The Total Synthesis of the Potent Anti-Bacterial
Agent Platencin and Various Analogues
A thesis submitted for the Degree of Doctor of Philosophy
of the Australian National University
by
Muhammad Nasrullah Rehmani
Research School of Chemistry
Canberra, Australia
i
Declaration
I declare that, to the best of my knowledge, the material presented in this thesis represents the result of original work carried out by the author during my PhD tenure and has not been presented for examination for any other degree. This thesis is less than 100,000 words in length. Established methodologies have been acknowledged, wherever possible, by citation of the original publications from which they derive.
ii
Acknowledgements
Firstly, I would like to express my deep and sincere gratitude to my supervisor, Professor Martin Banwell, for his invaluable mentorship and guidance while I conducted the work presented in this thesis. I am very thankful for his enormous support, encouragement and guidance during my time at the Research School of Chemistry (RSC).
I also would like to thank Dr Alistair Draffan and co-workers of Biota Holdings Ltd, for their great support. The general and technical staff of the RSC, especially those members of the mass spectrometry unit, the NMR centre and the workshop are acknowledged for all their assistance. I am especially appreciative of Tony Herlt’s training and other help with the operation of the HPLC and GC facilities located in the School.
The assistance and great friendship of past and present members of the Banwell Group, especially my lab mates Dr David Bon, Nadia Gao, Sylvia Yang, Dr Bora Lee and Dr Ee Ling Chang are also gratefully acknowledged, as are those offered by post-doctorals Drs Brett Schwartz, Eliska M and Xinghua Ma. Drs Mukesh Sharma, Ehab Taher and Aymeric Cervi have also been great colleagues and especially close friends in RSC over the years – I will miss you guys.
iii
Publications and Presentations
The following list details the publications and presentations that have resulted from research performed during the author’s PhD candidature.
Publications:
1) Rehmani, M. N.; Draffan, A. G.; Banwell, M. G.; Willis, A. C. ‘A Second-Generation Chemoenzymatic Total Synthesis of Platencin’, Synlett 2016, 27, 61. 2) Rehmani, M. N.; Chang, E. L.; Draffan, A. G.; Banwell, M. G.; Willis, A. C.; Carr, P. C. ‘Chemoenzymatic Syntheses of Some Analogues of the Tricarbocyclic Core of the Anti-bacterial Agent Platencin and the Biological Evaluation of Certain of Their N-Arylpropionamide Derivatives’ Aust. J. Chem. 2018, 71, 655.
Presentations:
1) Rehmani, M. N.; Banwell, M. G. Chemoenzymatic Total Synthesis of Platencin and Certain Derivatives. Poster presentation at the Southern Highland Conference on Heterocyclic Chemistry, August 28-30, 2016, NSW, Australia.
Presentation Awards:
iv
Abstract
For thousands of years Nature has provided humankind with the means for treating diseases. In more recent times, traditional knowledge of medicinally important natural products has gradually been linked to advances in chemistry. As a result, new methods for the isolation, chemical characterization and biological evaluation of the active principals associated with medicinal plants and other organisms have been developed. Single-compound and efficacious therapeutic agents have thus emerged for the treatment of an extraordinary range of human afflictions.
In this context, the identification of new antibacterial agents showing efficacy against the rapidly growing number of drug-resistant bacteria is becoming a worldwide priority in medicine and healthcare. Platencin (9), a recently discovered secondary metabolite of Streptomyces platensis, exerts its anti-bacterial effects through a novel mode of action. Specifically, it is a potent and selective inhibitor of bacterial fatty acid synthases. As such this compound and certain co-metabolites have emerged as promising leads for the development of new-generation anti-bacterial therapies. There is even a suggestion that it could form the basis of anti-diabetic therapies. On this basis, and given its unusual molecular architecture, platencin has become the subject of intense scrutiny as target for chemical synthesis.
v Chapter One (the Introduction) of this thesis provides a brief historical account of some concepts/theories related to infectious diseases and associated development of various antibacterial regimes. A brief introduction to the chemistry and pharmacokinetic properties of platencin and the structurally related metabolite platensimycin then follows. The last section of this chapter details previously reported approaches to platencin related to the current studies.
Chapter Two mainly describes previous research carried out within the Banwell Group that involved assembling the tricarbocyclic framework of platencin via a type-1 intramolecular Diels-Alder (IMDA) cycloaddition reaction involving substrate 46 derived from starting material 80. A series of conventional chemical transformations was then applied to the adduct of the IMDA process and so affording compound 99 that embodies the carbocyclic core of platencin.
vi Chapter Four details the implementation of a range of structural modifications to the tricyclic core of platencin 99 and thus delivering a “library” of variants of this important motif.
The research outlined in Chapter Five is an extension of that described in the preceding one and focussed on the elaboration, through side-chain attachment, of certain variants of the platencin core to analogues of the natural product. The antibacterial properties of some of these analogues are also reported.
vii
Glossary
The following abbreviations have been used throughout this thesis:
δ chemical shift (parts per million) °C degree Celsius
λ wavelength (nm) μg microgram(s) μL microliter(s) Å Angstrom ))) ultrasonication Ac acetyl
AIBN 2,2’-azobis(iso-butyronitritle) aq. aqueous
Ar (unspecified) aryl group atm atmosphere
BHT 2,6-di-t-butyl-methylphenol Bn benzyl
br broad Bu butyl
n-Bu normal-butyl t-Bu tertiary-butyl Bz benzoyl
9-BBN 9-borabicyclo[3.3.1]nonane c concentration
ca. circa (approximately) cat. catalytic/catalyst cf. confer (compare) cm centimeter(s) conc. concentrated
COSY correlation spectroscopy d doublet
viii DMAP 4-(N,N-dimethylamino)pyridine
DMF N,N-dimethylformamide DMP Dess-Martin periodinane 2,2-DMP 2,2-dimethoxypropane DMSO dimethyl sulfoxide E entgegen (opposite)
e.g. exempli gratia (for example)
EI electron impact (mass spectrometry) equiv. equivalents
ES electrospray (mass spectrometry) Et ethyl
et al. et alia (and others) eV electron volt(s)
FGI functional group interconversion g gram(s)
GC gas chromatography gem germinal
h hour(s)
HATU O-(7-azabenzotriazol-1-yl)-N,N,N’,N’- tetramethyluronium hexafluorophosphate HMPA hexamethylphosphoramide
HPLC high pressure liquid chromatography HRMS high resolution mass spectrometry Hz Hertz
IMDA intramolecular Diels-Alder IBX 2-iodoxybenzoic acid IR infra-red
J coupling constant (Hz)
KHMDS potassium bis(trimethylsilyl)amide L litre(s)
ix LiHMDS lithium bis(trimethylsilyl)amide
lit. literature value m multiplet M molar
M+ molecular ion Me methyl MHz mega-Hertz
MIC minimal inhibitory concentration min minute(s)
mL milliliter(s) mmol millimole(s) mol mole(s)
m.p. melting point (°C)
MPO 4-methoxypyridine-N-oxide MS mass spectroscopy
m/z mass-to-charge ratio nm nanometer(s)
NMO N-methylmorpholine-N-oxide NMR nuclear magnetic resonance nOe nuclear Overhauser enhancement ORTEP Oak Ridge Thermal Ellipsoid Plot P (unspecified) protecting group
p para
PCC pyridinium chlorochromate PDC pyridium dichromate Ph phenyl
pH logarithm of the reciprocal of the hydrogen ion concentration, – log10[H+]
plc preparative layer chromatography ppm parts per million
x quant. quantitative
ref. reference
R unspecified alkyl group
Rf retardation factor (in thin layer chromatography) s singlet
SAR structure activity relationship SEM 2-(trimethylsilyl)ethoxymethyl t triplet
ter/t tertiary
TASF tris(dimethylamino)sulfonium difluorotrimethylsilicate TBAF tetra-n-butylammonium fluoride
TBS/TBDMS t-butyldimethylsilyl temp. temperature (°C)
TEMPO 2,2,6,6-tetramethyl-1-piperidinyloxy free radical Tf trifluoromethanesulfonyl
TFA trifluoroacetic acid THF tetrahydrofuran TIPS triiso-propylsilyl
tlc thin layer chromatography TMS trimethylsilyl
TsOH toluenesulfonic acid UV ultra violet (spectroscopy) viz. videlicit (that is, namely) vs versus
V volt
v/v unit volume per unit volume (ratio) νmax infra-red absorption maxima (cm−1) Z zusammen (together)
xi
Table of Contents
Chapter One: Platencin: A New Antibiotic ……….. 1
1.1 Introduction ………. 1
1.2 Isolation and Structural Elucidation of Platensimycin and Platencin …… 6
1.3 The Biological Profiles of Platensimycin and Platencin ………... 8
1.4 Proposed Biogenesis of Platencin ………. 10
1.5 Previous Studies on the Synthesis of (–)-Platencin ………. 13
1.5.1 Overview ………. 13
1.5.2 Total and Formal Total Syntheses ………. 13
Nicolaou’s Asymmetric Synthesis of (–)-Platencin………..………. 13
Banwell’s Formal Total Synthesis of (–)-Platencin ……… 16
Mulzer’s Total Synthesis of (–)-Platencin ………. 18
Banwell’s Total Synthesis of (–)-Platencin ………... 20
1.6 Summary ………... 24
1.7 References ……….. 25
Chapter Two: Synthesis of the Platencin Core……… 30
2.1 Introduction ………... 30
2.2 Synthesis of the Dienophile and Diene Required for the IMDA Reaction … 31
2.2.1 Synthesis of Triene 46, a Precursor for the IMDA Reaction, using Negishi Cross-Coupling Chemistry ……….. 32
2.3 The IMDA Reaction ……….. 33
2.3.1 Elaboration of the IMDA Adducts to the Tricarbocyclic Core of (–)-Platencin ……… 34
2.4 Summary ………. 39
2.5 References ………... 40
Chapter Three: A Chemoenzymatic Synthesis of Platencin ………. 42
3.1 Introduction ………. 42
3.2 Two-fold Alkylation, at C-4, of the IMDA Adduct 99 ………. 43
xii
3.3 Completion of a Synthesis of Platencin (9) ………... 49
3.3.1 Reductive Cleavage of the −OBz Group ………..….... 56
3.3.2 Deoxygenation of Acetate 113 ……….... 59
3.3.3 Completion of a Synthesis of Platencinic Acid (28) ………... 66
3.3.4 Completion of a Second-Generation Chemoenzymatic Total Synthesis of Platencin ……….... 68
3.3.5 Synthesis of The Fully Deprotected Aniline Subunit (21) …………. 69
3.4 Endgame: Completion of a Second-Generation Synthesis of Platencin (9) 72
3.5 Next Generation Synthesis of Platencin (9) – An Alternate Route... 75
3.6 Summary ………... 83
3.7 References ………. 84
Chapter Four: Structural Variants of the Platencin Core ……….. 86
4.1 Introduction ……….. 86
4.2 Structural Variants of the Platencin Core Being Targeted ………. 87
4.3 Synthesis of Core Analogue 147 ………. 88
4.4 Efforts to Establish a Synthesis of the Platencin Core Homologue 148 90
4.5 Synthesis of Platencin Core Analogue 149 ………... 93
4.6 The Widespread Prevalence of Cyclopropyl Groups in Antimicrobial Compounds ………. 95
4.6.1 Synthesis of the Cyclopropanated Core, 150, of Platencin …………. 97
4.7 Synthesis of the β-Alkylated Core Variant, 151, of Platencin …………... 101
4.8 Synthesis the “More Advanced” β-Alkylated Core, 152, of Platencin ……. 105
4.9 Synthesis of a Hydroxylated Core, 153, of Platencin ……….. 108
4.10 Syntheses of the “Reverse Alkylation” Variants 154, 155 and 156 of the Platencin Core ……… 108
4.11 Synthesis of Platencin Core 157 ………... 115
4.12 Summary ………... 121
xiii
Chapter Five: Completing Syntheses of Some Platencin Analogues
and Their Biological Evaluation………..…. 125
5.1 Introduction ……… 125
5.2 The Synthesis of the Compound 158, an Oxygenated Platencin Analogue 125 5.2.1 End Game: The Pivotal Amidation Reaction ………. 131
5.2.2 An Alternate Synthetic Approach to the Compound 158 ………... 132
5.3 Elaboration of the Tricarbocyclic Core of Platencin to Compound 159, a “Reverse Alkylation” Analogue ……… 133
5.4 The Synthesis of the Epimer, 160, of Compound 159 ……….... 136
5.5 The Biological Evaluation of Platencin Analogues 159, 160 and 200 …… 142
5.6 Summary ………. 144
5.7 References ……….….. 145
Chapter Six: Experimental Procedures Associated with the Work Described in Chapter Two to Five ………...……… 146
6.1 General Experimental Procedures ……… 146
6.2 Experimental Procedures for Work Reported in Chapter Two ……….... 149
6.3 Experimental Procedures for Work Reported in Chapter Three ……….. 164
6.4 Experimental Procedures for Work Reported in Chapter Four ………… 189
6.5 Experimental Procedures for Work Reported in Chapter Five …………. 216
6.6 References ……… 231
Appendices
…
……….……….. 232A.1 X-ray Crystal Structure Report for Compound 98 ……… 232
A.2 X-ray Crystal Structure Report for Compound 100 ………. 239
A.3 X-ray Crystal Structure Report for Compound 147 ………. 245
A.4 X-ray Crystal Structure Report for Compound 162 ………..…… 254
A.5 X-ray Crystal Structure Report for Compound 150 ………... 265
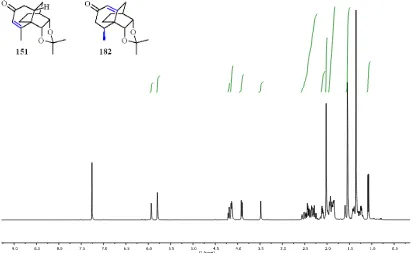
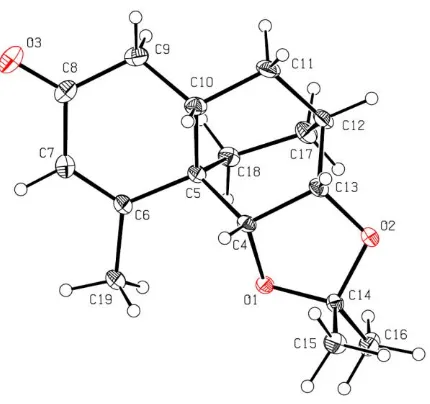
A.6 X-ray Crystal Structure Report for Compound 151 and 182 ………... 272
xiv A.8 Proposed mechanism for the dehydrogenation of enol ethers with the
IBX MPO complex ………... 289
A.9 Mechanistic rationalization of the SmI2-mediated reduction of
α-functionalized ketones ... 290
A.10 Probable mechanistic explanation for the reduction of α-functionalized carbonyl compound using a vanadium(II) complex ……… 291 A.11 Mechanistic explanation of Wharton rearrangement (transposition) …. 292 A.12 Copies of Publications ……….... 293
1) Rehmani, M. N.; Draffan, A. G.; Banwell, M. G.; Willis, A. C. ‘A Second-Generation Chemoenzymatic Total Synthesis of Platencin’,
Synlett 2016, 27, 61.
2) Rehmani, M. N.; Chang, E. L.; Draffan, A. G.; Banwell, M. G.; Willis, A. C.; Carr, P. C. ‘Chemoenzymatic Syntheses of Some Analogues of the Tricarbocyclic Core of the Anti-bacterial Agent Platencin and the Biological Evaluation of Certain of Their N-Arylpropionamide Derivatives’ Aust. J. Chem. 2018, 71, 655.
293
Chapter One – Platencin: A New Antibiotic
___________________________________________________
1 Chapter One
Platencin: A New Antibiotic
Introduction
Chapter One – Platencin: A New Antibiotic
___________________________________________________
2 consequence of the disease rather than the cause of it. Between 1845 and 1846 a mysterious disease wiped out silkworms around the world. Louis Pasteur proved that this chronic silkworm disease (pebrine) was caused by microorganisms found only in the tissues of diseased silkworms and their eggs. These tiny organisms could be seen using a microscope and he found a way of defeating the disease by identifying and then destroying the infected eggs and thus saving the silk industry. This discovery was the first convincing evidence that microorganisms were the cause of disease.7
The advent of the germ theory of disease, which suggested that microorganisms are the main cause of many diseases, brought about a revolutionary change in the understanding of the vital role of microbes in infectious diseases. Specific microbial pathogens were identified as the causative agents of some diseases and a race then began almost immediately to find effective means of killing the implicated microbes. Ernest Duchesne, who enrolled in medical school in 1894 and graduated in 1897 from L’Ecole du Service de Santé Militaire in Lyon, observed, while still a student, that the Arab stable boys at the army hospital stored their saddles in a dark and damp area so as to promote the growth of mould on them and did so because this microorganism helped to heal saddle sores on horses. As a result, Duchesne prepared a solution of the mould (now known to be Penicillium glaucum) and tested it as a possible cure for typhoid in guinea pig. In the event, he found that a competition for survival occurred between the mould and bacterium causing typhoid and such that the guinea pig did not die. This established the effectiveness of the mould as a means for killing certain bacteria. He concluded that this phenomenon could be further exploited for therapeutic use but, unfortunately, these observations were not pursued by the Pasteur Institute8 (then a renowned centre with the appropriate facilities and focused on research into infectious diseases).
Chapter One – Platencin: A New Antibiotic
___________________________________________________
3 he developed the first narrow-spectrum antibiotic, salvarsan (1), also named as arsphenamine or Ehrlich 606, for the treatment of syphilis and trypanosomiasis.9
Chapter One – Platencin: A New Antibiotic
___________________________________________________
4 Prontosil proved to be the first drug effective against bacterial infection and in 1939 Domagk earned the Nobel Prize in Physiology/Medicine for his achievements.
As noted earlier, in 1896 Ernest Duchesne discovered the antimicrobial properties of Penicillium. However, he failed to recognise that the fungus was producing a substance that had antibacterial properties. As a result, Penicillium was forgotten in the scientific community until Sir Alexander Fleming, a Scottish bacteriologist, discovered the antibiotic penicillin in 1928.12 The discovery that this antibiotic, produced by the fungus Penicillium notatum, was an effective treatment for bacterial infections such as gangrene, syphilis, and tuberculosis created a revolution. The discovery was entirely accidental. While conducting an experiment on the influenza virus Fleming observed that a common fungus, Penicillium notatum, had destroyed bacteria in a staphylococcus culture plate. Upon further investigation, he found that this antibacterial agent was effective even when diluted up to 800 times and he named the active ingredient penicillin and named the producing fungus “penicillium”. The latter name derives from the Latin “penicillus” meaning “a painter’s brush” because the “fronds” of this fungus resemble tiny paint brushes (the word pencil has the same origins). Fleming published the results of his research in 1929 and mentioned that penicillin might have therapeutic value if it could be produced in large amount. He was knighted in 1944 and awarded the Noble Prize in Medicine in 1945 for his extraordinary achievements that revolutionized the medical world. The penicillins proved to be β-lactam-containing compounds that act as suicide substrates for peptidoglycan transpeptidases involved in crosslinking the bacterial cell wall.13
Chapter One – Platencin: A New Antibiotic
___________________________________________________
5 well underway and an effective antibiotic was desperately needed to treat injured soldiers. In 1941, and in collaboration with Merck and the US Department of Agriculture, the mass production of penicillin was achieved. Penicillin was tested for military use in World War II and soon it became known as the “miracle drug,” by curing many an infectious disease and thus saving millions of soldiers’ lives.7,15 Later, Australia became the first country to make the drug available for civilian use. Penicillin proved effective against bacterial infections like pneumonia, syphilis, tuberculosis, gangrene and diphtheria. Indeed, penicillin conquered bacterial infection after bacterial infection to the extent that by the 1960s and 70s it was thought that the war against infectious diseases had been won. Notwithstanding such optimism, Alexander Fleming predicted, in a 1945 speech to the American Association of Penicillin Producers in New York, that as a result of the misuse of the drug “The microbes are educated to resist penicillin and a host of penicillin-fast organisms is bred out which can be passed to other individuals and perhaps from there to others until they reach someone who gets a septicaemia (invasion of the bloodstream by virulent microorganisms/bacteria) or a pneumonia which penicillin cannot save.”16 At about the same time, in the 1960s and 1970s, the science community found that many bacterial strains had already developed resistance by evolving enzymes, such as β-lactamase, that could metabolize the drug before it reached its biological target.17 The discovery of new antibacterial agents boomed between the 1950s and 1970s but since then no significant new classes of antibiotics have been developed by the research community. Instead, the main focus of research revolved around the relatively trivial modification of existing antibiotics to either counter their side effects or reduce their toxicities and so, with few exceptions, new antibiotics were only “next-generation” versions of established drugs.
Since the 1940s these drugs have been used so extensively and for so long that the organisms theseantibiotics are designed to kill have adapted to them with the result that many of these drugs have been rendered ineffective. As a result, the world is now facing a (potentially disastrous) future without effective antibiotics. This is, of course, because
pre-Chapter One – Platencin: A New Antibiotic
___________________________________________________
6 antibiotic era. As testimony to the gravity of this situation, in the last few decades approximately 13 million deaths related to infectious diseases have been reported worldwide according to World Health Organization (WHO) data. So the harsh reality is that the emergence of multidrug-resistant bacteria is a serious and urgent threat in contemporary society to the extent, as revealed in a recent study,18 that approximately 440,000 new cases of multi drug-resistant tuberculosis occur every year with 150,000 of these resulting in death. Each year in the United States alone at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die as a result. Accordingly, renewed efforts to discover new classes of antibiotics with novel modes of action are urgently needed. A high-level meeting on antimicrobial resistance at the United Nations General Assembly was held on 21 September 2016 to accelerate global commitments and enhance national multi-sectoral efforts to combat this global issue. The identification of new antibacterial agents showing efficacy against the rapidly growing number of drug-resistant bacteria is becoming a worldwide priority in the medical and healthcare sectors. A range of possible solutions to this profoundly challenging situation has been suggested,19 not the least being the identification of new, structurally unusual antibacterial agents displaying novel modes of action. In 2014 the UK Prime Minister commissioned “The Review on Antimicrobial Resistance” (AMR) and asked the chairperson, Jim O’Neill, to analyse the global issue of drug resistance and suggest some measures to tackle it. The final report (2016) recommended curtailing unnecessary use of existing antibiotics and increasing the supply of new ones. It also referred to the need for public awareness campaigns, the need to improve sanitation and hygiene, reduce pollution, introduce rapid diagnostic techniques and vaccines, the need to involve more people in this area and increase funding to generate more drugs. 1.2 Isolation and Structural Elucidation of Platensimycin and Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
7 The high degree of conservation of many of the component enzymes of the FAS II hold promise for the development of broad spectrum antibacterial agents. Moreover, the significant differences between the bacterial fatty acid synthase (FAS II) and the human fatty acid synthase (FAS I) system makes the bacterial FAS II an attractive target for antibacterial drug discovery.21 Systematic screening of 250,000 natural product extracts (83,000 strains examined in three growth conditions) led to the discovery of the potent and highly selective antibacterial agent platensimycin (8).22 This broad spectrum antibiotic acts against Gram-positive bacteria and was isolated as an amorphous powder from a strain of Streptomyces platensis MA 7327 derived from a soil sample collected in Eastern Cape, South Africa. Its structure was established using 2D NMR spectroscopic and mass spectrometric techniques and then confirmed, apart from its absolute configuration, by single-crystal X-ray analysis.23
Chapter One – Platencin: A New Antibiotic
___________________________________________________
8 Platensimycin is comprised of two distinct subunits, namely a hydrophilic 3-amino-2, 4-dihydroxybenzoic acid moiety that is linked, via an amide bond, to a hydrophobic oxabicyclo[3.2.1]octane-containing unit 10. Platencin on the other hand, contains a somewhat different hydrophobic core but the same hydrophilic 3-amino-2,4-dihydroxybenzoic acid residue. In this case the core is comprised of a cyclohexenone-fused bicyclo[2.2.2]octane framework 11. Accordingly, the significant variation in the biological profiles of these two newly discovered antibiotics must be attributed to rather subtle structural differences associated with these two core units.
1.3 The Biological Profiles of Platensimycin and Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
9 binding to the related enzyme FabH. These have culminated in the discovery of platencin and platensimycin.
Using high-throughput screening and in vitro experiments, it was found that both platencin and platensimycin exhibited broad-spectrum Gram-positive antibacterial activity against methicillin resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci faecalis (VREF) and extensively drug-resistant Mycobacterium tuberculosis.27 In addition, platencin exhibited significant activity against vancomycin-resistant Enterococcus faecium (with a MIC of < 0.06 μg/mL) and efflux-negative Eschericha coli (tolC) (with a MIC of 2 μg/mL). However, platencin was four times less effective against Streptococus pneumonia (MIC = 4 μg/mL) as compared with platensimycin. Platensimycin is believed to be less active against Gram-negative bacteria because of the operation of a selective extrusion mechanism.18
Chapter One – Platencin: A New Antibiotic
___________________________________________________
10 The in vivo efficacies of platencin and platensimycin were found to be very poor due to their limited metabolic stabilities.18,20d While there has been debate about the likely clinical benefit of inhibitors of fatty acid biosynthesis (FAS II) due to the presence of potentially ‘recruitable’ extracellular fatty acids, evidence is now emerging of their utility in vivo.28 Accordingly, platensimycin (8) and platencin (9) are regarded as important new leads in the development of urgently required, clinically effective new-generation antibacterial agents.29 In principle, the unfavourable pharmacokinetic profile of these compounds could be improved through an appropriate analoguing program.
1.4Proposed Biogenesis of Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
13 1.5Previous Studies on the Synthesis of (–)-Platencin
1.5.1 Overview
The unusual and (relatively) complex molecular structures and promising therapeutic profiles of platensimycin and platencin have resulted in their attracting significant interest as synthetic targets and several elegant routes to them have emerged as a result.31-47 Certain of these syntheses (most especially ones involving IMDA approaches), which are relevant to the work in this thesis, are discussed below.
1.5.2Total and Formal Total Syntheses
Nicolaou’s Asymmetric Synthesis of (–)-Platencin31
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
15 Reaction of compound 33 with the cuprate derived from allylmagnesium chloride resulted in a conjugate addition reaction and reduction of the product ketone using NaBH4 afforded a 2:1 mixture of the corresponding and diastereomerically-related alcohols. This mixture was converted into the corresponding mixture of xanthate esters 34 that was then treated with n-Bu3SnH and AIBN to bring about the pivotal homoallyl radical rearrangement reaction and thereby give the bicyclo[2.2.2]octadiene 35. This was contaminated with about 15% of a by-product presumed to come from a 5-exo-trig cyclization onto the pendant allyl group. Wacker oxidation50 of compound 35 and subsequent cleavage of the SEM protecting group afforded the corresponding hemiacetal 36. This last compound was subjected to a Ley-Griffith oxidation51 to afford the corresponding keto-aldehyde and exposure of this to ethanolic NaOH generated, through an intramolecular aldol condensation reaction, the desired enone 11 in 99% yield. The conversion of enone 11 into the desired carboxylic acid 28 started with the two-fold enolate alkylation reaction of the former compound with methyl iodide then allyl iodide. The resulting triene 37 underwent regioselective cross metathesis with vinyl pinacol boronate 38 in the presence of the 2nd generation Hoveyda-Grubbs’catalyst and benzoquinone to furnish boronate 39 as a ca. 3:1 mixture of E- and Z- isomers. Oxidation of the resulting boronate 39 with trimethylamine N-oxide followed by Pinnick oxidation of the aldehyde thus formed then afforded platencinic acid (28). The coupling of acid 28 with the aniline 40 was carried out using HATU [O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate] and Et3N in DMF at ambient temperature to afford the desired TMSE-protected derivative of platencin. Removal of the TMSE ether residue within this product was effected using TASF and so giving the natural or (–)-enantiomeric form of platencin in 22 steps and 0.76% overall yield.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
16 Barnwell’s Formal Total Synthesis of (–)-Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
keto-Chapter One – Platencin: A New Antibiotic
___________________________________________________
18 dibenzoate 51. Reductive removal of the benzoyloxy group adjacent to the ketone moiety using samarium iodide furnished the corresponding keto-ester, Wittig methylenation of which afforded the olefin 52 in 84% yield. The ester residue within compound 52 was saponified and oxidation of the resulting alcohol was effected using IBX and so generating ketone 53 in 95% yield.
Completion of the synthesis of the desired enone 11 required dehydrogenation of compound 53 and this was achieved following protocol defined by Lalic and Corey in their synthesis of platensimycin.53 This involved conversion of compound 53 into the corresponding TMS enol ether using Me3N and trimethylsilyl triflate (TMSOTf) and oxidation of the resulting silyl enol ethers with IBX and 4-methoxypyridine-N-oxide (MPO). The acquisition of enone 11 in 64% yield by such means represents a formal total synthesis of (–)-platencin by virtue of it being a late-stage intermediate associated with Nicolaou’s first synthesis of the natural product.31 This synthesis had certain desirable attributes. For example, by virtue of the highly enantioselective enzymatic process used to produce the c-DHC starting material, this synthesis produces a product of higher enantiomeric purity (>99% ee) than the asymmetric catalytic techniques used in Nicolaou synthesis of platencin (93%).
Mulzer’s Total Synthesis of (–)-Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
20 Banwell’s Total Synthesis of (–)-Platencin
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter One – Platencin: A New Antibiotic
___________________________________________________
23
Scheme 1.4:Reagents and Conditions: (i) HCO2H, MeOH, 18 °C, 3.5 h; (ii) 2-methoxypropene, p-TsOH•H2O, 18 °C, 2 h; (iii) N2H4, 120 °C, 2 h; (iv) (CF3CO)2O, C6H6, 80 °C, 5 h; (v) Lithium
diisopropylamide (LDA), THF, –78 to 18 °C, 16 h; (vi) 2 M aq. HCl, THF, 18 °C, 5 h; (vii) HCCMgBr, THF, –10 °C, 0. 5 h; (viii) Bu2Sn(OTf)H, hexane, 18 °C, 16 h; (xi) nBuLi, Et2O,
18 °C, 0.25 h; (x) [Pd(PPh3)4], CuI, CsF, dimethylformamide (DMF), 18 °C, 16 h; (xi) DMP,
pyridine, CH2Cl2, 0 to 18 °C, 1.5 h; (xii) toluene, 112 °C, 16 h; (xiii) H2 (1 atm), 5% Pd on C,
MeOH, 18 °C, 16 h; (xiv) 1 M aq. HCl, acetone, 0.25 h, 18 °C; (xv) PCC, NaOAc, CH2Cl2,
18 °C, 2 h; (xvi) NaClO2, NaH2PO4, 2 methyl-2-butene, t-BuOH/water, 18 °C, 1 h; (xvii)
DOWEX-50, MeOH/water, 65 °C, 48 h; (xviii) 4-NHAc-TEMPO, p-TsOH•H2O, CH2Cl2, 0 to
18 °C, 4 h; (xix) BzCl, DMAP, Et3N, CH2Cl2, 0 to 18 °C, 16 h; (xx) SmI2, THF, –78 °C, 0.25
h; (xxi) Ph3P=CH2, THF, 0 °C, 0.5 h; (xxii) TMSCl, LiI, KHMDS, CH2Cl2, 18 °C, 4 h; (xxiii)
IBX, NMO, DMSO, 60 °C, 16 h; (xxiv) 1 M aq. NaOH, THF, 18 °C, 24 h; (xxv) DCC, Et3N,
DMAP, MeCN, DMF, 18 °C, 38 h.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
24 demonstrate the utility of IMDA reactions involving substrates of the general form 70 (keto-form) and their conversion into adducts 71 incorporating a suitably constituted quaternary carbon centre adjacent to the carbonyl residue. Unfortunately, owning to certain difficulties, the reported reaction sequence required at total of nineteen steps.
1.6 Summary
Chapter One – Platencin: A New Antibiotic
___________________________________________________
25 1.7 References
1) (a) Forrest, R. D. J. R. Soc. Med., 1982, 75, 198; (b) Lindblad, W. J. Int. J. Low. Extrem. Wounds. 2008, 7,2, 75; (c) Wainwright, M. Mycologist. 1989. 3, 1, 21.
2) (a) Last, J. M. “Miasma Theory.” Encyclopedia of Public Health. Ed. Lester Breslow. 2001, New York: Macmillan Reference: 765; (b) Demaitre, L. Bull. Hist. Med. 2004, 78, 466; (c) Halliday, S. BMJ. 2001, 323, 7327, 1469; (c) Robert, H. F. Epidemiology 101, 2nd Ed; Chapter 6, 131, Jones and Bartlett Learning, 2018. 3) Byrne, J. P. “Daily Life during the Black Death.” Westport, CT: Greenwood Press:
London. 2004, 23.
4) Cook, G. C. Postgrad. Med. J. 2001, 77, 914, 802.
5) (a) Pasteur, L. Science. 1881, 2, 62, 420; (b) Conn, H. W. Science. 1888, 11, 257, 5; (c) Gaynes, R. P. Germ theory: medical pioneers in infectious diseases, ASM Press, 2011, 143.
6) Gest, H. Notes Rec. R. Soc. London. 2004, 58, 2, 187.
7) (a) Amsterdamska, O. International Encyclopedia of Public Health, 2008, 282; (b) Pasteur, L. Science. 1881, 2, 62, 420; (c) Conn, H. W. Science. 1888, 11, 257, 5.
8) (a) Pouillard, J. Hist. Sci. Med, 2002, 36, 1, 11; (b) Serge, D. The Lancet, 1999, 354, 9195, 2068; (c) “The History of the Germ Theory”, The Br. Med. J. (The BMJ). 1888, 1415, 312.
9) Ehrlich, P.; Bertheim, A. Berichte der Deutschen Chemischen Gesellschaft (European Journal of Inorganic Chemistry). 1912, 45,756; (b) Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz. B. M. J. Am. Chem. Soc., 2006, 128, 11348; (c) Riethmiller, S. Bull. Hist. Chem. 1999, 23, 28.
10)Raju, K.; Tonse, N. The Lancet (British edition), 1999, 353, 9153, 681.
11)(a) Fuller, A. T. The Lancet 1937, 1, 194; (b) Jourova, L.; Anzenbacher, P.; Anzenbacherova, E. Biomed Pap Med Fac Univ Palacky Olomuc Czech Repub. 2016, 160, 3, 317; (c) Fouts, J. R.; Kamm, J. J. Brodie, B. B. J Phrmacol Exp Ther. 1957, 120, 3, 291.
12)(a) Drews J. Drug discovery: a historical perspective, Science. 2000, 287, 5460, 1960; (b) Fleming, A. Br. J. Exp .Pathol., 1929, 10, 3, 226.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
26 Goldsworthy, P. D.; McFarlane. A. C. Med. J. Aust. 2002, 176, 178; (e) Wong, J. Prim. Care Update Ob Gyns. 2003, 10, 3, 124; (f) Chain, E. Trends. Biochem. Sci., 1979, 4, 6, 143.
14)Chain, E.; Florey, H. W.; Gardner, A. D.; Heatley, N. G.; Jennings, M. A.; Orr-Ewing, J.; Sanders, A.G. The Lancet, 1940, 236, 6104, 226; (b) Ligon, B. L. Semin Pediatr Infect Dis. 2004, 15, 1, 58; (c) Berger, H. Clin. Cardiol. 1989, 12, 110. 15)Radetsky, M. Pediatr. Infect Dis. J. Aust. 2002, 176, 178.
16)(a) Fleming A. Speech at a banquet in his honour at the Waldorf Astoria, New York, 25 June, 1945. American Association of Penicillin Producers. Typewritten. British Library Add. MS 56122, 232; (b) “Resistance in Futile”, British Library, retrieved on (http://britishlibrary.typepad.co.uk/science/2015/03/resistance-is-futile.html accessed on 01/09/2018); (c) Stolberg, S.G. 1998. “Superbugs”, New York Times (Magazine, retrieved on http://www.nytimes.com/1998/08/02/magazine/ Superbugs. html (accessed on August 24, 2010).
17) Abraham, E. P.; Chain, E. Nature. 1942, 149, 328; (b) Neu, H. C. Science. 1992, 257, 1064.
18)Allahverdiyev, A. M.; Bagirova, M.; Abamor, E. S.; Ates, S. C.; Koc, R. C.; Miraloglu, M.; Elcicek, S.; Yaman, S.; Unal, G. Infect. Drug Resist. 2013, 6, 99. 19)(a) Payne, D. J.; Gwynn, M. N.; Holmes, D. J.; Pompliano, D. L. Nat. Rev. Drug
Discov. 2007, 6, 29; (b) Lewis, K. Nature (London, U.K.) 2012, 485, 439; (c) Lewis, K. Nat. Rev. Drug Discov. 2013, 12, 3781; (d) Reardon, S. Nature (London, U.K.) 2015, 521, 402; (e) Garber, K. Nat. Rev. Drug Discov. 2015, 14, 445.
20)(a) Campbell, J. W.; Cronan, J. E. Annu. Rev. Microbiol. 2001, 55, 305; (b) Heath, R. J.; Rock, C. O. Curr. Opin. Investig. Drugs. 2004, 5, 146; (c) Zhang, Y.-M.; Marrakchi, H.; White, S. W.; Rock, C. O. J. Lipid Res. 2003, 44, 1; (d) Hӓbich, D.; Von Nussbaum, F. Chem Med Chem. 2006, 1, 951; (e) Wright, H. T.; Reynolds, K. A. Curr. Opin. Microbiol. 2007, 10, 5, 447; (f) White, S. W.; Zheng, J.; Zhang, Y. M.; Rock, C. O. Annu. Rev. Biochem. 2005, 74, 791.
21)Brinster, B.; Lamberet, G.; Trieu-Cuot, S. P.; Gruss, A.; Poyart, C. Nature 2009, 458, 83.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
27 Jayasuriya, H.; Ondeyka, J.; Herath, K.; Zhang, C.; Hernandez, L.; Allocco, J.; Basilio, A.; Tormo, J. R.; Genilloud, O.; Vicente, F.; Pelaez, F.; Colwell, L.; Lee, S. H.; Michael, B.; Felcetto, T.; Gill, C.; Silver, L. L.; Hermes, J. D.; Bartizal, K.; Barrett, J.; Schmatz, D.; Becker, J. W.; Cully, D.; Singh, S. B. Nature 2006, 441, 358.
23)Singh, S. B.; Jayasuriya, H.; Ondeyka, J. G.; Herath, K. B.; Zhang, C.; Zink, D. L.; Tsou, N. N.; Ball, R. G.; Basilio, A.; Genilloud, O.; Diez, M. T.; Vicente, F.; Pelaez, F.; Young, K.; Wang, J. J. Am. Chem. Soc. 2006, 128, 11916.
24)Smanski, M. J.; Peterson, R. M.; Rajski, S. R., Shen, B. Antimicrob. Agents Chemother., 2009, 53, 4, 1299.
25)(a) Revill, W. P.; Bibb, M. J.; Scheu, A.-K.; Kieser, H. J.; Hopwood, D. Q. J. Bacteriol. 2001, 183, 3526; (b) Lai, C.-Y.; Cronan, J. E. J. Biol. Chem. 2003, 278, 51494.
26)Schujman, G. E.; Choi, K.-H.; Altabe, S.; Rock, C. O.; Mendoza, D. de; J. Bacteriol. 2001, 183, 3032; (b) Price, A. C.; Choi, C.-H.; Heath, R. J.; Li, Z.; White, S. W.; Rock, C. O. J. Biol. Chem. 2001, 276, 6551.
27)Moustafa, G. A. I.; Noima, S.; Yamano,Y.; Aono, A.; Arai, M.; Mitarai, S.; Tanaka, T.; Yoshimitsu, T. Med. Chem. Commun. 2013, 4, 720.
28)Parson, J. B.; Yao, J.; Frank, M. W.; Rock, C. O. Antimicrob. Agents Chemother. 2015, 59, 849; and references cited therein.
29)For some reviews see: (a) Tiefenbacher, K.; Mulzer, J. Angew.Chem. Int. Ed. 2008, 47, 2548; (b) Palanichamy, K.; Kaliappan, K.P. Chem. Asian J. 2010, 5, 668; (c) Martens, E.; Deamin, A. L. J. Antibiot. 2011, 64, 705; (d) Saleem, M.; Hussain, H.; Ahmed, I.; van Ree, T.; Krohn, K. Nat. Prod. Rep. 2011, 28, 1534; (e) Allahverdiyev, A. M.; Bagirova, M.; Abamor, E. S.; Ates, S. C.; Koc, R. C.; Miralogu, M.; Elcicek, S.; Yaman, S.; Unal, G. Infect. Drug. Resist. 2013, 6, 99.
30)(a) Herath, K. B.; Attygalle, A. B.; Singh, S. B. J. Am. Chem. Soc. 2007, 129, 15422; (b) Herath, K. B.; Attygalle, A. B.; Singh, S. B. Tetrahedron Lett. 2007, 48, 5429. 31)Nicolaou, K. C.; Tria, G. S.; Edmonds, D. J. Angew. Chem. Int. Ed. 2008, 47, 1780. 32)Hayashida, J.; Rawal, V. H. Angew. Chem. Int. Ed. 2008, 47, 4373.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
28 34)Waalboer, D. C. J.; Schaapman, M. C.; Van Delft, F. L.; Rutjes, F. P. J. T. Angew.
Chem. Int. Ed. 2008, 47, 6576.
35)Nicolaou, K. C.; Toh, Q. Y.; Chen, D. Y. J. Am. Chem. Soc. 2008, 130, 11292. 36)Austin, K. A. B.; Banwell, M. G.; Willis, A. C. Org. Lett. 2008, 10, 4465. 37)Varseev, G. N.; Maier, M. E. Angew. Chem. Int. Ed. 2009, 48, 3685. 38)Ghosh, A. K.; Xi, K. Angew. Chem. Int. Ed. 2009, 48, 5372.
39)Tiefenbacher, K.; Mulzer, J. J. Org. Chem. 2009, 74, 2937.
40)Nicolaou, K. C.; Tria, G. S.; Edmonds, D. J.; Kar, M. J. Am. Chem. Soc. 2009, 131, 15909.
41)Li, P.; Yamamoto, H.; Chem. Commun. 2010, 46, 6295.
42)Yoshimitsu, T.; Nojima, S.; Hashimoto, M.; Tanaka, T. Org. Lett. 2011, 13, 3698. 43)Hirai, S.; Nakada, M. Tetrahedron. 2011, 67, 518.
44)Yadav, J. S.; Goreti, R.; Pabbaraja, S.; Sridhar, B. Org. Lett. 2013, 15, 3782. 45)Zhu, L.; Zhou, C.; Yang, W.; He, S.; Cheng, G. J.; Zhang, X.; Lee, C. S. J.Org.
Chem. 2013, 78, 7912.
46)Singh, V.; Das, B.; Mobin, S. M. Synlett 2013, 24, 1583.
47)Chang, E. L.; Schwartz, B. D.; Draffan, A. G.; Banwell, M. G.; Willis, A. C. Chem. Asian. J. 2015, 10, 427.
48)Nicolaou, K. C.; Li, A.; Edmonds, D. J., Angew. Chem., Int. Ed. Engl., 2006, 45, 7086.
49)Nicolaou, K. C.; Edmonds, D. J.; Li, A.; Tria, G. S., Angew. Chem., Int. Ed. Engl., 2007, 46, 3942.
50)Park, P. K.; S. J. O’Malley, S. J.; Schmidt, D. R.; Leighton, J. L. J. Am. Chem. Soc. 2006, 128, 2796; (b) Smidt, J.; Sieber, R. Angew. Chem. 1959, 71, 261; (c) Takacs, J. M.; Jiang, X. -t. Curr. Org. Chem. 2003, 7, 369; (d) Tsuji, J. Synthesis 1984, 369. 51)Griffith, W. P.; Ley, S. V.; Whitcombe, G. P. White, A. D. J. Chem. Soc. Chem.
Commun. 1987, 1625.
52)Ma, Z.; Bobbitt, J. M. J. Org. Chem. 1991, 56, 6110. 53)Lalic, G.; Corey, E. J. Org. Lett., 2007, 9, 4921.
54)Mino, T.; Saitoh, M.; Yamashita, M. J. Org. Chem. 1997, 62, 3981. 55)Nordin, I. C.; Thomas, J. A. Tetrahedron Lett. 1984, 25, 5723.
Chapter One – Platencin: A New Antibiotic
___________________________________________________
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
30 Chapter Two
Synthesis of the Platencin Core
2.1 Introduction
As detailed in the Introduction, in 2008 a formal total synthesis of platencin was achieved by Banwell and co-workers.1 This involved an IMDA approach to establish the 1,2-cyclohexannulated bicyclo[2.2.2]octane framework 11 in an enantioselective manner. A pivotal aspect of this work was the use of the enzymatically-derived and enantiomerically pure cis-1,2-dihydrocatechol 80 as starting material.
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
31 2.2 Synthesis of the Dienophile and Diene Required for the IMDA Reaction
The first stage of the author’s efforts (Scheme 2.1) were concerned with the synthesis of the dienophile side-chain required in the substrate to be used in the IMDA reaction. To this end, a procedure established by Hutchison et al.2 was followed and involved treating allyl bromide 81 and ethyl formate 82 with magnesium turnings in dry THF. The dienol 41 (87%) thus obtained was then readily converted into the corresponding and previously reported tert-butyldimethylsilyl (TBDMS) ether 83 (91%). The next step involved conversion of diene 83 into alcohol 42 by treating the former compound with a controlled supply of ozone3 in the presence of 2,6-lutidine.ɠ The yield of alcohol 42 was maximised by monitoring the progress of the reaction by TLC. After warming the reaction mixture to 0 ºC, granular NaBH4 was added portion-wise over 1 h. This procedure reliably afforded a chromatographically separable mixture of the staring material 83 (11% recovery), the unwanted diol 43 (46% at 89% conversion) and the required mono-ol 42 (48% at 89% conversion) which was obtained in racemic form. This sequence is superior to the previous1 one, at least in the sense that comparable results were obtained by replacing the four-fold cheaper and less hazardous reagent TBSOTf with TBDMSCl.
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
32 Alcohol 42 was subjected to an Appel reaction6 involving its treatment with molecular iodine in the presence of triphenylphosphine and imidazole (Scheme 2.1) and thus providing the corresponding and required iodide 84 (78%). Once again, some useful modifications to the previous method1 were identified including lowering the reaction temperature from 18 °C to 0 °C and, in particular, replacing the original solvent benzene with THF. Pleasingly, a slightly better yield was observed upon employing these modifications. The diene necessary for coupling with compound 84 was obtained by converting the enantiomerically pure cis-1,2-dihydrocatechol780 into its corresponding acetonide1,8 45 (95%)through treatment of the former compound (Scheme 2.2) with 2,2-dimethoxypropane (2,2-DMP) and in the presence of catalytic amounts of p-toluenesulfonic acid (p-TsOH).
2.2.1 Synthesis of Triene 46, a Precursor for the IMDA Reaction, using Negishi Cross-Coupling Chemistry
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
33 The conditions required for achieving this coupling successfully were rather specific. So, for example, if a THF rather than an ethereal solution of ZnCl2 was used a variable mixture (Figure 2.1) of products 85 - 87 was formed along with compound 46.10
2.3 The IMDA Reaction
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
34 intramolecular variants of such processes are rare, one reason being the necessary but challenging task of tethering a dienophile to the fragile c-DHC core. While rather acid sensitive, triene 46 was readily engaged in an IMDA reaction by heating it in refluxing toluene (Scheme 2.4). By such means a 10:10:1:1 mixture of the four endo-type cycloaddition products 47, 48, 88 and 89 was obtained in 97% combined yield. The major pair of cycloadducts, namely compounds 47 and 48, was generated via an anti-addition process involving the dienophile approaching from the less hindered face of the diene. The cycloaddition reaction leading to the minor pair of adducts, 88 and 89, proceeded via approach of the tethered dienophile to the sterically more hindered face of diene, viz the same face as occupied by the acetonide moiety. Subjection of this mixture of adducts to column chromatography allowed for the isolation of clean samples of cycloadducts 48 and 88. In contrast, congeners 47 and 89 were obtained as an inseparable mixture. The lack of activation of the dienophilic portion of substrate 46 meant that there was concern about whether or not an endo-selective cycloaddition reaction would take place and whether the activation energy required to initiate such a process might be prohibitively high.
2.3.1 Elaboration of the IMDA Adducts to the Tricarbocyclic Core of (–)-Platencin
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
35 (13%), that could now be readily separated from one another using conventional chromatographic techniques (Scheme 2.5).
Adduct 48 was readily converted into corresponding alcohol 92 (97%) by the same means and obtained as a crystalline solid (Scheme 2.6).
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
36 With compounds 93 and 94 to hand attention now turned to their elaboration into the core structure of (–)-platencin as embodied within compound 99. A one-pot and two-fold oxidation procedure devised by Nicolaou et al13was pursued in the first instance. Thus, a mixture of alcohols 93 and 94 was treated with IBX under a variety of conditions but, unfortunately, the desired α,β-unsaturated ketone 99 could not be obtained by such means (Scheme 2.8). Even though some evidence for partial oxidation of these alcohols to the corresponding ketone was obtained, even freshly prepared IBX failed to produce the targeted enone. Accordingly, other protocols for effecting the desired conversion were explored.
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
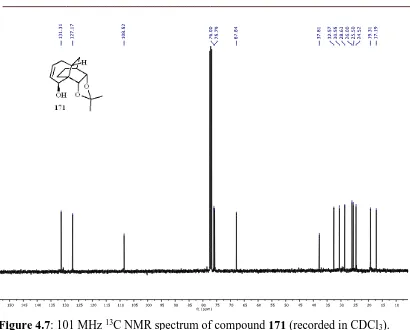
37 The IR spectrum of product 95 showed a strong C=O absorption band at 1717 cm−1 while the ESI mass spectrum featured a molecular-associated ion at m/z 273 [(M + Na)+]. The 13C NMR spectrum exhibited 15 distinct carbon resonances including one for the carbon of the carbonyl unit atδ 211.3.
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
38 enones 98 (21%) and 99 (65%) was obtained (Scheme 2.9) (a commonly accepted mechanism for the dehydrogenation of enol ethers by this means is presented in Appendix A.8).
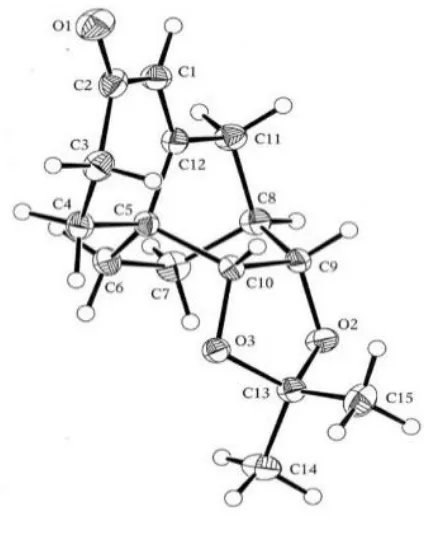
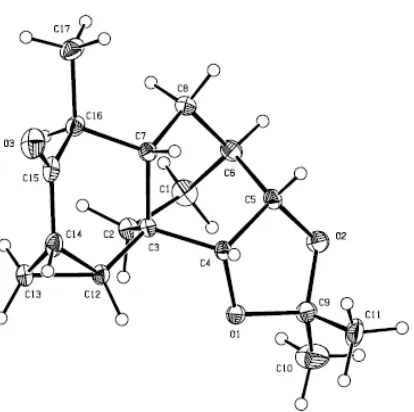
The various spectral data obtained on compound 99 clearly indicated the desired compound had been obtained. So, for example, in the 1H NMR spectrum two mutually coupled one-proton doublets (J = 10.1 Hz) appeared at δ 6.89 and 5.91 and these are attributed to the hydrogens associated with the newly installed double bond of the enone moiety. The 13C NMR spectrum exhibited 15 distinct carbon resonances including one due to the carbon of the carbonyl unit at δ 199.5. The EI mass spectrum of this enone displayed a molecular ion at m/z 248, while the IR spectrum displayed a strong C=O absorption band at 1712 cm–1. The solid-state structure of compound 98 was established by single-crystal X-ray analysis and the derived ORTEP is shown in Figure 2.2 (additional details are provided in Appendix 1).
[image:60.595.164.376.410.678.2]Chapter Two – Synthesis of the Platencin Core
___________________________________________________
39 2.4 Summary
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
40 2.5 References
1) Austin, K. A. B.; Banwell, M. G.; Willis, A. C. Org. Lett. 2008, 10, 4465.
2) Parks, B. W.; Gilbertson, R. D.; Domaille, D. W.; Hutchison, J. E. J. Org. Chem. 2006, 71, 9622.
3) (a) Veysoglu, T.; Mitscher, L. A.; Swayze, J. K., Synthesis, 1980, 807; (b) Ferraboschi, P.; Canevotti, R.; Grisenti, P.; Santaniello, E. J. Chem. Soc., Perkin Trans. 1, 1987, 2301; (c)Isayama, S. Bull. Chem. Soc. Jpn. 1990, 63, 1305.
4) Slomp, G.; Johnson, J. L. J. Am. Chem. Soc. 1958, 80, 915.
5) Trost, B. M.; Machacek, M. R.; Tsui, H. C. J. Am. Chem. Soc. 2005, 127, 701. 6) (a) Appel, R. Angew. Chem. Int. Ed. Engl. 1975, 14, 801; (b) Wood, J. L. et al. J.
Am. Chem. Soc. 2004, 126, 16300.
7) (a) Entwistle, D. A.; Hudlicky, T. Tetrahedron Lett. 1995, 36, 2591; (b) Boyd, D. R.; Sharma, N. D.; Llamas, N. M.; O'Dowd, C. R.; Allen, C. C. R. Org. Biomol. Chem. 2006, 4, 2208; (c) Hudlicky, T.; Boros, E. E.; Olivo, H. F.; Merola, J. S. J. Org. Chem. 1992, 57, 1026.
8) (a) Boyd, D. R.; Sharma, N. D.; Byrne, B.; Hand, M. V.; Malone, J. F.; Sheldrake, G. N.; Blacker, J.; Dalton, H. J. Chem. Soc., Perkin Trans.1 1998, 1935; (b) Boyd, D. R.; Sharma, N. D.; Malone, J. F.; O’Dowd, C. R.; Allen, C. C. R, Org. Biomol. Chem. 2005, 3, 1953; (c) Banwell, M. G.; Lehmann, A. L.; Menon, R. S. Willis, A.
C. Pure Appl. Chem. 2011, 83, 411.
9) (a) Negishi, E.; Hu, Q.; Huang, Z. H.; Qian, M. X.; Wang, G. W. Aldrichimica Acta, 2005, 38, 71; (b) Negishi, E.; Valente, L. F.; Kobayashi, M. J. Am. Chem. Soc. 1980, 102, 3298; (c) Smith, A. B.; Beauchamp, T. J.; Lamarche, M. J.; Kaufman, M. D.; Qiu, Y.; Arimoto, H.; Jones, D. R.; Kobayashi, K. J. Am. Chem. Soc., 2000, 122, 8654.
Chapter Two – Synthesis of the Platencin Core
___________________________________________________
41 11)Clark, J. H. Chem. Rev. 1980, 80, 429; (b) Corey, E. J. J. Am. Chem. Soc. 2005,
127, 701.
12)Garg, N. K.; Caspi, D. D.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 9552. 13)Nicolaou, K. C.; Zhong, Y. L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596. 14)(a) Ito, Y.; Hirao, T.; Saegusa, T. J. Org. Chem. 1978, 43, 1011; (b) Uchida, K.;
Yokoshima, S.; Kan, T. ; Fukuyama, T. Org. Lett. 2006, 8, 5311.
15)(a) Vedejs, E.; Piotrowski, D. W.; Tucci, F. C. J. Org. Chem. 2000, 65, 5498; (b) Moriarty, R. M.; Prakash, O. Org. React. 1999, 54, 273; (c) De Munari, S.; Frigerio,
M.; Santagostino, M. J. Org. Chem. 1996, 61, 9272.
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
42 Chapter Three
A Chemoenzymatic Synthesis of Platencin
3.1 Introduction
In the preceding Chapter the synthesis of an advanced precursor, 99, to platencin was described. This was obtained after a sequence of conventional functional group transformations was applied to carbocycle 47, the product of a thermally promoted IMDA cycloaddition reaction (Figure 3.1).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
43 precursor diol being obtained in enantiomerically pure form via the enzymatic dihydroxylation of iodobenzene. The work described in this chapter details the author’s efforts focused on the elaboration of compound 99 into platencin. This involved, inter alia, two-fold alkylation of compound 99 at C-4, as well as a series of relatively conventional functional group interconversions (Figure 3.1) to complete the synthesis. Specifics are provided in the following sections.
3.2 Two-fold Alkylation, at C-4, of the IMDA Adduct 99
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
44
٭Prolonged reaction times, stirring at higher temperatures and/or using slightly more equivalents of MeI/KHMDS resulted in co-production of significant quantities of dimethylated product.
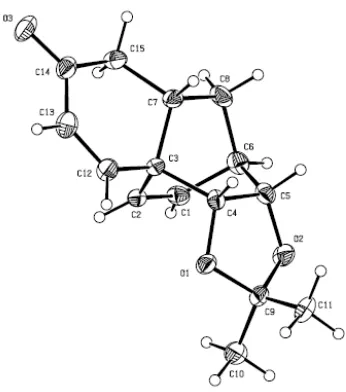
Figure 3.2: ORTEP derived from the single-crystal X-ray analysis of compound 100 (anisotropic displacement ellipsoids display 30% probability levels while hydrogen atoms are drawn as circles with small radii.)
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
45 Figure 3.3: 400 MHz 1H NMR spectrum of compound 101 (recorded in CDCl3).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
46 The next step of proposed synthetic plan was the olefin cross-metathesis of compound 101 and vinyl pinacol boronate (38) (Scheme 3.2). However, this proved to be
problematic and failed to deliver the hoped-for unsaturated boronate ester 103.
Thus, under the wide range of conditions investigated only complex mixtures of products were obtained. As such a new approach for completion of the installation of the propanoic acid side of platencin was required.
3.2.1 A Michael Addition Strategy for Side Chain installation
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
47 Table 3.1: Stereochemical outcomes of the1,4-addition of the potassium enolate
derived from compound 100 to t-butyl acrylate.
Entry Conditions dr
107:108
Combined Yield
1
t-BuOK (1 M in t-BuOH) 2.5 mole equiv., t-butyl acrylate 3.0 mole equiv., THF,
0 ºC, 1 h.
2 : 1 82%
2
t-BuOK (1 M in t-BuOH) 2.0 mole equiv., t-butyl acrylate 3.0 mole equiv., THF,
18 ºC, 1 h.
2.5 : 1 79%
3
t-BuOK (1 M in t-BuOH/Et2O, 1:1 v/v) 2.0 mole equiv., t-butyl acrylate 2.0 mole
equiv., THF, 18 ºC, 0.7 h.
4 : 1 85%
4
t-BuOK (1 M in t-BuOH) 2.0 mole equiv., t-butyl acrylate 3.0 mole equiv., THF,
18 ºC, 0.5 h.
3 : 1 83%
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
48 nine-proton singlet appearing at δ 1.42 is attributed to the t-butyl group. The 13C NMR spectrum (Figure 3.6) displayed the expected twenty-one carbon resonances with the most diagnostic ones appearing at δ 203.4 and 172.9 and being assigned to the carbonyl carbons of the ketone and ester moieties, respectively. The EI mass spectrum of compound 107 exhibited a molecular ion at m/z 390 and the illustrated configuration at C-4 was assigned using NOESY techniques. In particular, a strong correlation was observed (Figure 3.7) between the signals due to the methylene protons of C-6 and that arising from the protons of the methyl group at C-4.
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
49 Figure 3.6: 101 MHz13C NMR spectrum of compound 107 (recorded in CDCl3).
3.3 Completion of a Synthesis of Platencin (9)
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
50 was explored. Thus, compound 107 was reacted with this resin in a methanol-water-THF mixture at 65 °C. Under these conditions a 63% yield (at 83% conversion) of the required diol 109 was obtained. Co-production of the corresponding methyl ester was also seen as a result of a trans-esterification process and increasing amounts of this by-product were observed as the reaction time was increased.
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
51 Figure 3.8: 400 MHz 1H NMR spectrum of compound 109 (recorded in CDCl3).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
53 Figure 3.10: 400 MHz 1H NMR spectrum of compound 110 (recorded in CDCl3).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
54 Given its propensity to dimerize,3 compound 110 was immediately used in the next step of the reaction sequence. Specifically, then, it was subjected to O-benzoylation,4 (Scheme 3.5) using benzoyl chloride in the presence of triethylamine and 4-(N,N-dimethylamino)pyridine (DMAP) as the first step in an effort to remove the residual OH group via reductive cleavage. By such means, the corresponding benzoate 111 was obtained in 91% yield.
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
55 Figure 3.12: 400 MHz 1H NMR spectrum of compound 111 (recorded in CDCl3).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
56 3.3.1 Reductive Cleavage of the –OBz Group
With the desired benzoate 111 to hand, the next step of the synthesis required reductive cleavage of −OBz group. Samarium(II) iodide (SmI2) has been used extensively for such purposes (a mechanistic rationalisation of the SmI2-mediated reduction of α-functionalized carbonyl compounds is shown in Appendix A.9) within the Banwell Group5 as well as by others.4a,4c,6 Accordingly, compound 111 was treated with four mole equivalents of samarium(II) iodide in THF/MeOH between −78 °C and 18 °C for a total of 3 h. Unfortunately, under such conditions a complex mixture of non-UV active compounds was obtained with little evidence being obtained for the formation of the target ketone 112 (Scheme 3.6).
Chapter Three – A Chemoenzymatic Synthesis of Platencin
___________________________________________________
57 Table: 3.2 Conditions used in effort to effect the reductive cleavage of
the −OBz group within compound 111
Entry Reagents and reaction conditions Outcome
1 SmI2 (4.0 mole equiv.), THF: MeOH (2:1 v/v), −78 ºC, 1→3 h.
complex mixture 2 SmI2 (2.5 mole equiv.), THF: MeOH (3:1 v/v),
−78 ºC, 1→3 h.
complex mixture 3 SmI2 (1.5 mole equiv.), THF: t-BuOH (2:1 v/v),
−78 ºC, 1→3 h.
complex mixture
4 SmI2 (1.5 mole equiv.), THF: MeOH (2:1 v/v ), −78 ºC, UV,٭ 1.5 h.
complex mixture
٭Recently it has been found that light plays an important role in controlling the reducing power of SmI 2.
Thus, Hoz etal. demonstrated that many reactions involving SmI2 are inhibited when put under a
UV-lamp at 254 nm.7Accordingly, a UV-lamp available in lab for TLC examination (and operating at 254
nm) was used in an attempt to slow down unwanted side reactions but to no useful effect.