THESES SIS/LIBRARY TELEPHONE: +61 2 6125 4631 R.G. MENZIES LIBRARY BUILDING NO:2 FACSIMILE: +61 2 6125 4063
THE AUSTRALIAN NATIONAL UNIVERSITY EMAIL: library.theses@anu.edu.au CANBERRA ACT 0200 AUSTRALIA
USE OF THESES
This copy is supplied for purposes
of private study and research only.
Passages from the thesis may not be
copied or closely paraphrased without the
T H E N E U R 0 N A L C 0 N T R 0 L 0 F
D R A G 0 N F L Y F L I G H T.
PETER JOHN SIMMONS
A thesis submitted for the degree of Doctor of Philosophy of the Australian National University.
DECLARATION .
I declare that all parts of this thesis describe my own original work.
Most of chapters 2 and 3 form the basis of two papers which have been accepted for publication, and a paper describing crevice organs (chapter 4) is in preparation.
During the course of my PhD I also studied the mechanism of singing in the cicada Cystosoma saundersii. One paper on this, written in collaboration with
Dr David Young of Melbourne University, is in preparation. Another, concerned with intracellular recordings from
motoneurons and interneurons, has been submitted for publication, and complements chapter 3 of this thesis in that i t deals with a much simpler rhythm generator than the neuronal machinery that controls dragonfly
ACKNOWLEDGEMENTS
It is a pleasure to acknowledge the advice and encouragement given to me by my two supervisors,
Professor G. Adrian Horridge and Dr Eldon E. Ball. Professor Horridge initially suggested that I should investigate dragonfly flight and helped to initiate the study. Dr Ball patiently gave much time to
consideration of numerous problems which arose during. the work. Drs Peter Snow, Willi Ribi, Gert Stange, Steve Shaw, David Sandeman and David Dvorak gave useful guidance, and Paul Whitington provided many helpful comments.
Bruce Ham helped to catch dragonflies; Bob Jackson, Kevin Downing, Andrew Welsh and Bob Trewin willingly endured requests and questions about equip-ment; and Rod Whitty and his staff provided technical assistance for the scanning electron microscopy of chapter 4. Rosemary Drury speedily and accurately typed the manuscript.
"Thus the dragonfly enters updn a more noble life than i t had hitherto
led in the water, for in the latter i t was obliged to live in misery, creeping and swimming slowly, but now i t wings the air."
ABSTRACT
The mechanical action and innervation of the
major flight muscles of aeshnid dragonflies are described. All flight muscles investigated are innervated by at
least three motoneurons, and one by as many as fifteen. Cell bodies of motoneurons that innervate the same
muscle are clustered together, and have similar, wide-spread dendritic branching patterns. Motoneurons of leg muscles have greater variety in cell body size and position than the major flight motoneurons.
Striking similarities between the organisation of motoneurons in dragonflies and other insects raise interesting questions about the evolution of insect nervous systems.
Intracellular recordings have been made from identified motoneurons, singly and in pairs, in tethered flying and non-flying dragonflies. During flight large rhythmical fluctuations in membrane potential, usually
motoneurons, and each motoneuron receives inputs from a separate set of interneurons. Motoneurons have in-puts to central neurons, forming feedback loops. Many receptors provide information about wing movements to the central nervous system. As in the locust,
populations of non-spiking and spiking interneurons dfive the flight motoneurons.
In the hard cuticle of the basal region of one wing vein there are groups of small, elongate innervated indentations called crevice organs. They differ in
structure from any previously described insect
CHAPTER l·
CHAPTER 2
CHAPTER 3
CHAPTER 4
BIBLIOGRAPHY ADDENDUM
CONTENTS
INTRODUCTION
Introduction 1
The Neuronal Generation of
Rhythmical Movements 1
The Neuronal Control of Locust
Flight 6
Dragonflies - Flight, Nervous
System and Natural History 9
The Experimental Approach Adopted
in This Study 13
ANATOMY Methods Results Discussion PHYSIOLOGY Methods Results Discussion CREVICE ORGANS
Methods Results Discussion
The Neuronal Generation of a Simple Rhythmical Behaviour: . Singing in a Cicada
C H A P T E R 1
INTRODUC'l'ION
Dragonflies have been called the "birdwatcher's insect" (Corbet et al., 1960). They are excellent at
flying and much of their behaviour as adults is con-spicuous because i t is performed on the wing. The neuronal control of flight in a completely different type of insect, the locust, has been the subject of much investigation and has become a classical example of a
movement where the temporal pattern of muscular contraction is completely encoded within the central nervous system. ,Locusts are considered by some to be excellent animals
for investigation into the neuronal mechanisms that control behaviour (e.g. Hoyle, 1975;1976) and the significance of work on locusts can be increased if i t can be shown that ,
results obtained are applicable to the nervous systems of other animals. Phylogenetically dragonflies are not closely related to locusts and they are capable of a greater
diversity of aerobatic manoeuvres. This study
deals with the neuronal control of flight in dragonflies and enables comparisons to be made with findings on locusts. It also reveals a number of constant features in the way that motoneurons are arranged in insect ganglia and prompts some speculation on the evolution of insect nervous
systems.
THE NEURONAL GENERATION OF RHYTHMICAL MOVEMENTS
- 2
-that is repeated at regular intervals. Two mechanisms by which central nervous systems can generate repeated cycles of events are shown diagrammatically in figure 1.1. One method relies on endogenous variation in membrane
potential in a single neuron called a pacemaker to
generate the repetition. The other relies on interaction between neurons that are not individually pacemakers. In reality many rhythmical movements may be generated by mechanisms that incorporate features bf both of these extreme types.
It is difficult in practice to distinguish
between these two mechanisms. In order to test rigorously the endogenous pacemaking properties of a neuron i t is necessary to remove all synaptic inputs to it. No way is known to isolate a whole neuron in this manner but somata of identified neurons have been isolated from Aplysia ganglia and exhibit oscillations in membrane potential similar to those that can be recorded from their neurons when intact (Alving, 1968; Chen et al., 1971). The
treatment of bathing neurons in a calcium-deficient, magnesium-rich solution, which blocks many synapses, has often been used to determine whether repeated potentials recorded from a neuron are of synaptic or intrinsic
a. b.
Pacemaker
I nterneu rons
Motoneurons Motoneurons
- 3
-(Barker and Gainer, 1974; Meech and Standen, 1975;
Eckert and Lux, 1976) and second because i t is impossible to be certain that all synapses are blocked. Another method of testing the endogenous pacemaking capabilities of a neuron is to apply pulses of current to it. These should reset the timing of potentials generated entirely within the neuron but affect only the amplitudes of
potentials that are transmitted synaptically to it. How-ever, current pulses applied to a non-pacemaker neuron that interacts with other neurons to produce a rhythmical output will also affect timing of potentials.
Using both of these treatments Woollacott and Hoyle (1977) have found that a locust motoneuron, the anterior adductor of the coxa,has endogenous pacemaking properties. When the metathoracic ganglion is perfused
with magnesium-rich saline, which abolishes reflex movements of the hind leg, this motoneuron spikes regularly. Timing of spikes is altered when an experimenter causes an
antidromic spike in the motoneuron. This finding is important because i t shows that a motoneuron can play an active role in determining its own output rather than passively converting a sum of synaptic inputs into a particular frequency of spikes.
4
-ganglion of the spiny lobster which controls the gastric mill cycle (Mullaney and Selverston, 1974). In this ganglion all the neurons and many strong connections between them have been thoroughly studied. There is little possibility that activity recorded from a neuron originates in unidentified antecedent neurons. In contrast to the gastric mill section the pyloric section of the
stomatogastric ganglion is driven by neurons which have
endogenous pacemaking properties {Maynard, 1972).
A most important recent discovery is that neurons that do not produce spikes are involved in the generation of rhythmical movements. Such neurons were first reported from the neuronal mechanism that controls ventilatory move-ments in a hermit crab {Mendelson, 1971). Subsequently four types of non-spiking neuron have been found to be involved in the walking of cockroaches {Pearson, Fourtner and Wong, ·1973; Pearson and Fourtner, 1975) and non-spiking
inter-neurons have been discovered in cicadas (described in the adden-dum, p. 73i. Some inhibitory interactions are mediated
without spikes in the lobster stomatogastric ganglion
(Maynard and Walton, 1975). In locusts Burrows and Siegler (1976) have shown that injection of graded current into a non-spiking interneuron results, after a delay of about
lOms, in a proportionally graded polarisation of motoneuron membrane potential. Postsynaptic potential is driven in a
- 5
-synaptic transmission involves modulation of the continuous release of transmitter from presynaptic terminals
(Dowling and Ripps, 1973).
Investigators now have the arduous task of penetrating and staining small processes of interneurons
and motoneurons simultaneously. Co-ordinating non-spiking
interneurons will not be revealed by recording of coincident unitary PSPs in pairs of rnotoneurons, a method that has
revealed a number of (presumably) spiking interneurons involved in walkirig of locusts before their anatomical identity is known (e.g. Hoyle and Burrows, 1973 a, b; Burrows and Horridge, 1974)~
Because many synapses in central nervous systems may operate without spikes (this is argued for vertebrates in Rakic, 19 74) ·it is reasonable to compare the relative advantages of spiking and non-spiking transmission of
information. In transmission over distance the advantage of spikes is clear. The threshold property of spikes may also be important. But pathways that employ spikes are limited in the speed with which they can register change in their input since such change must be translated into an
alteration in spike frequency. Also non-spiking neurons can register continuous ranges of signal amplitude more accurately than spiking neurons. The amplification of signals that occurs without spikes between the retinula cells and monopolar cells in the dragonfly compound eye
- 6
-transmitted into the nervous system. Pearson (1976) has suggested that accurate regulation of motoneuron spiking
frequency is better achieved by non-spike mediated excitation and inhibition than by spike-caused PSPs.
THE NEURONAL CONT.ROL OF LOCUST FLIGHT
There is much information on the aerodynamics and muscular control of locust flight {e.g. Weis-Fogh and
Jensen, 1956; Wilson and Weis~Fogh, 1962; Wilson, 1961). Wings are elevated by the direct action of dorso-ventral muscles that are placed centrally in the thorax,and
depressed by the indirect action of dorsal longitudinal muscles in deforming the thorax and the direct action of
laterally-positioned dorso-ventral muscles. The pronation
and supination of the wings, which are important for main-taining l i f t and propulsion throughout a wingbeat cycle, are controlled by the lateral depressor muscles. Promotion and remotion occur passively during a wingbeat cycle. The large power-providing flight muscles are each innervated
by one or two motoneurons that produce fast twitch-contractions. The positions within ganglia and the geometry of the
neuropilar processes of these motoneurons are known (Bentley, 1970; Burrows, 1973a; Tyrer and Altman, 1974).
7
-be generated within the thoracic ganglia when the wings and the sense organs that monitor their movements are
removed. In such preparations the cycle repetition frequency is lower than that in intact animals and there is a
reduction in the elevator-to-depressor phase-lag. Most attention has been paid to the single-celled stretch receptors in the region of the wing hinge (which may be peculiar to Orthoptera) as providing information about wing movements (Gettrup, 1962, 1963; Wilson and Gettrup, 1963). Burrows (1975a) has demonstrated that a spike from a stretch receptor causes a large unitary EPSP on ipsilateral depressor motoneurons and a unitary IPSP on ipsilateral elevator
motoneurons. Such reflexes probably regulate and reinforce the centrally determined flight rhythm. Near to the stretch receptor there is a chordotonal organ (Gettrup, 1963) but nothing is known of its response to wing movements and role in co-ordinating flight. Input from campaniform.sensilla
on the wing veins affects the angle of attack of the forewings (Gettrup and Wilson, 1964; Gettrup, 1965, 1966).
The involvement of interneurons in the generation of flight first became apparent when Bentley (1969) recorded compound subthreshold excitatory potentials at the same
frequency as normal wingbeats from flight motoneurons
- 8
-·Further, Burrows found that a spike in an elevator motoneuron (no. 113) is sometimes followed, after a delay equal to the usual inter-wingbeat period, by a wave of excitatory
potentials in the elevator motoneuron and in its contralateral partner. Occasionally a spike in this elevator motoneuron causes, after a delay approximately equal to half the normal wingbeat period, excitation in an antagonist motoneuron.
Interneurons are most likely involved in the pathways of such delays. Such pathways could generate and control flight
rhythms but i t is more likely that they reinforce a more centra1ly...,.determined programme.
It is not surprising that no strong direct connec-tions between flight motoneurons have been discovered because such connections would interfere with simple reflexes
involving individual motoneurons and with the independence
of rnotoneurons that is necessary for the control of bifunctional muscles. Bifunctional muscles are used in different
com-binations for di£ferent movements (Wilson, 1962; Elsner, 1974). In flies there is evidence for strong direct coupling between the motoneurons which innervate the myogenic
muscles (Mulloney, 1970).
- 9
-motoneurons in all three thoracic ganglia. One rhythm occurs in phase with ventilation and the other rhythm has a much higher frequency, equal to the wingbeat frequency in flight. Potentials of the faster rhythm are seen only
during the expiratory phase of ventilation. It is interesting that the same interneurons convey the rhythms of two different movements, flight and ventilation, because such neuronal
economy has been suggested from observations on rhythmical behaviours of crickets. Rhythms of different movements in crickets, such as singing, walking and ventilation, are related to each other (Kutsch, 1969). However, i t is not obvious how these interneurons could co~ordinate spiking in motoneurons during flight because in quiescent locusts they depolarise antagonistic and serially homologous
motoneurons in phase with each other. Also the rhythms
conveyed by these interneurons originate in the rnetathoracic ganglion but an isolated rnesothoracic ganglion has been shown to be capable of producing a flight rhythm (Wilson, 1961). Orthopterans have rather stereotyped rhythmical behaviours and i t will be interesting to see if other insects possess interneurons that convey information about more than one rhythm.
DRAGONFLIES - FLIGHT, NERVOUS SYSTEM AND NATURAL HISTORY
10
-other manoeuvres. Variations in the twisting and fore-and-aft movements of each wing and in the relative motions of the four different wings must be important. I know of no
attempt to describe the wing movements that accompany manoeuvres that dragonflies make during flight although recent developments in the high-speed photography
of free-flying insects may soon rectify this (e.g. Dalton, 19 75) •
In a recent study on the kinematics of wing
movements in a species of Zygoptera, Rudolph (1976b) states th~t the £our ~ings of anisopteran dragonflies show
considerable variation in their relative movements and considerable variation in wingbeat frequency.
Clark's (1940) report on the anatomy of the
flight musculature of dragonflies is the most complete and accuraj:.e and I have used his nomenclature.
Neville (1960) studied the mechanics of wing action during steady flight of aeshnid dragonflies. He related twisting and fore-and-aft movements of ·a wing to a rapid separation of the major sclerites of the wing base, the axillary and humeral complexes, along a line of weakness between them. This is largely controlled by a small elevator muscle, dvm6 , or the anterior coxoalar muscle. In locusts
11
-Action of dragonfly flight muscles is further described in Chapter 2.
Flight in anisopterous dragonflies can start with either an upstroke or a downstroke (Pond, 1973), unlike the
where
ti::"'"'!:-
sl-a.r~ wi~h Cl d.owflsko}:.esituation in damselflies; (Rudolph, 1976a) and other insects that fold their wings (Pond, 1972) where i t always starts with
an
upsfrroke ' Some sensory organs have specific control over wing movements during the flight of dragonflies. There are mechanoreceptors'in
the antennae which affect thedegree of dorsal ~xcursion made by the wings (Gewecke et al., 1974). Hair plates in the neck region, which are stimulated by movements of the head, may play a role in the stabilisation of flight (Mittelstaedt, 1950). Sense organs in the wing
bases have received little attention, except for a brief report by Erhardt (1916).
The ventilatory movements of adult and larval dragonflies h~ve been studied. Miller (1962) investigated ventilation in a number of adult African dragonflies.
Spiracles remain open during flight and generally open and close in phase with abdominal pumping movements during rest. The rhythmical movements of ventilation originate in
- 12
-Zawarzin's (1924) description of the cellular structure of the mesothoracic and fourth abdominal ganglia of dragonfly larvae has become a crassical account of the organisation of a nervous system. I was able to put names to motoneurons in his diagrams of the mesothoracic ganglion.
(Fig. l.2a). The main value of his painstaking work is that i t describes many types of interneuron on the basis of
structure alone (Fig. l.2b).
Most of my observations and experiments were made on male Hemianax papuensis (Burmeister) , but I also used females of this species and males of another locally-occurring aeshnid, Aeshna brevistyla (Rambur). Both sexes and both species average 65mm in length. I found no
differences in flight machinery between sexes or species.
My casual observations on the behaviour of these dragonflies indicate that i t is similar to that described for the European species, Aeshna cyanea (Miiller) by Kaiser
(1974b) . Females are rarely seen except when flying "in
tandem" with males during copulation and egg-laying (Fig. 1. 3c). Males spend much of the time "hawking" - flying at varying
heights with varying turns catching and eating smaller
insects and performing various cleaning activities (Fig. l.3a,b). While hawking,males show little reaction to other dragonflies. However, "fightsi; between males are common during "patrolling"
flights. These flights are thought to be for the purpose of finding females. Patrolling flights are generally made at a constant height, parallel to the edge of a stream or
b.
c.
9
Figure 1.3
. Aeshnid dragonflies in flight. (a) and (b) are after Kaiser (1974b) ~
(a): the .dragonfly is described as grooming its wings. (b)·: the dragonfly is grooming its
abdomen.
(c}: a male and a female fly"in tandem". With processes on the tip of his abdonen the male
[image:27.602.21.594.31.803.2]13
-Unlike the Zygoptera and smaller Anisoptera the larger aeshnids cannot be considered to be territorial animals and do not have courtship rituals (e.g. Kaiser, 1974a, b; Green, 1974; Heymer, 1912; Waage, 1973). Males probably recognise females of the same species by the distinctive colouration oftheir species - the body of H. papuensis is
coloured shades of green and brown while that of A. brevistyla has striking vertical yellow and brown stripes.
THE 'EXPERIMENTAL APPROACH ADOPTED IN THIS STUDY
In the approach that I chose for examination of the neuronal control of dragonfly flight the first stage was to identify the motoneurons that innervate the large flight muscles. It has been known since the 1890's that certain invertebrate ganglia contain several large cells which can be recognised from individual to individual in the same species (Retzius, 1891). This me.ans that, in many species, i t is feasible to draw up a map of reidentifiable neurons showing the characteristic position, shapes ano sizes of cell bodies and larger neuropilar processes. The construction of such maps is greatly facilitat~d by the recently-developed technique of back-filling neurons with stain (Iles and Mullaney, 1971). Surprisingly, since the
introduction of this technique few reports on the organisation and geometry of motoneurons in different species of
14
-their fast, slow or inhibitory character.
I found no report on the morphology of nerves in the dragonfly thorax. It was necessary to describe this before proceeding with the back-filling of neurons. During my study of the morphology of the nerves I examined sense organs in the wing base and found one type that differs in structure from any s~nse organ previously described in insects. (Chapter 4) .
Most experimental effort in this study went into intracellular recordings from neurons. These were most
informative when I managed to penetrate a pair of motoneurons simultaneously in a dragonfly that could be induced to
perform tethered flights. This allowed me to test for possible interactions between the motoneurons and to draw conclusions about the interneurons that drive them. ~uccess
in making these intracellular recordings was spasmodic but exciting.
I also made extracellular recordings from motor s
and se1ory nerves and from muscles. The recordings from muscles were often hard to interpret, whereas recordings
C H A P T E R 2
- 15
-METHODS
Dragonflies were caught locally on warm summer days by netting. Following capture they were kept in a moist atmosphere at l0°c for up to a week. Attempts to .raise adults from larvae were unsuccessful because we
found no way to predict or to induce emergence of adults, and adults which did emerge in captivity were small and weak compared with wild dragonflies.
For study of nerve tr.unk anatomy leuco-methylene blue was injected into living specimens (Pantin, 1946) , which were left for an hour before dissection and fixation in cold ammonium molybdate solution. Specimens preserved in 70% alcohol and in alcoholic Bouin's fixative were also examined. Photographs and drawings made from dissected preparations form the basis for the diagrams in this paper.
To investigate axonal content of nerve trunks
thoraces were fixed in phosphate-buffered 2.5% glutaraldehyde at pH 7.4 for at least 24 hours and kept for several weeks in buffer at
o
0c. Muscles and nerves were dissected from the thoraces, stained in 5% osmium tetroxide for one hourto improve the contrast of later staining, dehydrated, embedded in Araldite and sectioned with glass knives at l-2µrn.
Sections were stained with toluidine blue and examin~d
16
-5µm and stained with Mann's stain (Pantin, 1946) provided a check on axon counts, as did the methylene blue-stained preparations.
To visualise neuron cell bodies ganglia were removed from live dragonflies and sta.ined with toluidine blue using the method of Altman and Bell (1973). Some ganglia prepared in this way were embedded in wax and sectioned serially at 5µm.
Two methods were used to stain identified neurons. Neurons in about one hundred ganglia were stained by the method of filling with cobaltous ions through cut ends of axons (Sandeman and Okajima, 1973; Tyrer and Altman, 1974). 5% cobaltous chloride was used - higher concentrations produced extensive clumping of stain in neurons. No current was used, and the
preparation was left in a moist chamber for 10-24 hours
0
at 22 C. After treatment with ammonium sulphide and mounting for examination good preparations were photo-graphed immediately at several depths of focus using a Zeiss Photoscope. This was done to save time when live dragonflies were available for experimentation, with the object of reconstructing dendritic shapes of neurons by
tracing serial photographs later. Many preparations
did not deteriorate markedly with time, however, and drawings of neurons here were almost all made from
17
-Motoneuron cell body positions for most major flight muscles were also determined by injecting Procion brown (H3R) or brilliant red (H3B) dye into them. For this dragonflies were opened ventrally. and neurons in thoracic ganglia were impaled with micropipettes filled with a 5%s.olution of Procion dye and identified using methods described in Chapter 3. Dye was expelled from
an electrode by applying a brief pulse of 180 volts across it, switched manually. Filling could be observed visually, and usually required only one pulse less than a second long. Good recordings could be obtained with Procion-filled
electrodes. Ganglia with injected cells were further treated by staining with toluidine blue to allow positions of other cell bodies to be visualised, the injected neurons standing out as bright red blobs.
18
-RESULTS
Neuromuscular Morphology
Each wing of a dragonfly is moved by five major muscles that contract in a dorso-ventral direction (Fig.
2.1 Table 2.1). The action of these muscles was
confirmed by stimulating each one with electrical shocks. Elevation and depression occur about a pivot. The depressor muscles dvm3 and pm1 attach directly to the wing distal to
the pivot; the main elevator muscle, dvm1 , acts via the scutum on the proximal part of the wing; and the smaller elevator muscles, dvm6 and dvm7 , attach directly to the
wing proximal to the pivot. Important for prod,ucing lift and propulsion throughout a wingbeat are wing supination
and pronation, and wing promotion and remotion (e.g. Pringle, 1957) . . Supination is achieved largely by a rapid separation of the axillary and humeral complexes caused by contraction of the elevator muscle dvm6 (Neville, 1960). Pronation
is achieved by contraction of the large depressor muscle dvm3 , but is opposed by the much smaller depressor muscles pm2 and pm3 • The degree_of pronation or of supination is, therefore, directly controlled by muscles. Promotion of a wing occurs passively during depression, and remotion occurs passively during elevation, although remotion is reinforced by dvm6 through its action on the axillary and humeral
[image:34.595.35.583.25.825.2]19
-The axes of the wing base muscles (dlm, pm4a'
pm4b' pm5 ) are parallel to the thorax roof. Their function appears to be to affect the form of the wingbeats by
TABLE 2.1
MUSCLES OF THE DRAGONFLY THORAX
A. Wing elevators
Major:
Minor:
dvm1 (=first tergosternal).
dvm6 (= anterior coxoalar) - main action is to supinate the wing. Also responsible for active remotion .. dvm7 (=posterior coxoalar).
dvm2 (second tergosternal) - very small fan-shaped muscle situated on the anterior border of dvm 1.
B. Wing depressors
Major: dvm3
pm1
Minor: dvm4
pm2 pm3
(= second basalar) - also pronates the wing~
(= first subalar depressor).
(= first basalar) - small muscle situated adjacent to dvm3. (= second subalar depressor) - also supinates the wing. (= third subalar depressor) - a minute, watery muscle
with a dorsal tendon of pure resilin (Weis-Fogh, 1960).
Supinates the wing.
C. Wing base muscles
dlm
D. Muscles that raise the abdomen
E. Leg muscles
Abbreviations:
The abbreviations used in this thesis are taken from Clark (1940).
II mesothoracic
III me ta thoracic
dvm dorso-ventral muscle
pm pleural muscle
vlm ventral longitudinal muscle
dlm dorsal longitudinal muscle
[image:36.595.11.584.23.823.2]Pleural
ridge
.
.
.
. . ..
..
. ..
.
.
. ...
. .. . . . . . .....
.
: :.
:
:
. . ....
. . ...
·...
---~Axillary
c.
Humeral
c.
~~---Scutum
'L---dvm7
f
L---dvme
Q.
L---dvm
1
A
- 20
-The Innervation Pattern of the Flight Muscles
Figure 2.2 and table 2.2 summarise the innervation pattern of the flight muscles. Nerves leaving each ganglion are numbered 1 to 3 from anterior to posterior.
letters.
Major branches are labelled with capital
· The first abdominal ganglion is completely fused with the metathoracic ganglion, except for a small hole. A large nerve trunk from the first abdominal ganglion innervates III vlm1 and III ism. ·
Anatomical relations between thoracic nerves, muscles and skeleton are shown in Figs. 2.3; 2.4 and 2.5. Ganglia are enclosed in a chitinous box formed by the floor of the thorax and the heavily sclerotised endoskeleton
(Fig. 2.1) .. Nerves fit snugly into spaces between cuticular struts that form the sides of this box •
. Nerves always bifurcate as they enter muscles and commonly run along tracheae inside them. Except for dvm1 , major flight mu~cles are entered by nerves about one third of the distance between their ventral and dorsal attachments.
[image:39.597.20.584.25.821.2]- 21
MESO.
META.
<rf:dvm
4. . .
1
s---~==
d
v m3
____
!/!---""<-~w
i
~g
t:<:
veins
dim
3C-.~:::pm'
---<
ending in muscle
.
~
Mec:lian
pm3
-
ending in sense
organ~
.
I
sp1rac e
NERVE
2C
3A
3 except 3A
3B
3C
MEDIAN
I
ORIGIN
Near the ganglion
N3 branches into N3A and N3B at the posterior border of dvm7
Dorsal surf ace of ganglion posterior to centre
ROUTE
Dorsally, laterally and posteriorly to the anterior border of dvm6 • Branch 2 travels dorsally along the anterior border of dvm6.
DESTINATION
1. · dvm6 2. a
-b c
pm5
joins branch of N3B; innervates pm4
Fine fibres enter connective tissu~
surrounding dvm6 and dvm7. Methylene blue stains cell bod.ies in this tissue
To the leg
I
LegMesothorax: dorso-laterally.
Metathorax: posterior along the
endocuticular box that encloses the ganglion
Enters dvm7 at its posterior border. One branch travels dorsally.
Around the posterior border of pm2 to enter pm1 and pm2.
Branches into 2 on exit from ganglion. Each branch travels laterally and dorsally.
1. dvm7
2. Joins branch of N2C and innervates pm4
1. pm2
2. pm1
3. pm3
4. Small branches join the median nerve.
Spiracle - closer muscle and other
structures. (Maybe heart.)
Il NlD
n
dimn
d v m3---ir.-+r--Tn
d v m,.--7i!Lf--..,.-+-i7--Tvim
5 mm
To ID
pmsTo ID
pm4 •. bm
dvm8m
dvm7n~':!-P-~=---tr--mp~ :.l...ff-_____c~-+----m pm2
f!--<;:,~,__--f:,___-m pm3
f-iii~rnL+-f++-1:;'-'--'c~---"'---m dvm4
.9---m
sp.~
~~~~~~~~~==~T}o~m
Tom
dvm,.2pm,,2,3 5II
dvrl\---~i/.T
Ganglion II
Ir
pm
75
mm
.
I.
.
.
.
I
• • I
~
- 22
-Axons of Nerves Supplying Muscles
Table 2.3 shows the number and diameters of axons that innervate each major flight muscle.
No axon was seen to branch and innervate more than one muscle.
Each elevator muscle is innervated by axons of the same diameter (Figs. 2.6c,e,g}. Among the depressor muscles, pm1 is innervated by axons of different diameter - two being 12-lSµm in diameter, and two being about ~m
in diameter (Fig. 2.6f}. The depressor muscle dvm3 receives many more axons than any other muscle; 15 in each of two metathoraces examined, and 13 in another (Fig. 2.6b}. At least 10 axons enter dvm3 in the mesothorax
(Fig. 2.6a}. Axons that innervate dvm3 muscles have diameters ranging from about 7µm to 14µm (Figs. 2.6a,b}.
[image:49.600.17.584.23.687.2]- 23
-Cellular material surrounding branching points of nerves contains cell bodies that stain with methylene blue, and probably has a secretory or sensory function
TABLE 2.3
NUMBERS AND SIZES OF AXONS SUPPLYING MUSCLES
Depressors Elevators Minor depressors Muscle dvm5 dvm7
Spiracle closers
No. of axons
10-15 (see text)
4 3 3 5 2 4 2 1
Diameter, µm
[image:51.597.20.587.137.829.2]c •
\
(
\d
- 24
-General Features of Motoneuron Organisation
Most motoneuron cell bodies lie on the ventral surfacesof ganglia, but several are lateral or almost dorsal. Their general arrangement can be seen clearly in ganglia stained with toluidene blue (Fig. 2.7).
Cell bodies of motoneurons that innervate the same muscle lie very close to each other, and homologous motoneuron cell bodies occupy similar positions in the meso-and metathoracic ganglia. Fig. 2.8 summarises cell body positions for all major flight motoneurons. Cell bodies of motoneurons that innervate different muscles are often of similar size and their possible positions overlap, so that i t is not always possible to identify a particular toluidene blue-stained cell body with absolute certainty. This is particularly true for motoneurons of the elevator muscles dvm1 and dvm6 • It is also difficult to back-fill motoneurons of only one of these muscles in a single
preparation because the nerve that innervates dvm6 branches
from nerve 2 just as i t enters dvm1 • Positions of cell bodies of dvm1 and dvm6 were confirmed by injecting them with Procion dye through microelectrodes, and dvm1
motoneurons generally have cell bodies that are anterior and medial to those of dvm6 (Fig. 2.9).
[image:54.598.30.594.21.812.2]notoneurons :>toneurons :>toneurons
ELEVATORS
m
dvm,
3
m
dvm6
3
m
dvm7
5
DEPRESSORS
(10)
m
dvm3
13-15
m
pm,
MESO.
_j
v
L
l
) •• ,
,, c
(
)
dvm
6 (I'"
I (100 J.Jm
META.
I
,.v
=~
/-:
v •••Figure 2.9
Variations in the p::>sitions of the cell bodies of rrotaneurons of b\o elevator muscles in the :n:eso- and :n:etathorax. Each
[image:59.607.37.564.19.776.2]- 25
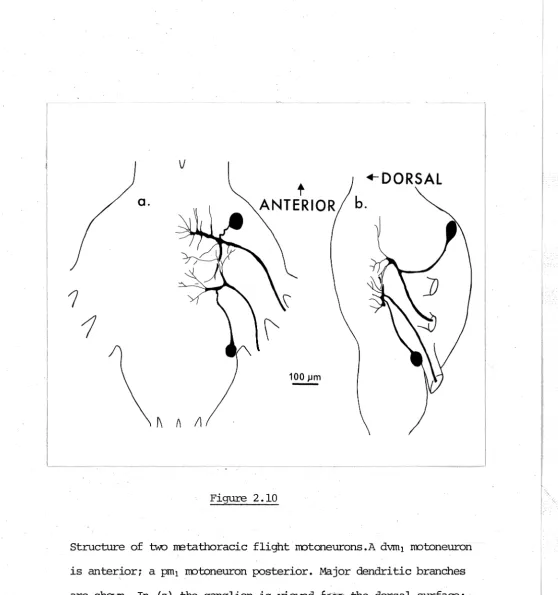
-(Fig. 2.10). Proximal to the region where the axon of
a motoneuron originates there is usually a swollen region
from which originate major dendritic processes and a narrow process which connects the cell body to the rest of the motoneuron. In preparations where i t was not badly distended with stain this region was lOµm to 15µm wide, slightly wider than the axon. Axons of motoneurons that innervate the same muscle originate close to each
other~. and sometimes twist around each other before they
leave the ganglion (particularly axons of dvrn3 - Fig. 2.13). Third- and fourth-order dendritic processes, about lµm
wide, branch from the larger processes. The largest processes of flight motoneurons that have axons in the same nerve run parallel to each other. Commonly beading was seen along the finest branches, and swelling occurred at branch points.
All major flight motoneurons have dendritic processes that extend over much of the side of the ganglion ipsilateral to the axon and all have a few processes that cross the ganglion midline. Processes · from contralateral homologues cross each other but stain was never seen to have passed from one motoneuron to
another. Passage of stain· into other sinall cell bodies, possibly of interneurons (Strausfeld and Oberrnayer, 1976) or glial cells did occasionally occur. Some idea of variability in branching pattern between homologous
r:
I
[image:60.603.12.587.18.805.2]- 26
1
1
a.
v
I\ {\
/I
t
ANTERIOR
100 µm
-Figure 2.10
.-ooRSAL
b.
Structure of two rretathoracic flight notaneurons.A dvm1 rrotoneuron
is anterior; a pm1 rrotoneuron posterior. Major dendritic branches
are shown. In (a) the ganglion is viewed
fJf•
the dorsal surface;· [image:62.602.25.584.24.620.2]- 27
-Major Flight Motoneurons
Elevator motoneurons
Of the motoneuron cell bodies, the largest in ,the ganglia are dvm1 (Figs. 2.10; 2.12) and dvm6 (Fig. 2.12). Usually they are 60-65µm across in toluidene blue preparations. The dendritic processes of dvm1 and dvm6 motoneurons are similar in shape and the axons
of the dvm6 motoneurons originate slightly posterior to those
of the dvm1 motoneurons (Figs.2.10;2.lla,c,d).Characteristical one large process runs posteriorly and another laterally
towards the ganglion midline, but this pattern was not always clearly seen because one process usually swelled more than the other.
The five cell bodies of dvm7 motoneurons (Fig.
2.12) are not clustered as closely as those of other motoneurons innervating a common muscle. They are
posterior to nerve 3, near and medial to the prn1 cell bodies
(see later). Too few dvm7 motoneurons were filled to
draw conclusions about the shape of their dendritic processes, but they usually resembled those of the depressor motoneurons, pm1 , which also have their axon in nerve 3.
Depressor motoneurons
)
Ildvm
1u
L
(
r
)
r
v
fildvm
1\I\
fl/1
) u
l___
)
)
u
,\__
\~
flA
c
10011m
- 28
-of their dendritic processes, axon and muscle. They are situated on the edges of the ganglia, anterior to nerve 1. Twelve, 40µm - 45µm across, have been filled in a single metathoracic ganglion. Some of these may have innervated dvm 4 - I. did not succeed in distinguishing dvm4 from dvm3 motoneurons. The major process traverses the ganglion from the axon to the midline, where the narrow process joined to the cell body originates. Other
secondary processes branch from the major transverse process and extend posteriorly and slightly medially or laterally.
Four pm1 motoneurons (Figs. 2.10; 2.lla,d; 2.13) were filled in some preparations, and had cell bodies 50-60µm in diameter. They are closely-packed on the
edges of ganglia, posterior to nerve 3. One major process extends. towards the midline and another towards the
u
) u
l_
/
v
- 29
-Remaining Motoneurons That Were Studied
.Median nerve. The motoneurons of the closer muscles of spiracles and a number of other neurons were filled from the median nerves. All of these neurons have axons that branch as they leave the ganglion, one branch
travelling along each nerve. Spiracle motoneurons (Fig. 2.14) in each thoracic ganglion have cell bodies 40 - SOµm in diameter near the midline and between the levels of nerves 2 and 3, easily recognisable in toluidene blue-stained preparations (Fig. 2.7). The overall shape of spiracle motoneuron dendritic processes is distinct from that of flight motoneurons. Processes from left and right motoneurons overlap extensively, but I have seen no evidence of direct contact between them.
In each thoracic ganglion three cell bodies less than Sµm in diameter, in a lateral position near nerve 2 and clearly joined to axons, were often filled from a median nerve. Near the cell bodies of the spiracle motoneurons a number of small cell bodies, not clearly
joined to axons, were also filled.
- 30
-Nerve 2. Back-fills of this nerve to stain dvm1 and
dvm6 motoneurons often stained a number of smaller cell
bodies as well. Nerve 2 innervates dvm2 , dvm5 , pm4
(see also under nerve 3C), and pm5 and these smaller cell
bodies are likely to belong to motoneurons of these ·muscles. Most of them lie near and posterior to dvm1
motoneuron cell bodies.
Nerve 3A. Leg motoneurons have cell bodies that are scattered in position and va.riable in size (Fig. 2.14). None is as large as those of the major flight motoneurons. Many leg motoneurohs have cell bodies in anterior parts 0£ ganglia, in contrast to pm1 and dvm7 , which are flight motoneurons of nerve 3. Dendritic processes of leg motoneurohs are not as extensive as those of flight
motoneurons, and none have been seen to cross the ganglion midline.
Nerve 3B. Often more than the five cell bodies of moto-neurons that innervate dvm7 were filled from nerve 3C.
Some of these were median and near the spiracle motoneuron cell bodies. Some fills of nerve 2C also stained cell bodies in this region, and these probably belong to pm4
motoneurons.
1
/j
v
Spiracle
.l\ttll
(b)
1
1
100µm
-Figure 2.14
v
Leg
Non-flight notoneurons in the rretathoracic ganglion.
...
••
•
(a): one spiracular notoneuron. Its dendritic processes show
considerable overlap with its contralateral partner, which is
not shown. Axons branch into both the rredian nerves. The cell ·
body is considerably swollen. (b) : consensus of three fills
[image:73.607.17.583.33.812.2]- 31
-First abdominal ganglion. III ism motoneuron cell
-bodies are 40µm in diameter and contralateral to their axons.Central Projections of Sense Organs of the Wings
Cobalt back-fills from nerves le and lD, which innervate sense organs of the wing base, were attempted only five times. Sufficient lengths of thes~ nerves for filling are difficult to dissect from animals. Bundles of fibres run towards the midline of the ganglion and then parallel to the midline in anterior and posterior directions (Fig. 2.15). Short branches project from
the fibres, and I have not seen any that cross the midline of the ganglion. Sensory processes from the mesothoracic ganglion run through ipsilateral connectives into the
t
DORSAL
~
ANT.
Me so.
100µm
-',-:
...
I\
Figure 2.15
Central projections of rretathoracic wing base sense organs.
Fibres travel up the medial part of the ipsilateral connective
to the rresothoracic ganglion, where one of them is shown in
[image:75.605.15.574.21.631.2]- 32
-DISCUSSION
Dragonfly Motoneurons and Flight
Each of the major flight muscles of dragonflies must act as a single functional unit because all the
axons that innervate a particular muscle have similar
distributions within it. Motoneurons that innervate
the same muscle cannot be distinguished by the sizes or positions of their cell bodies, or by the overall
b~anching pattern of their dentritic processes, and all the major flight motoneurons appear to produce fast ·twitches in muscles. If there are differences between
the motoneurons that innervate a particular muscle -which is to be expected if dragonflies make full use of opportunities to regulate the power output from muscles - they must reside largely in the central connections made by the motoneurons.
One dragonfly muscle, dvm3 , is innervated by an unusually high number of motoneurons, almost as many as the total that· innervate all other major flight muscles. A possible interpretation ·is that dvm3 plays the.main part
- 33
-It is disappointing that the overall branching pattern of different flight motoneurons is so similar,
as differences might provide clues to the neural mechanisms that generate flight. The structural information does tell a physiologist where to place electrodes and, because of the complexity of branching patterns, that he will have to probe in several places before he fully understands the relationship between a motoneuron's input and output. In one arthropod motoneuron, involved in the eye withdrawal reflex of a crab, spikes are initiated at the region where the axon originates from a major process (Sandeman, 1969). The LGMD of the locust, however, has two separate spike initiation regions, each associated with a clearly-defined dendritic region (O'Shea, 1975), and the spike initiation region in cicada tymbal motoneurons is probably not at the anatomically recognisable origin of the axon (Simmons, in preparation) .
Comparison of the Organisation of Flight Motoneurons in Dragonflies and in Locusts
- 34
-Flight muscles of dragonflies are generally
innervated by more axons than flight muscles of locusts, a characteristic probably related to the greater control required by _dragonflies for aerobatic manoeuvres.
There are important differences in the mechanical
action of the power-providing flight muscles in the two types of insect. In locusts the largest depressor muscle is the dorsal longitudinal muscle, which does not attach to the wing base but exerts force on the wings indirectly, whereas in dragonflies all flight muscles pull almost directly on the wing bases. Wing supination in locusts is a passive event during the up-stroke (Wilson and
Weis-Fogh, 1962) whereas in dragonflies i t is actively controlled by the small elevator muscle, dvm6 (Neville,
1960).
The evolutionary origin of the Odonata is unknown (Corbet et al., 1960) which makes i t impossible to be certain how differences in the flight musculature of dragonflies and locusts have evolved. All the major flight muscles iI1 dragonflies have been homologised with muscles in locusts, apparently only on the basis of their general position (Clark, 1940). Homologies can be
extended to the positions of the cell bodies of the
dim
(4)
DEPRESSORS
dvm3 2nd basalar
(10)
pml
lst subalar
(4)
DRAGONFLY
LOCUST
e
Depressor motoneuron cell body 0 Elevator motoneuron cell bodyELEVATORS dvm1 tergosternal (3) ~vm6 anterior coxoalar (6) ~vm7 posterior coxoalar (5)
{ coxoalar -tergocoxal )
83
lst tergosternal
(1)
84
2nd tergosternal
(1)
. 90
posterior
tergocoxal
- 35
-First, this muscle is by far the largest muscle in dragonflies, and is innervated by at least ten moto-neurons, whereas i t is not exceptionally large in locusts and ·is innervated by only two motoneurons
(Wilson and Weis-Fogh, 1962). Second, in dragonflies i t is innervated through nerve 1, but in locusts i t is innervated through nerve 3 - the significance of this is discussed in the next section.
The Organisation of Motoneurons in Insect Ganglia in General
Some strikingly constant features in the way moto-neurons are organised in ganglia of insects, and perhaps of arthropods in general, are beginning to emerge. The clearest of these concern positions of cell bodies of
motoneurons that have axons in nerve 1, but generalisations about differences between the innervation of different
types of muscle are also possible.
In contrast to flight muscle motoneurons, leg muscle motoneurons of locusts (Burrows and Hoyle, 1973)
and dragonflies have cell bodies that are diverse in
36
-Dragonflies use their legs to grasp perches and prey; actions requiring fine control of muscle contraction rather than fast twitches.
All motoneurons which have cell bodies contra-lateral to the axon have axons in nerve 1 or, in fused ganglia, in nerves that can be homologised with it. This is true for one motoneuron that innervates each thoracic dorsal longitudinal muscle i~ locusts (Tyrer and Altman, 1974) and cicadas (Simmons, unpublished observation) , for locust abdominal ganglia (Lewis et al., 1973), moth abdominal ganglia (Taylor and Truman, 1974), and dragonfly abdominal ganglia (Zawarzin, 1924i Mill, 1964; Sinunons, unpublished observations). Moto-neurons of dvm3 in the dragonfly thorax emphasise the
point in that they are so numerous (Fig. 2.llb). No nerve other than nerve 1 has been found to contain motoneurons with axons contralateral to their cell bodies, and this tendency extends also to other arth-ropods, as indicated by recent work on Limulus (Levy et al., 1975) and crayfish (Wine et al., 1974).
Another constant feature 0£ the organisation of motoneurons in insects is the innervation of the dorsal longitudinal muscle. Four cell bodies of motoneurons that innervate this muscle in insects as diverse as
- 37
-1974), cicadas (Simmons and Young, unpublished) and dragonflies are situated in the next anterior ganglion. In locusts and cicadas the axons travel up the recurrent nerve, but this is absent in dragonflies and the axons enter a ganglion by nerve 1 and then travel anteriorly by the ipsilateral connective. As the recurrent nerve has been found in most insect orders this feature is
probably common to them all. The dorsal longitudinal muscle is of prime importance in providing power for flight·in
locusts and cicadas, but in dragonflies is minute in comparison, particularly in the metathorax, although its pointsof attachment are similar. No explanation
can be offered as to why such a small muscle is innervated by five motoneurons, but possibly the muscle is a vestige from an ancestral flying insect in which i t was large.
There may be constant features of neuronal organisation throughout the insects. Evolution of muscular function may well be primarily a matter of change in functional central connections. Study of the motoneuron organisation in further insect types can be expected to lead to generalisations about the
. C H A P T E R 3
Ganglion support and saltne flow
Figure 3.1
Microelectrode
Drawing of a dragonfly tethered and prepared for experirrentation.
For clarity only one miqroelectrode and one pair of extracellular
[image:85.600.26.596.34.806.2]- 38
-METHODS
To prepare a dragonfly for an experiment, legs and ventral parts of the cuticle were removed to expose the thoracic ganglia and allow direct access to some muscles (see Fig. 2.5). The dragonfly was attached to a holder made from watchmaker's forceps and suspended
upside~down (Fig. 3.1). Tethered in this way a dragon-fly could still flap its wings in a flight-like manner. A platform, fashioned from a flattened 30-gauge
hypodermic needle covered with hard pliable plastic and attached to a 26 gauge needle through which saline (Eibl, 1974) passed, was micromanipulated into place beneath the meso- and metathoracic ganglia to stabilise them against movements. The platfo~m was inserted between the pro-mesothoracic connectives, which were held by micromanipulated hooks during insertion. Tips of
small pins inserted in the plastic helped reduce movements of the ganglia. The chance of successfully preparing a dragonfly to this stage did not exceed forty percent, and preparation took between one and two hours. In this chapter, representative recordings from 82 successful preparations are presented.
at 22°c.
Experiments were performed
- 39
-connectives, and pairs of such wires were placed in various muscles. Micromanipulation was the only satisfactory way of positioning the wires, and they were used both for recording extracellular electrical activity and stimulating axon endings of motoneurons in muscles. A chloridised silver wire inserted in the abdomen served as an indifferent electrode for intracellular recording. An airjet directed at the head increased the tendency to fly and was monitored
using a thermistor. Platinum hooks or suction electrodes were used to record from and stimulate nerve trunks.
When wings were manipulated their angular movement was registered using a ten-turn potentiometer the spindle of which was attached to a crank moving the wing. Move-ments of wings produced by the animal were sometimes monitored by a capacitance device (Stange and Hardeland,
1970). Abdominal movements were measured as imbalance
in a bridge circuit of which a semiconductive strain gauge attached to the abdomen formed two arms.
To record intracellular activity from motoneurons microelectrodes drawn with glass fibres inside, filled with two molar potassium acetate, and having resistances of forty to eighty megohms were usually used. The
shanks of these electrodes were strong enough to allow the tip to penetrate the ganglionic sheath, but flexible enough to follow some movements of the ganglia occurring
- 40
-via probes to low-level D-C amplifiers incorporating a bridge circuit which allowed some current to be passed through the electrode without unduly disturbing the recording.
On penetration of the ganglionic sheath, recorded potential fell by thirty to fifty millivolts. No further fall in resting potential accompanied penetration of a motoneuron cell body but this was signified by appearance of ripples in potential or by spikes. The following criteria, similar to those used by Hoyle and Burrows
(1973a), were used to identify a motoneuron as innervating a particular muscle:
(a) Strict correlation of spikes recorded in the cell body with spikes in an axon innervating a particular muscle (e.g. Figs. 3.4a; 3.Sd), with post-synaptic
potentials in muscle fibres (Figs. 3.Sf ,g), or with twitches in the muscle.
(b) Production of antidromic spikes by stimulating axon terminals in muscles with low voltage pulses lms long.
(c) Position of the electrode tip in the ganglion, and general shape of activity recorded - these were not rigid criteria for identification, but served as reliable guides in later experiments after establishment of a
motoneuron map.
All
the
rnoboneu.ronsdescribed
in fh.e,results
.secf;(<M.we-re.
iclerit-;fiecl
h~
(:he, c,riberill.
se,b ov.b in
Gt>
c.u1c1{b), (,\hove.
Most recordings were filmed directly from the oscilloscope screen as they occurred, but sometimes- 41
-RESULTS
Patterns of Motoneuron Spikes During Flight and Their Central Origin
During tethered f ligh~bursts of spikes at about twenty per second occur in motoneurons that innervate elevator and depressor muscles, bursts to the elevator muscles alternating with bursts to the depressors. Observations with a stroboscope show that wingbeat
frequency in tethered Jragonf lies varies between ten and forty beats per second, and that all four wings can show considerable independence in their movements.
All the flight motoneurons studied produce fast twitches in the muscles they innervate. Myograms are often difficult to interpret because of the number of motoneurons, each of which may spike several times in a wingbeat, and because electrodes often move when the muscles contract vigorously. Myogr~ms in Fig. 3.2 show some features of recordings during flight.
v--'
fetevation
c.
dvm
1d.
dvm
1Figure 3.2
Myograms from rretathoracic muscles in tethered flying
dragon-flies. (a): alternate activity in an elevator muscle, dvm6 ,
and a depressor muscle, dvm3 • Al though dvm3 is inneJ:Vated by
four to five tines as many rrotoneurons as dvm6 , the dvm3 myograrn
is · simpler. (b) : there is a phase difference between
tines of spikes to two depressor muscles, dvm3 and prn1 •
(c): correlation between the myograrn from the elevator muscle,
dvrn1 and rroverrent in the wing. (d) : recording with a suction
electrode from a few fibres of dvrn1 • Note the variation in the
number of spikes per burst. 'Ihis muscle is innervated by three
[image:90.597.33.596.33.797.2]