Synthesis of Dihydroquinoline Derivatives Using Biodegradable Cellulose Sulfuric Acid as a Solid Acid Catalyst
Full text
Figure
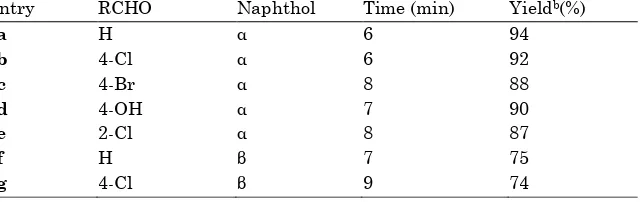
Related documents
In the UK, the most commonly used measure of available phosphorus involves an Olsen (sodium bicarbonate) extraction, and currently target phosphorus values are generally recommended
• The AIP:c.9T>G variant encodes for an amino acid change from aspartic acid to glutamic acid in a region of the AIP protein considered to be important for its tumour
A conventional accounting ap- proach, and a four-input translog cost function and as- sociated share equations are used to estimate total and partial factor productivities, own
We then search for the core (generally the charm hadron remnant) by reclustering the muon and calorimeter towers with total jet energy fraction f min.. tower
Um die Einflussgröße „Geschwisteranzahl“ auf das Verhalten der Late Talkers zu testen, verglichen wir das Verhalten der Late Talkers, die als Einzelkinder aufwuchsen mit jenen, die
In the present study, the Socio-Economic factors of the selected investors attitude towards the investments are discussed with the help of following variables such as gender
GIS descriptor Data T ype Date Le gend Author Access T opograph y 1 m bare-earth DTM a of Claduègne catchment Raster 2012 Lidar campaign Sintégra géomètres Public experts 25