin: A primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA. 90, 6473–6477.
Pierson, D.E. and Falkow, S. (1993). The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect. Immun. 61, 1846–1852.
Redfield, R.J. (2002). Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10, 365–370. Reverchon, S., Bouillant, M.L., Salmond, G., and Nasser,
W. (1998). Integration of the quorum-sensing system in the regulatory networks controlling virulence factor synthesis in Erwinia chrysanthemi. Mol. Microbiol. 29, 1407–1418.
Salmond, G.P.C., Bycroft, B.W., Stewart, G.S.A.B., and Williams, P. (1995). The bacterial enigma – cracking the code of cell-cell communication. Mol. Microbiol. 16, 615–624.
Shadel, G.S., Young, R., and Baldwin, T.O. (1990). Use of regulated cell-lysis in a lethal genetic selection in Escherichia coli – identification of the autoinducer-binding region of the LuxR protein from Vibrio fischeri
ATCC-7744. J. Bacteriol. 172, 3980–3987.
Shaw, P.D., Ping, G., Daly, S.L., Cha, C., Cronan, J.E., Rinehart, K.L., and Farrand, S.K. (1997). Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA. 94, 6036–6041.
Smith, R.S., Fedyk, E.R., Springer, T.A., Mukaida, N., Iglewski, B.H., and Phipps, R.P. (2001). IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa β and activator protein-2. J. Immunol. 167,
366–374.
Stevens, A.M. and Greenberg, E.P. (1997). Quorum sensing in Vibrio fischeri: Essential elements for activation of the luminescence genes. J. Bacteriol. 179, 557–562.
Swift S., Williams P., and Stewart, G.S.A.B. (1999). N-acylhomoserine lactones and quorum sensing in proteobacteria. In Cell-Cell Signaling in Bacteria, G.M. Dunny and S.C. Winans, eds. (Washington DC: ASM Press), pp. 291–314.
Swift, S., Downie, J.A., Whitehead, N.A., Barnard, A.M.L., Salmond, G.P.C., and Williams, P. (2001). Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45, 199– 270.
Swift, S., Isherwood, K.E., Atkinson, S., Oyston, P., and Stewart, G.S.A.B. (1999). Quorum sensing in Aeromonas and Yersinia. In Microbial Signalling and Communication, R. England, G. Hobbs, N.J. Bainton, and D.M. Roberts, eds. (Cambridge, UK: Cambridge University Press), pp. 85–104.
Swift, S., Winson, M.K., Chan, P.F., Bainton, N.J., Birdsall, M., Reeves, P.J., Rees, C.E.D., Chhabra, S.R., Hill, P.J., Throup, J.P., Bycroft, B.W., Salmond, G.P.C., Williams, P., and Stewart, G.S.A.B. (1993). A novel strategy for the isolation of luxI homologs – evidence for the widespread distribution of a luxR luxI superfamily in enteric bacteria. Mol. Microbiol. 10, 511–520.
Tan, M.W., Rahme, L.G., Sternberg, J.A., Tompkins, R.G., and Ausubel, F.M. (1999). Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA. 96, 2408–2413.
Telford, G., Wheeler, D., Williams, P., Tomkins, P.T., Appleby, P., Sewell, H., Stewart, G.S.A.B., Bycroft, B.W., and Pritchard, D.I. (1998). The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect. Immun. 66, 36–42.
Throup, J.P., Cámara, M., Bainton, N.J., Briggs, G.S., Chhabra, S.R., Bycroft, B.W., Williams, P., and Stewart, G.S.A.B. (1995). Characterization of the yenI/yenR locus from Yersinia enterocolitica mediating the synthesis of two N-acyl homoserine lactone signal molecules. Mol. Microbiol. 17, 345–356.
von Bodman, S.B., Majerczak, D.R., and Coplin, D.L. (1998). A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA. 95, 7687–7692.
Watson, W.T., Minogue, T.D., Val, D.L., von Bodman, S.B., and Churchill, M.E.A. (2002). Structural basis and
specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 9, 685–694. Whiteley, M. and Greenberg, E.P. (2001). Promoter
specificity elements in Pseudomonas aeruginosa quorum- sensing-controlled genes. J. Bacteriol. 183, 5529–5534.
Williams P. (2002). Quorum sensing: an emerging target for antimicrobial chemotherapy? Expert Opinion on Therapeutic Targets 6, 257–274.
Williams, P., Cámara, M., Hardman, A., Swift, S., Milton, D., Hope, V.J., Winzer, K., Middleton, B., Pritchard, D.I., and Bycroft, B.W. (2000). Quorum sensing and the population-dependent control of virulence. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 355, 667–680.
Winson, M.K., Swift, S., Hill, P.J., Sims, C.M., Griesmayr, G., Bycroft, B.W., Williams, P., and Stewart, G.S.A.B. (1998). Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol. Lett. 163, 193–202.
Winzer, K., Hardie, K.R., Burgess, N., Doherty, N., Kirke, D., Holden, M.T.G., Linforth, R., Cornell, K.A., Taylor, A.J., Hill, P.J., and Williams, P. (2002a). LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology-Sgm 148, 909–922.
Winzer, K., Hardie, K.R., and Williams, P. (2002b). Bacterial cell-to-cell communication: Sorry, can’t talk now – gone to lunch! Curr. Opin. Microbiol. 5, 216–222.
Yates, E.A., Philipp, B., Buckley, C., Atkinson, S., Chhabra, S.R., Sockett, R.E., Goldner, M., Dessaux, Y., Cámara, M., Smith, H., and Williams, P. (2002). N-acylhomoserine Lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70, 5635–5646.
Young, G.M., Badger, J.L., and Miller, V.L. (2000). Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68, 4323–4326.
Young, G.M., Smith, M.J., Minnich, S.A., and Miller, V.L. (1999). The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 181, 2823–2833.
Zhang, R.G., Pappas, T., Brace, J.L., Miller, P.C., Oulmassov, T., Molyneauz, J.M., Anderson, J.C., Bashkin, J.K., Winans, S.C., and Joachimiak, A. (2002). Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417, 971–974.
• MALDI-TOF Mass Spectrometry in Microbiology
Edited by: M Kostrzewa, S Schubert (2016)
www.caister.com/malditof
• Aspergillus and Penicillium in the Post-genomic Era
Edited by: RP Vries, IB Gelber, MR Andersen (2016)
www.caister.com/aspergillus2
• The Bacteriocins: Current Knowledge and Future Prospects
Edited by: RL Dorit, SM Roy, MA Riley (2016)
www.caister.com/bacteriocins
• Omics in Plant Disease Resistance
Edited by: V Bhadauria (2016)
www.caister.com/opdr
• Acidophiles: Life in Extremely Acidic Environments
Edited by: R Quatrini, DB Johnson (2016)
www.caister.com/acidophiles
• Climate Change and Microbial Ecology: Current Research
and Future Trends
Edited by: J Marxsen (2016)
www.caister.com/climate
• Biofilms in Bioremediation: Current Research and Emerging
Technologies
Edited by: G Lear (2016)
www.caister.com/biorem
• Microalgae: Current Research and Applications
Edited by: MN Tsaloglou (2016)
www.caister.com/microalgae
• Gas Plasma Sterilization in Microbiology: Theory,
Applications, Pitfalls and New Perspectives
Edited by: H Shintani, A Sakudo (2016)
www.caister.com/gasplasma
• Virus Evolution: Current Research and Future Directions
Edited by: SC Weaver, M Denison, M Roossinck, et al. (2016)
www.caister.com/virusevol
• Arboviruses: Molecular Biology, Evolution and Control
Edited by: N Vasilakis, DJ Gubler (2016)
www.caister.com/arbo
• Shigella: Molecular and Cellular Biology
Edited by: WD Picking, WL Picking (2016)
www.caister.com/shigella
• Aquatic Biofilms: Ecology, Water Quality and Wastewater
Treatment
Edited by: AM Romaní, H Guasch, MD Balaguer (2016)
www.caister.com/aquaticbiofilms
• Alphaviruses: Current Biology
Edited by: S Mahalingam, L Herrero, B Herring (2016)
www.caister.com/alpha
• Thermophilic Microorganisms
Edited by: F Li (2015)
www.caister.com/thermophile
• Flow Cytometry in Microbiology: Technology and Applications
Edited by: MG Wilkinson (2015)
www.caister.com/flow
• Probiotics and Prebiotics: Current Research and Future Trends
Edited by: K Venema, AP Carmo (2015)
www.caister.com/probiotics
• Epigenetics: Current Research and Emerging Trends
Edited by: BP Chadwick (2015)
www.caister.com/epigenetics2015
• Corynebacterium glutamicum: From Systems Biology to Biotechnological Applications
Edited by: A Burkovski (2015)
www.caister.com/cory2
• Advanced Vaccine Research Methods for the Decade of Vaccines
Edited by: F Bagnoli, R Rappuoli (2015)
www.caister.com/vaccines
• Antifungals: From Genomics to Resistance and the Development of Novel
Agents
Edited by: AT Coste, P Vandeputte (2015)
www.caister.com/antifungals
• Bacteria-Plant Interactions: Advanced Research and Future Trends
Edited by: J Murillo, BA Vinatzer, RW Jackson, et al. (2015)
www.caister.com/bacteria-plant
• Aeromonas
Edited by: J Graf (2015)
www.caister.com/aeromonas
• Antibiotics: Current Innovations and Future Trends
Edited by: S Sánchez, AL Demain (2015)
www.caister.com/antibiotics
• Leishmania: Current Biology and Control
Edited by: S Adak, R Datta (2015)
www.caister.com/leish2
• Acanthamoeba: Biology and Pathogenesis (2nd edition)
Author: NA Khan (2015)
www.caister.com/acanthamoeba2
• Microarrays: Current Technology, Innovations and Applications
Edited by: Z He (2014)
www.caister.com/microarrays2
• Metagenomics of the Microbial Nitrogen Cycle: Theory, Methods
and Applications
Edited by: D Marco (2014)
www.caister.com/n2
Caister Academic Press is a leading academic publisher of advanced texts in microbiology, molecular biology and medical research. Full details of all our publications at caister.com