618 Clinical Chemist’y 42:4
618-624 (1996)
Laboratory testing for recent alcohol
consumption: comparison of ethanol, methanol,
and 5-hydroxytryptophol
ANDERS HELANDER,l* OLOF BECK,2
and A.
WAYNE JoNEs3The ratio of 5-hydroxytryptophol to 5-hydroxyindole-3-acetic acid (5HTOIJSHIAA) in urine was compared with concentrations of ethanol and methanol as a way to monitor recent alcohol consumption. During detoxification of alco-hol-dependent subjects, ethanol persisted longer in urine than in breath or plasma. Blood and urinary methanol remained increased for 2-6 h after blood ethanol had returned to background concentrations, whereas 5HTOLI
5HIAA remained increased fir 6-15 h. In healthy
volun-teers who had ingested alcohol (range 3-98 g) the previous afternoon or evening, 87% (for men) and 59% (for women) of all drinking occasions exceeding 7 g of alcohol were
identified by an increased 5HTOIJ5HIAA in the first
morning urine void. This compares with 32% and 12%,
respectively, identified by analysis of ethanol (>200 xmol/ L). No gender difference in the excretion pattern of SHTOIJSHLAA was seen. The results demonstrate that SHTOLJ5IJIAA provides a specific and more sensitive method to detect recent alcohol consumption than does ethanol or methanol.
INDEXING mIt11s: alcoholism. 5-hydroxyindole-3-acetic acid. alcohol metabolism #{149}serotonin #{149}aldehyde dehydrogenase
alcohol dehydrogenase
A major problem in the early detection and treatment of alcohol dependence is obtaining reliable information about a person’s drinking habits. Self-reports and diagnostic questionnaires are widely used for this purpose, but people with alcohol-related problems may deliberately deny or underreport the actual
Karolinska Institute, Department of Clinical Neuroscience, Psychiatry Sec-tion at St. GoransHospital, Stockholm, Sweden.
2Department of Clinical Pharmacology, Karolinska Hospital, Stockholm, Sweden.
‘Department of Forensic Toxicology, University Hospital, Linkoping,
Swe-amounts they consume [1, 2]. For this reason, underdiagnosis of alcohol misuse is not uncommon by these methods. Well-controlled population surveys, for example, have accounted for only about half of the known consumption of alcohol [3]. To help overcome this problem, a large number of biochemical and hematological tests have been developed to identify alcohol-dependent subjects or those engaged in hazardous drinking [4, 5]. However, when biochemical markers are evaluated in terms of diagnostic sensitivity and specificity, most studies still rely on self-reports as the “gold standard” for alcohol consump-tion, which creates a problem.
The limited reliability of interview data underscores the need for more objective tests of recent alcohol consumption, permit-ting frequent monitoring of individual drinking patterns. The most obvious way to prove that a person has been drinking is by demonstrating the presence of ethanol in blood, urine, saliva, or breath [6]. However, because ethanol is rapidly eliminated from the body, this approach is practical only for consumption of alcohol within the past few hours. Increased concentrations of the metabolites of ethanol oxidation, acetaldehyde and acetic acid, have been proposed as alternative indicators of recent drinking. Acetaldehyde, however, is rapidly converted to acetic acid by aldehyde dehydrogenase (ALDH), and the concentra-tion in peripheral blood is normally very low f7]4 Its use is further hampered by problems associated with artifactual for-mation after sampling the blood. The concentration of acetic acid, on the other hand, increases with the development of metabolic tolerance to alcohol, and might therefore be more suitable as a marker of chronic alcoholism [8].
A metabolic interaction between ethanol and serotonin (5-hydroxytryptamine) can be utilized for detection of recent drinking [9-11]. 5-Hydroxytryptophol (5HTOL) is normally a minor metabolite of serotonin, but its formation increases dramatically after ingestion of ethanol, whereas 5-hydroxyin-dole-3-acetic acid (5HIAA), the major metabolite under normal
Address correspondence to this author at: Alcohol & Drug Dependence Unit, St. Gorans Hospital, Box 12500, S-112 81 Stockholm, Sweden. Fax (+46) 8 6721994.
Received October 10, 1995; accepted January 24, 1996.
Nonstandard abbreviations: ALDH, aldehyde dehydrogenase; 5HTOL, 5-hydroxytryptophol; 5HIAA, 5-hydroxyindole-3-acetic acid; and ADH, alcohol dehydrogenase.
conditions, is concomitantly decreased. This metabolic shift can be monitored by analysis of blood or urine, and the excretion of 5HTOL will not normalize until several hours after blood and urinary ethanol have reached background concentrations [11].
On the basis of this observation, increased urinary concentration of 5HTOL was introduced as a sensitive biochemical marker of recent alcohol consumption [11, 12].
An abnormally high concentration of methanol in body fluids or breath has also been suggested as an indicator of recent drinking [13]. Methanol accumulates in the body during the metabolism of ethanol [14, 15], and, as for 5HTOL, the con-centration remains increased for some time after ethanol is no longer detectable [16, 17]. Thus measurement of either 5HTOL or methanol will provide a higher sensitivity than analysis of ethanol alone for the detection of recent alcohol consumption.
The present study was carried out to evaluate further the usefulness of 5HTOL as a marker of recent alcohol consump-tion, and to compare its sensitivity and specificity with those of ethanol and methanol.
Materials
and
MethodsDETERMINATION #{248}FETHANOL, METHANOL, AND ACETONE The concentrations of ethanol, methanol, and acetone in blood and urine samples were determined by headspace gas chroma-tography essentially as described elsewhere [18]. To increase sensitivity for determination of methanol, we used a salting-out procedure, adding 1.2 g of sodium chloride to 0.5 mL of specimen [19]. The limits of detection for analysis of ethanol and methanol were 200 j.molIL and 15 /.Lmol/L, respectively. The concentration of ethanol in breath was determined with a hand-held analyzer (Alcolmeter S-D2; Lion Labs., Barry, UK) and the result reported as presumed blood alcohol concentra-tion.
DETERMINATION OF 5HTOL AND 5HIAA
The concentration of 5HTOL in urine is expressed as a ratio to the concentration of 5HIAA, because ingestion of foods rich in serotonin (such as banana, pineapple, kiwifruit, and walnuts) can otherwise cause false-positive results [20]. 5HTOL and 5HIAA were determined with sensitive gas chromatographic-mass spec-trometric and HPLC methods [10, 12, 2/]. The reference limit used to indicate an increased urinary 5HTOL/5HIAA ratio was set at 15 pmol/nmol [12, 22, 23].
CLINICAL PROCEDURES
Male alcohol-dependent patients (ages 40-62 years) were re-cruited from the detoxification unit at St. G#{246}ransHospital. Blood and urine samples (10 mL each) for determination of ethanol, methanol, and the 5HTOL/51-IIAA ratio were col-lected from 10 subjects with a blood alcohol concentration of 23.7-57.5 mmollL (mean 43.6) on admission to the hospital. Thereafter, the breath ethanol concentration was measured every 4-6 h, and blood and urine samples were also collected. When breath measurements indicated zero ethanol concentra-tion, sampling of blood and urine was continued at 3-h intervals for another 12-15 h.
In a previous experimental protocol [11], blood and urine
samples were collected from healthy volunteers (social drinkers without any history of previous excessive consumption of alco-hol). Ethanol (160 g/L in orange juice) was administered at 0.8 g/kg body weight to five women and five men (ages 23-39 years) after an overnight fast, and the drink was finished within 30 mm. From all subjects participating in that experiment, the plasma samples collected at the start of drinking and then at 2-h intervals for a total of 10 h, and the urine samples collected at the start and then at every 2-3 h for 22 h, were also used for the present study. All samples had since (for -30 months) been stored in l-mL portions at -80 #{176}C.The urinary 5HTOL/ 5HIAA ratio and the concentrations of plasma and urinary ethanol and of urinary methanol (which was not analyzed in the previous study) were determined as described above.
In a new experiment on healthy volunteers, consecutive urine samples were obtained daily from four women and four men (ages 24-49 years) over a period of 4-6 weeks. A lO-inL sample of the first morning void was collected, and the subjects kept a record of the amount of alcoholic beverages, if any, consumed the previous day throughout this period. The concentration of ethanol and the SHTOL/5HIAA ratio were determined.
In an earlier study on the use of 5HTOL as a marker of recent drinking [24], the possible interference by metabolic acidosis in a subject with diabetes mellitus could not be ruled out. To examine this further, we assayed urine samples collected from 20 diabetic patients (12 women and 8 men, ages 38-91 years) admitted to St. G#{246}ransHospital for treatment of ketoac-idosis or coma. Thereafter, we assayed additional samples collected at 1-week intervals for a total of 3-5 weeks, having asked the patients not to consume any alcohol during this period. The concentrations of ethanol and acetone and the 5HTOL/5HIAA ratio were determined.
All blood samples were collected from an antecubital vein into Vacutainer Tubes containing EDTA (Becton Dickinson, Meylan Cedex, France) for preparation of plasma by centrifu-gation. The urine samples were collected into plastic tubes without preservative. Before analysis, the plasma and urine samples were stored at -80 #{176}C.Samples were thawed overnight at 4 #{176}C.The study was approved by the local Ethical Committee (VSO/SSO) in Stockholm.
Results
Figure 1shows the time course of concentrations of ethanol in breath, plasma, and urine, and of methanol in plasma and urine, as well as the 5HTOL/5HIAA ratio in urine, for each of the 10 alcohol-dependent subjects during detoxification. Ethanol was usually detectable longer in urine than in breath or plasma. The concentration of methanol was markedly increased in the first sample [mean 0.57 ± 0.15 (SD) mmol/L in plasma and 0.62 ±
0.17 mmol/L in urine] and did not start to fall until the blood ethanol had returned to background concentration. Thereafter, methanol remained increased for a further 2-6 h (mean 3.5 h). The urinary SHTOL/51-HAA ratio in the first sample ranged between 331 and 1081 pmol/nmol (mean 684 ± 259 pmol/ nmol), and remained above the cutoff for 6-15 h (mean 10 h) after ethanol was no longer detectable in blood samples.
80 1.0 60 0.8 0.6 40 0.4 20 0.2 0 0 80 1.0 60 0.8 0.6 40 0.4 20 02 0 0 12 16 20 24 ..,.
.T1TTT7
1000 100 50 40 30 20 l0 0 80 1.0 :; 0.8 60 o E E E06 40 #{149}6 0A C #{149} #{149} -20 Ui 0 0 80 1.0 = 0.8 60 o E E E0.6 40 -0.4 = _ #{149} .= 20 o.z Ui 0 -80 -J .. 60 0 E 40 0 C 20 Ui 0 80 1.0 0.8 60 o E E E0’6 .4Q -0.4 = C #{149} .c 20 uj 0.2 0 0 #{149}#{182}000E 100 0 E 50 -40 30 20 10 = - U, #{149}#{182}0006 #{149}I00 0 a so -40 30 20 3 10 0 u, -#{182}000 -100 0 a 0. = U) -j 0 I-U) 80 80 40 20 0 1.0 0.8 0.6 0.4 02 A Tim. (hI -1000 -100 50 40 30 20 to 0 0 4 8 12 16 20 24 28 80 80 0 a 40 0 = 20 UJ 0 80 1.0 60 0.8 0.6 40 0.4 20 0.2 0 0 80 1.0 60 0.8 0.6 40 0.4 20 02 0 0 -J 0 E a 0 C -1000 a tOO 0 a 50 40 30 U, 20 #{149}I0 0ii,
#{149}iooo;:I
40 30 = U, 20 0 to_
0 v, 0 4 8 12 #{182}620 24 28 Tim. (hIAg. 1. Time course of concentrations of ethanol in breath, plasma, and urine, and of methanol in plasma and urine, as well as the ratio of 5HTOL/5HIAA in urine, in samples collected from 10 male alcohol-dependent subjects (A-fl during detoxification.
Thedashed line represents the cutoff limit usedtoindicate an abnormal5HTOL/51-IIM ratio (>15 pmol/nmol). Helander et al.: Lab testing for alcohol consumption
620
0 Breath Ethanol D Urinary Ethanol U Urinary Methanol
Plasma Ethanol A Plasma Methanol * Urinary 5HTOL/5HIAA
in plasma and urine, and of methanol in urine, as well as the 5HTOL/5HIAA ratio in urine, for the five female and five male social drinkers after administration of ethanol at 0.8 g/kg body weight. The concentration of methanol was highest in the 6-h sample (-5 times baseline) and decreased again to the baseline concentration (<0.04 mmollL) 10-12 h after the start of
drinking. The 5HTOL/5HIAA ratio was increased >100-fold in the 6-h sample and did not return to baseline until after
14-16 h. No significant gender differences in ethanol elimina-tion rate or in the urinary excretion patterns of methanol and 5HTOL/5HIAA were seen.
25 20 15 10 5 0.20--J ‘..-. 0.15 -0 E - 0.10 -iO 0.05 0 -J 0 E 0 C Ui 0 2 6 10 14 18 22 Time (h)
Table 1. Sensitivity and specIficity of urinary (U) ethanol and 5HTOL/5HIAA ratio to detect recent alcohol consumption in
healthy social drinkers.
Increased U-ethanol Normal U-ethanol
No.of days 5lITOL/5HIAA >200 amoi/L 5HIOL/5HIAAb <200 gemol/L
No. of
Age, Total With >7 g Alcohol intake No. No. alcohol- No. No.
years study ethanol range, g(mean) days Sens., % days Sen.., % free days days Spec., % days Spec., %
20 87 5 22 20 20 100 14 67 7 33 12 12 100 20 95 4 19 6 6 100 29 97 16 53 5 4 80 87 32 24 30 9 30 32 15 38 28 18 41 28 18 Mean >15 pmol/nmol. ‘<i pmol/nmol. 8-50 (27.1) 8-71 (27.1) 8-60 (21.4) 9-64 (24.0) 3 33 1 11 19 11 73 2 13 16 14 78 1 6 10 9 50 3 17 10 59 12 19 100 16 100 10 100 10 100 19 100 16 100 10 100 10 100 100 100
0 P-ethanol 0 U-ethanol U-methanol U-5HTOL/5HIAA
U P-ethanol 9 #{149}U-ethanol 9 A U-methanol 9 #{149}U-5HTOL/5HIAA g
urine samples, an average of 29 g of alcohol (range 3-98 g/day) had been consumed the previous day on 155 of a total of 261 sampling days, according to the self-reported drinking proto-cols. Usually, drinking occurred in the evening or late after-noon. Analysis of the urinary 5HTOL/5HIAA ratio in the first
morning void identified on the average 73% (sensitivity) of all drinking occasions exceeding 7 g of alcohol, as compared with 22% by analysis of ethanol (>200 mol/L) (Table 1). On some occasions when relatively large amounts of alcohol had been consumed, the second and, in one case, even the third void of urine the next day confirmed that the 5HTOL/5HIAA ratio remained increased for considerably longer than ethanol could be detected (Fig. 3). The mean sensitivity for analysis of ethanol and the 5HTOL marker was somewhat less for women than for men (Table 1). However, two women reported that they occa-sionally voided during the night. The specificity was 100% for
1000
#{149}000E
Ag. 2. Concentration-time profiles of ethanol in plasma (P) and urine (U), methanol in urine, and the ratio of 30 5HTOL/5HIAA in Urine, in samples collected from healthy
20 social-drinking volunteers (five women and five men, ages I- 23-39 years) after oral administration of ethanol at 0.8
10 g/kg body weight.
The drink was finished within 30 mm in the morning after an 0 overnight fast. Data represent meanvalues ± SD for the 5HTOL/
5HIAA ratio, but, forclarity, SOs have been omitted for the other variables. Thedashed line represents the cutoff limit used to indicate an abnormal 5HTOL/5HIAA ratio (>15 pmol/nmol(.
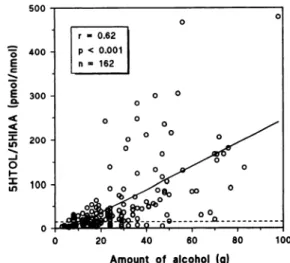
both ethanol and 5HTOL/5HIAA in all subjects except one. This male subject showed one slightly increased metabolite ratio (21 pmol/nmol), although no alcohol consumption was reported the previous evening. We observed a significant correlation between the amount of alcohol consumed and the urinary
5HTOL/5HIAA ratio in the first morning void the next day (Fig. 4).
In the urine samples collected from 20 diabetic patients with metabolic ketoacidosis or admitted to hospital in diabetic coma, the highest concentration of acetone was 4.4 mmol/L. The 5HTOL/5HIAA ratios were 6.5 ± 3.4 pmol/nmol (mean
± SD, n = 85), and all samples except one showed ratios below the cutoff limit of 15 pmol/nmol. This single value was only slightly increased (17 pmol/nmol) and was present in the urine sample collected immediately upon admission to the hospital. Men 32 43 23 9-98 (29.8) 39 38 21 8-48 (24.5) 40 27 21 8-74 (27.8) 49 35 30 14-83 (45.0) Mean Women 20 100 12 100 6 100 5 100 95 100
0 A-40 30 20 to
H
11 ‘4-‘4 to 00 too 1000‘!
I
0 E = 0 “4 “4 = U) -J 0 I-U, 0 20 40 60 80 100622 Flelander et al.: Lab testing for alcohol consumption
o lot void
#{149}2nd void e 3rd void
Fig. 3. Urinary ethanol concentrations and 5HTOL/5HIAA ratios in the first, second, and, in one case, third morning voids collected on 16 days from three of the social drinkers.
The clashed line represents the cutoff limit used to indicate an abnormal 5HT0L/5HlA ratio(>15 pmol/nmol).
Discussion
Hitherto, the determination of ethanol in body fluids or breath has represented the only generally available objective method to prove recent consumption of alcohol. However, because ethanol is rapidly eliminated from the body, this approach will only detect very recent drinking, even though ethanol can be de-tected for some hours longer in urine than in blood or breath, owing to retention of urine in the bladder. The use of even more sensitive analytical assays may increase diagnostic sensitivity, but also the risk of false-positive results, because several microor-ganisms occasionally found in humans are capable of producing ethanol by metabolic conversion of glucose or other endogenous substrates /25].
An increased concentration of methanol in body fluids or breath has been suggested as an alternative indicator of recent drinking [13]. The concentration of methanol increases during a drinking spree owing to competitive inhibition of alcohol dehy-drogenase (ADH). The methanol might originate from endog-enous sources, but it is also present as a congener in alcoholic beverages and can be derived from various foodstuffs (e.g., fruits and fruit juices) [26]. In the present experiments, methanol remained increased even after the blood ethanol concentration had returned to background concentrations in both alcohol-dependent subjects and healthy volunteers. This time lag be-tween ethanol and methanol clearance agrees with previous reports [14-17]and implies that methanol is more sensitive than ethanol as indicator of recent drinking. However, an abnormally high concentration of methanol in blood or urine could reflect recent as well as long-term continuous drinking. After acute intake of alcohol by healthy volunteers, the concentration of methanol increased to -0.14 mmol/L on average before return-ing to baseline values after 10-12 h. During chronic drinking, methanol accumulates gradually to reach much higher concen-trations, and complete clearance after termination of drinking might require several days [14]. This was further confirmed in the alcohol-dependent subjects during detoxification, where methanol leveled out within the concentration range reached in
Amount of alcohol (g)
Fig.4. Correlation between the urinary 5HTOL/5HIAA ratio in the first morning void and the amount of alcohol consumed (range 3-98 g) on the day before sampling in eight social-drinking female and male subjects.
Accordingtothe self-report protocols, drinking usually occurred in the evening or
late afternoon. The dashed line represents the cutoff limit used to indicate an abnormal 5HTOL/5HIAA ratio (>15 pmol/nmol).
social drinkers after the ingestion of ethanol at 0.8 g/kg body weight. Accordingly, an increased methanol concentration is not specific for recent alcohol consumption, but may be useful for detection of chronic drinking
[27, 28].
The results of the present study demonstrate that the urinary 5HTOL/5HJAA ratio provides a more sensitive method to detect recent alcohol consumption compared with analysis of ethanol or methanol in blood, breath, or urine. The specificity for detecting alcohol was also very high with the 5HTOL/ 5HIAA ratio [29]. A dose-dependent increase in the urinary excretion of 5HTOL is seen after each drink, owing to compet-itive inhibition of ALDH by ethanol-derived acetaldehyde and (or) the change in hepatic redox state [30]. In contrast to methanol, the baseline value for 5HTOL/5HIAA is not in-creased after prolonged misuse and can therefore be used to identify recent drinking in both social drinkers and chronic consumers. The major advantage of 5HTOL/5HIAA compared with ethanol and methanol is that the serotonin metabolite ratio stays increased for several hours longer than ethanol and methanol, thereby increasing diagnostic sensitivity. However, the exact time available for detection of acute alcohol consump-tion by analysis of urine depends not only on the dose and time since the last drink, but also on voiding between the time of drinking and sampling.
To enhance the specificity of the marker, 5HTOL is ex-pressed as a ratio with 5HIAA rather than creatinine, because dietary serotonin might otherwise cause false-positive results owing to a general increase in the urinary output of serotonin metabolites [20].To discriminate between normal and increased values of the urinary 5HTOL/5HIAA ratio, the cutoff limit was originally set at 20 pmollnmol
[12].
However, after careful study of a large number of abstinent subjects of both Caucasian and Oriental origin [22/, a lower limit of 15 pmollnmol was intro-duced into clinical practice. Actually, all those subjects had ratios<10 pmol/nmol, but we used a higher cutoff to reduce the risk of reporting false positives. The ratio is stable both within day and between days during periods of abstinence [11, 12, 31], and both metabolites are relatively stable in urine samples during transport, handling, and storage [21, 31].Variation in ADH and ALDH isoenzyme pattern does not influence the 5HTOL/ 5HIAA basal value [22], despite the well-known genetic effects on the metabolism of ethanol [32]. The present results further indicate that gender and diabetic ketoacidosis do not markedly influence the urinary ratio of 5HTOL/5HIAA. Treatment with the alcohol-sensitizing drugs disulfiram (Antabuse#{174};Dumex, Copenhagen, Denmark) and cyanamide (Dipsan#{174};Lederle, Wayne, NJ), both potent inhibitors of ALDH, represent the only known cause for false positives [33]. However, the change in ratio resulting from taking ALDH inhibitors was less pro-nounced than after drinking alcohol. Moreover, large interindi-vidual differences in response were evident, and, in some subjects, there was no effect at all on the 5HTOL/5HIAA ratio. The effect of disulfiram lasted longer than for cyanamide, which is in good agreement with their respective clinical effects [34]. In conclusion, the possibility of detecting recent intake of alcohol by laboratory tests decreases in the order: urinary 5HTOL/5HIAA > urinary or plasma methanol > urinary ethanol > blood or breath ethanol. Measuring 5HTOL/5HIAA can detect consumption of even moderate amounts of alcohol within the preceding -24 h and, by frequent urine sampling, a detailed picture of a person’s drinking pattern can be obtained. This new biochemical marker can be useful for investigating single relapses in alcoholic outpatients, to prove a person actually has abstained from alcohol [35, 36], and possibly in connection with workplace alcohol testing, e.g., as a postacci-dent test of recent heavy drinking (possible impairment caused by hangover). Furthermore, 5HTOL is a much more objective measure than self-reported alcohol consumption when new treatment models and new biochemical markers of alcohol misuse are being evaluated. Further research should focus on the clinical usefulness of 5HTOL/5HIAA measurements for mon-itoring relapse in drinking.
This work was supported in part by the Karolinska Institute. We thank Ulla Lindstr#{246}mfor the collection of samples, and Tina Bur#{233}nius,Gun Jacobsson, and Catharina L#{246}wenmofor skillful technical assistance.
References
1. Fuller RK, Lee KK, Gordis E. Validity of self-report in alcoholism research: results of a Veterans Administration Cooperative Study. Alcohol Clin Exp Res 1988;12:201-5.
2. Ness DE, Ende J. Denial in the medical interview: recognition and management. JAMA 1994:272:1777-81.
3. Nilssen 0, Huseby NE, H#{248}yerG, Brenn T, Schirmer H, F#{248}rdeOH. New alcohol markers-how useful are they in population studies: the Svalbard Study 1988-1989. Alcohol Clin Exp Res 1992:16:
82-6.
4. Mihas AA, Tavassoli M. Laboratory markers of ethanol intake and abuse: a critical appraisal. AmJ Med Sci 1992;303:415-28.
5. Conigrave KM, Saunders JB, Whitfield JB. Diagnostic tests for
alcohol consumption. Alcohol Alcohol 1995:30:12-26.
6. Jones AW. Pharmacokinetics of ethanol in saliva: comparison with blood and breath alcohol profiles, subjective feelings of intoxica-tion, and diminished performance. Clin Chem 1993;39:1837-44. 7. Eriksson CJP, Fukunaga T. Human blood acetaldehyde (update
1992). Alcohol Alcohol 1993;2(Suppl):9-25.
8. KorriUM, Nuutinen H, Salaspuro M. Increased blood acetate: a new laboratory marker of alcoholism and heavy drinking. Alcohol Clin Exp Res 1985;9:468-71.
9. DavisyE, BrownH, HuffJA,Cashaw JL.The alteration of serotonin metabolism to 5-hydroxyttyptophol by ethanol ingestion in man. J Lab Clin Med 1967:69:132-40.
10. Beck 0, Borg S, Eriksson L, Lundman A. 5-Hydroxytryptophol in the cerebrospinal fluid and urine of alcoholics and healthy subjects. Naunyn Schmiedebergs Arch Pharmacol 1982:321:293-7.
Ii.. Helander A,Beck 0, Jacobsson G, L#{246}wenmoC, Wikstr#{246}mT. Time course of ethanol-induced changes in serotonin metabolism. Life Sd 1993:53:847-55.
12. Voltaire A,Beck 0, BorgS. Urinary5-hydroxytryptophol: a possible marker of recent alcohol consumption. Alcohol Clin Exp Res 1992;16:281-5.
13. Jones AW. Abnormally high concentrations of methanol in breath: a useful biochemical marker of recent heavy drinking [Letter]. Clin Chem 1986:32:1241.
14. Majchrowicz E, Mendelson JH. Blood methanol concentrations during experimentally induced ethanol intoxication in alcoholics. J Pharmacol Exp Ther 1971:179:293-300.
15. Glig T, von Meyer L, Liebhardt E. Zur Bildung und Akkumulation von endogenen Methanol unter Athanolbelastung. Blutalkohol 1987;24:321-32.
16. Jones AW, Sternebring B. Kinetics of ethanol and methanol in alcoholics during detoxification. Alcohol Alcohol 1992:27:641-7. 17. Haffner H-T, Batra A, Wehner HD, Besserer K,Mann K.
Methanol-spiegel und Methanolelimination bei Alkoholikern. Blutalkohol 1993:30:52-62.
18. Jones AW, Lovinger H.Relationship between the concentrations of ethanol and methanol in blood samples from Swedish drinking drivers. Forensic Sd Int 1988:37:277-85.
19. Jones AW, Schuberth J. Computer-aided headspace gas chroma-tography applied to blood-alcohol analysis: importance of on-line process control. J Forensic Sci 1989:34:1116-27.
20. Helander A, Wikstr#{228}mT, L#{228}wenmoC, Jacobsson G, Beck 0. Urinary excretion of 5-hydroxyindole-3-acetic acid and 5-hy-droxytryptophol after oral loading with serotonin. Life Sci 1992: 50:1207-13.
21. Helander A, Beck 0, Wennberg M, Wikstr#{246}mT, Jacobsson G. Determination of urinary 5-hydroxyindole-3-acetic acid by high-performance liquid chromatography with electrochemical detec-tion and direct sample injecdetec-tion. Anal Biochem 1991:196:170-3. 22. Helander A,Walzer C, Beck 0, Balant L, BorgS, von Warthurg J-P.
Genetic variation in alcohol dehydrogenase and aldehyde dehydro-genase and serotonin metabolism. Life Sci 1994;55:359-66.
23. Helander A,Beck 0, Borg S. The use of 5-hydroxytryptophol as an alcohol intake marker. Alcohol Alcohol 1994:2(Suppl):497-502.
24. Helander A, Beck 0, Jones AW. Distinguishing ingested ethanol from microbial formation by analysis of urinary 5-hydroxytryptophol and 5-hydroxyindoleacetic acid. JForensic Sd 1995:40:95-8.
25. Saady JJ, Poklis A, Dalton HP. Production of urinary ethanol after sample collection. JForensic Sci 1993:38:1467-71.
26. GrUner 0, Bilzer N. Zum Methanolgehalt von Fruchts#{228}ften.Seine Bedeutung bei der Begleitstoffanalyse. Blutalkohol 1983:20: 241-52.
624 Helander et al.: Lab testing for alcohol consumption
Methanol as a marker of alcohol abuse. Alcohol Clin Exp Res
1989:13:172-5.
28. Iffland R, Balling P, Borsch G, Herold C, Kaschade W, LOffler T, et al.Zur Wertung erh#{246}hterSpiegel von GGT, CDT, Methanol, Aceton und Isopropanol im Blut alkoholauffalliger Kraftfahrer. Blutalkohol 1994;31:273-314.
29. Helander A, Beck 0, Boysen L. 5-Hydroxytryptophol conjugation in man: influence of alcohol consumption and altered serotonin turnover. Life Sci 1995:56:1529-34.
30. Walsh Mi. Role of acetaldehyde in the interactions of ethanol with neuroamines. Adv Mental Sci 1973:3:233-66.
31. Helander A, Beck 0, Borg S. Determination of urinary 5-hy-droxytryptophol by high-performance liquid chromatography with electrochemical detection. J Chromatogr 1992:579:340-5.
32. Thomasson HR, Crabb DW, Edenberg Hi, Li T-K. Alcohol and aldehyde dehydrogenase polymorphism and alcoholism. Behav Genet 1993:23:131-6.
33. Beck 0, Helander A, Carlsson S. Borg S. Changes in serotonin metabolism during treatment with the aldehyde dehydrogenase inhibitors disulfiram and cyanamide. Pharmacol Toxicol 1995;77: 323-6.
34. Levy MS, Livingstone BL, Collins DM. A clinical comparison of disulfiram and calcium carbamide. Am J Psychiatry 1967;123: 1018-22.
35. Voltaire-Carlsson A, Hiltunen AJ, Beck 0, Stibler H, Borg S. Detection of relapses in alcohol-dependent patients: comparison of carbohydrate-deficient transferrin in serum, 5-hydroxytryptophol in urine, and self-reports. Alcohol Clin Exp Res 1993:17: 703-8.
36. Helander A, Voltaire-Carlsson A, Borg S. Longitudinal comparison of carbohydrate-deficient transferrin and gamma-glutamyl trans-ferase: complementary biochemical markers of excessive alcohol consumption. Alcohol Alcohol 1996;31:lOi.-7.