JOURNAL OF VIROLOGY, May1979,p.610-623 Vol.30,No. 2 0022-538X/79/05-0610/ 14$02.00/0
Tryptic Peptide
Analysis of gag and
gag-pol
Gene
Products
of
Rauscher Murine
Leukemia
Virus
J. J. KOPCHICK, W. L.KARSHIN, AND R. B. ARLINGHAUS*
Departmentof Biology, The University of Texas System Cancer Center, M. D. AndersonHospitaland TumorInstitute, Houston, Texas 77030
Received forpublication19January1979
[3H]tyrosine-labeled viral precursor polyproteins and knownmatureviral pro-teinsderivedfrom the Rauscher murine leukemia virus gag and pol geneswere
examinedby two-dimensional tryptic peptide mapping.
PrMy0gaP"
wasfoundtocontainpeptidesequences of the viralcoreproteins p30, p15, p12, andp1O,aswell
aspeptidesequences found in the cell-associated reversetranscriptase.
Interme-diate reverse transcriptase precursor Pr125P'° lacked peptide sequences of the four-core proteins but contained reverse transcriptase-specific tryptic
peptides
plus twoadditionaltyrosine-containing tryptic peptidesnotrelatedtogag orpolgeneproducts. Methionine-containingtryptic peptide analysis also suggested the
presenceofadditionalprotein material in Pr125,"' (Kopchick etal., Proc. Natl. Acad.Sci. U.S.A.75:2016-2020, 1978).
Pr20&gagP"',
althoughcontaining bothviral core and reverse transcriptase-associated methionine and tyrosine tryptic pep-tides, also contained additional tryptic peptides. These are of two classes: (i)tryptic peptides associated with the Pr125P'l but not
Pr8"0"'
and (ii) trypticpeptidesnotfound inPr125"'P orin any known viralprotein. Oneinterpretation
of these results is that
Pr200g'g-1°'
contains additional gene products aside from the gagand pol genes.Pr80QagandPr659'9peptide maps were also examinedandfound tohave sequences ofall four core proteins. Pr65gagwasfound to contain
two p30 tyrosine tryptic peptides that were absent in
Pr808'g,
suggesting thatPr8Og`gmaynot be the precursortoPr65. Pr8Og",asexpected from itslarger
size, alsocontainedtryptic peptidesnotfound inPr659"(.Twoof theseadditional
Pr8g5ag
tryptic peptideswerefoundinPr80P°o aswell butnot in any of theviralcoreproteins, suggestingthatPr8fgagand Pr80I may have overlappingpeptide
sequences. ConSistentwith thisfindingis the conclusion that
Pr80g'9
terminates within the pol gene. A model that describes the relationship of these recentfindingstoviralgene productsispresented.
The genome of type C nondefective retrovi-ruses is knownto containthreegenetic regions required for replication, known as gag (group
antigens, or core proteins),pol [viral
RNA-di-rected DNA polymerase, or reverse
transcrip-tase(RT)],andenv(envelope proteins) (4). The
proteins encoded in these genetic regions are
known to besynthesized by way of high-molec-ular-weight precursor polyproteins that are cleaved and processed in infected cells to form
matureviral proteins (1-3, 13, 20, 25, 31, 32). In
cells infected with Rauscher murine leukemia
virus(R-MuLV), gag gene-derived core polypro-tein precursors of 80,000 daltons (Pr80ag) and 65,000 daltons (Pr659a') are found (1-3, 20), along witha 200,000-dalton RT precursor poly-protein
(Pr2009a9P")
thatcontains core protein, RTantigenicdeterminants, andp30tryptic pep-tides (1, 2, 10).Theseprecursorpolyproteinsaresynthesized
in vivo (10), as well as in a cell-free protein
synthesisprogrammedwith 35S R-MuLV RNA,
inaratio of about1 mol of
Pr200ga'.Pol
to20molof
Pr8Ogas
plus Pr659'9 (10, 19). A translationalcontrol modelhasbeenproposedtoexplainthe
differential level ofsynthesisofcoreprotein as
comparedwith theviralpolymerase (10, 13, 19).
Peptide mapping studies of precursors and
matureviralproteinshavebeenperformed (13).
Tosimplifythecomplexityof thepeptide maps
of higher-molecular-weight proteins,
methio-nine-labeledviralproteinswereexamined;
how-ever, it was found that the only core proteins that contain methionine are
p30
andp12
(11).Thelattercontainsanacidic methionine tryptic
peptide that does not bind to the
cation-ex-change column (1) usedto generate thepeptide
maps previously published (1). Under reducing
conditions,two
p30
methionine-containingtryp-tic peptides, aswell asseveral
methionine-con-610
on November 10, 2019 by guest
http://jvi.asm.org/
PEPTIDE MAPS OF gag-pol GENE PRODUCTS 611
taining tryptic peptides derived from the viral
polymerase (13),areresolved bythisprocedure.
Inthis regard, M00ga9P°wasfoundtocontain
both p30 methionine-containing tryptic
pep-tides, as well as the viral polymerase-specific
tryptic peptides (13). However, the viral RT
(p8OPoI) contained one methionine-containing
tryptic peptide that comigrated with one of the
p30methionine-containing tryptic peptides. To
investigate the protein content of Pr2009a9-Po
further, we labeled virus-specific proteins and
theirprecursors with radioactive tyrosine,since
tyrosine was found to be present in all of the
core proteins and RT (1). We present results
whichshow that the core proteins p30, p15, p12,
and plO, as well asthe DNApolymerase of
R-MuLV, are containedinthe
200,000-molecular-weight polyprotein RT precursor, Pr200f'.
Most importantly, we found that
Pr2009g-P`
contains additional tryptic peptide sequences
notfoundinthemature corestructuralproteins
or virion polymerase. Furthermore, these pep-tides mappingstudies suggestthat Pr809agand
cell-associatedRThavepartialoverlapping
pep-tidesequences. In addition, a comparison map
ofPr809agandPr659aR suggeststhatPr809agmay
notbeprocessedtothe coreproteinPr65Rag.
MATERLALS AND METHODS
Cells and virus. NIH Swiss mouseembryo cells
(JLS-V16) infected with R-MuLV were used in this study (20). The culture mediumwasamodifiedEagle
formulacontaining 10% fetal calf serum,asdescribed by Syrewiczetal. (28). Cellswere grownin 2-ounce (ca.60-ml) prescriptionbottlesor2-quart(ca.1.9-liter)
roller bottles. Cellsweresubconfluent beforeuse.
Vi-rus waspurifiedasdescribedpreviously(28).
Labelingof cells and virus. Cellswererinsedin warmHanks balanced salt solution andpulse-labeled at37°C.RadioactiveR-MuLVwasobtainedby label-ingaroller bottle ofJLS-V5 R-MuLV-infected cells (28) with 50 mCi of
[3H]tyrosine
for48h ingrowthmediumcontaininglxEagle amino acids.
Immunoprecipitation of viral proteins from cells. Thepreparationofcytoplasmicextractswas as
described previously (20). Briefly, cell lysiswas per-formed in lysisbuffer containing 0.5% Nonidet P-40
(Particle Data Laboratories, Ltd.) and 0.5% sodium
deoxycholate (Schwarz/Mann) (1).Monospecificgoat antiserapreparedagainstR-MuLVp30,p15, p12,plO,
gp69/71, and
p80IW0'
wereobtainedthroughthe Office ofProgram Resources andLogistics, ViralOncology,National Institutes of Health. Pulse-labeled cellswere
lysedby homogenizationin2to 10ml oflysisbuffer. All antisera were absorbed with excess uninfected
JLS-V16 cellcytoplasmicextracts(2).Also,antiserum
directed against
p80P"
wasabsorbed with disrupted virus as describedpreviously (10).For indirectimmu-noprecipitation,themonospecificantiseraweremixed
withcytoplasmic extracts. Incubation was for15min at roomtemperature and overnightat 4°C. For the second immunereaction, 20volumes of rabbit
anti-goat serum (relative to the volumes of antiserum used in thefirst immune reaction) was added for 15minat room temperatureand 4°C overnight. In a few exper-iments, indirectimmunoprecipitation was carriedout by using the staphylococcal protein A method as de-scribedby Kessler (12). The indirect immunoprecipi-tates and the staphylococcal protein A precipitates werecollected by centrifugation at 10,000 rpm for 20 min through a1.5-ml cushion of immune buffer (0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.02 M Tris-hydrochloride [pH 7.5], and 0.05 M NaCl) containing 1 M sucrose, 1% Triton X-100, and 1% tyrosine ina Sorvall GSAswinging bucket rotor.
Gelelectrophoresis. Sodium dodecyl sulfate-poly-acrylamide gelelectrophoresis (SDS-PAGE) was per-formed on gel slabs, using the buffer system described by Laemmli (14). Viral proteinsp30, p15, p12, and plO were separated on an 11.25% gel, and precursor pro-teins
MW0"",
Pr80Y"'9, pMY5, and Pr80/"' were resolved on a 6 to 12% gradient gel. The gels were subjectedtofluorography as described previously (5). Toobtain a linear response to radioactivity, the X-ray films werepreflashed (15).Purification of viral proteins. Viral proteins p30, p15, p12, and plO were first purified by guanidine-hydrochloride agarose chromatography (9). The pro-teins were purified further by SDS-PAGE as part of thetrypticdigestion procedure.
Peptide mapping. Tryptic digestion was carried
outbyincubating the dry slab gel band at 37°C in 2 to
3mlof0.05 MNH4HCO3 (pH 8.5) containing 50jigof trypsin per ml. After 15 h, an additional 50 jig of trypsin per ml was added, and the incubation was continued for 8 h.Tolylsulfonyl phenylalanyl chloro-methyl ketone (TPCK)-trypsin (Worthington Bio-chemicalsCorp.) wasstoredat -20°Cat 5mg/ml in
0.01M HCIcontaining1mMCaCl2. Fragments of gel wereremoved by filtration through0.45-jimcellulose nitrate filters, and the supernatant fluids containing the soluble tryptic peptides werelyophilized. Radio-activity recovery from thegel varied from60 to80%. Thepeptidesweredissolved in0.2mlof bufferA(33), and the sample was clarified by centrifugation at
10,000 x g for 10 min. The tryptic peptides were resolved by cation-exchange chromatographyas de-scribed previously (1) orbytwo-dimensional finger-printing. The latter procedurewasperformed by spot-ting the[3H]tyrosine-containingtrypticpeptidesona
cellulosethin-layer plate (20 by20cm). Thepeptides were separated in thefirst dimension by electropho-resis at 150 V in a 28% formic acid solution. After drying, the peptides wereresolved in the second di-mensionbyascendingchromatography ina butanol-pyridine-acetic acid-water (6.2:1:3.3:2.8) buffer system. 2,5-Diphenyloxazole (PPO) wasapplied to the cellu-losethin-layer platesbyascending chromatography in
anacetonesolutioncontaining10% PPO(wt/vol).To obtain a linear response to radioactivity, the X-ray filmswerepreflashed (15).
RESULTS
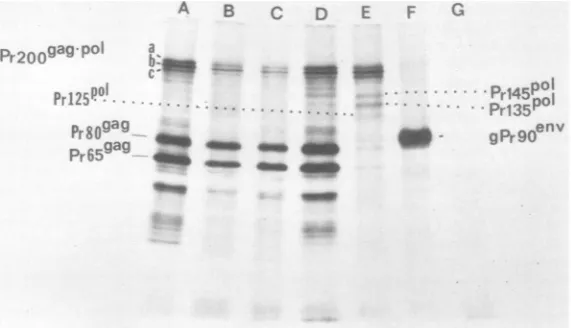
Immunoprecipitation of pulse-labeled
cell extracts with
monospecific
antiserapreparedagainst
gag,pol,
andenvproteins.VOL. 30, 1979
on November 10, 2019 by guest
http://jvi.asm.org/
612
KOPCHICK, KARSHIN, AND ARLINGHAUSWereportedpreviouslythatviralstructural
pro-teinsaremadeininfected cellsby synthesisand
proteolytic cleavage oflarger-molecular-weight
precursorpolyproteins (1-3, 10, 13, 20). In Fig.
1, infected cells were pulse-labeled for 15 min
with[3H]tyrosine and immunoprecipitated with
antiserum to the core, RT, and envelope
pro-teins.Anti-ovalbumin was used as a control(Fig.
1, lane G). Theimmunoprecipitates were
ana-lyzedon a 6 to 12%gradient gel. These
experi-ments have identified several rapidly labeled
polypeptides termed
Pr200g'g-P"
(Fig.1, a,b, andc), Pr145P°Z-Pr125POI,
Pr809as,
Pr65ag,
andgPr9oen. It is evident that anti-p30, anti-p15,
anti-p12, and anti-plO recognized Pr20Og'g-P°
(Fig. 1,lanesAthrough D). This indicatesthat
Pr200ea'PoI shares antigenic determinants with
all of the viral
group-specific
antigens, asre-ported
previously
(10). Antiserum madeagainst
the viral DNA polymerase also
precipitated
Pr2Offas'Po (Fig. 1, lane E), as previously
re-ported,aswell asPr145Po,
Pr135P'1,
andPr125PI(10, 13). Antiserum directed against the major
viralglycoprotein did not recognize
Pr200eaggPo
but didimmunoprecipitate a 90,000-dalton
en-velope precursordesignated gPr9oen', whichhas
beenshowntocontainp15(E) and gp69/71
tryp-tic peptides (11). Two additional polyprotein
precursors were precipitated by antiserum to
core proteins. They are termed Pr80Sa9 and
Pr65sas
(Fig. 1, lanes A through D) and have been shownbytryptic mappingtobestructur-ally relatedtothe fourviral coreproteins (1, 3).
Anti-plOserum (Fig. 1, lane D) recognized two
protein bands with approximate molecular
weights of 170,000 and 145,000,respectively,the
smaller of which
migrated slightly
slower thanPr145PoI
(comparelane D with lane E, Fig. 1). Wehaveshownpreviously by pulse-chaseex-perimentsthat
pM20fyasPOI, Pr8099a,
andgPr9Oenl
areunstableproteins (1-3, 10, 11, 13, 20,21). In
pulse-chase incubations with various
monospe-cific antisera, we have shown that the mature
structural proteins, as
exemplified
by p30 andp15,
accumulate
under chase conditions, withtheconcomitant deceasein
Pr80Sa9
andPr65SaS
(2, 10, 20). Theintermediate stable polymerase
precursors
Pr135PoI
andPr125Po,
aswellastheintracellular 80,000-molecular-weight
RT-re-latedprotein
Pr8OPoI,
whichhasbeen shown tobenearly identicalto virionpolymerase
(p8OPoI)
(13), also accumulate during the chase at the
expense of
Pr200gagPo
(10, 13) (see Fig. 2 of reference 13). The virion envelope proteinsgp69/71
andp15(E)
also appear in the chaseincubation and are derived from
gPr90en',
aspreviously reported (21).
Comparison of the tryptic
peptide
se-quencesof thecoreprecursor
polyproteins
with thematurestructural
proteins.
Cation-exchangechromatography of tryptic digestshas
revealed thatmethionine-containing
p30
trypticpeptides are present in
Pr89ya9,
Pr65SaS,
andPr200easgIg
(2). Also, by the same procedure,Pr809as
andPr65sas
were shown to sharetyro-sine-containing tryptic peptides with the viral
structural proteins
p30,
p15, p12, and plO (1).Pr8O9a9
wasshowntocontainone extratyrosine-containing tryptic peptide (1). To examine
fur-ther the relationship of thegag precursors to
A B C D E F G
p
20flgag
rpol
Pri2200I
Pr
t2;5P°'
Pr8gfag
P,65gag
a
Pt145po PO
'35P'
-gPr9O
en-.
-am _0Memm~
FIG. 1. Kineticsofformation of R-MuLV precursor polypeptides. R-MuL V-infected JLS-V16cells (=5 x
10' cellsin l-quart [ca. 0.9-liter]prescription bottle) were pulsed-labeled for 15min with 20 ml of Hanks balanced salt solution containing[3H]tyrosine (150juCi/ml). Thecytoplasmic extracts were treated with 50
Al
ofthefollowingantisera: (A) anti-p30, (B)anti-p15,(C)anti-p12, (D) anti-plO, (E) anti-RT, (F)anti-gp69/71,(G)anti-ovalbumin. Theimmunoprecipitates were analyzed by SDS-PAGE on a 6 to 12% slab gel.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.497.119.405.429.593.2]PEPTIDE MAPS OF gag-pol GENE PRODUCTS 613
B
p15
A
p30
279 *
96
4
0
PlO
10
D
p12
FIG. 2. Two-dimensional trypticpeptide maps of[:H]tyrosine-labeled viralcoreproteins.
[;'Hityrosine-labeled viral structuralproteinsp30(A), p15, (B), plO (C), and p12 (D)werepurified by guanidine-hydrochlo-ride-agarose chromatography, followed by SDS-PAGE. The purified proteins were digested with TPCK-trypsin andseparatedon cellulosethin-layer plates in the first dimension by electrophoresis (left toright), followed by ascending chromatography. PPO was applied to the plates by ascending chromatography. Approximately 30,000 cpmofeachtryptic digestwereappliedtothethin-layer plate. The plateswereexposed
topreflashed X-rayfilms for2 to 3weeks.
the virionstructural
proteins
p30,p15, p12, andplO, wedigestedthese
tyrosine-labeled
proteinswith trypsin andfractionated themby
electro-phoresisinonedimension andchromatography
in a second dimension.
Figure
2Athrough
Dshows theresults ofanalyses oftryptic digests
ofp30, p15, plO,andp12,respectively.Viralp30
wasfoundtocontain sixmajor
tyrosine-contain-ing tryptic peptides (no. 2, 4, 5, 9, 27, and 28)
and several minor spots. Viralp15andp12each consisted oftwomajorandtwominor
tyrosine-containingpeptides.Themajor p15peptidesare
numbered6and8; those fromp12arenumbered
7 and 25. Viral plO contained one major and
severalminorpeptides.The minor
tyrosine-con-taining trypticpeptidespotswere notidentified
because ofvariability in detection from
experi-ment to experiment. Mixing experiments were
performed with p30-p15, p3O-p12, and p15-plO
tryptic digests (data not shown). This allowed
the location of the trypticpeptides of the indi-vidualproteins with respectto each other. For
example,themajorplOtryptic peptide (spot 3)
liesdirectly beneath spot 6, which isoneof the
two p15 tyrosine-containing tryptic peptides. The plO peptide (spot 3) was also found to lie
on adiagonalbetweenp30spots2and 4, andso
on(Fig.2).
VOL. 30, 1979
a
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.497.48.447.73.447.2]614 SOPCHICK, KARSHIN, AND ARLINGHAUS
Figures 3 and 4 show the two-dimensional
peptidemaps ofPr8099aand
P65gas,
respectively.Analysis of the results indicated that Pr809'9
(Fig. 3) and Pr65,as (Fig. 4) contained
p30-spe-cific peptides (spots 2, 4, 5, and 9), one p15
peptide (spot 6), p12 peptides (spots7 and
25),
andthe plOpeptide (spot 3).These
two-dimen-sional maps confirm our findings that
Pr80Sa9
and Pr65sasshare much of theirproteinsequence
information (1, 2, 10) and that they contain
sequences of all four viral core proteins.
Al-though spot 8 ofp15 appears tobe present in
both Pr8O9a9 andpM5 ag, thisregionof themaps
is not well resolved. This lack of resolution,
combinedwith ourpreviousstudieswith column
chromatography (1), which indicated thatonly
one ofthe two p15 tyrosine-labeled peptides is
present in Pr809ag and
pM55ag,
raises some doubt as to whether both spots 6 and 8 of p15 are present in these precursors. We marked spot 8with a question mark to indicate this
uncer-tainty. We note that both Pr809ag and
Pr65sag
contained
tyrosine-containing
tryptic peptides(spots 26 and 34) that are not found in any of
the core proteins. Another interesting point is
that
Pr65sag
contained tryptic peptides(spots 27,28, and 33) not found in Pr809ag.
Specifically,
tryptic peptide spots 27 and 28 of
p30
originweremissingin
Pr809ag,
andspot 33appearedtobeuniqueto
Pr65sag.
Pr80yag
also containedad-ditionalspots not found in
Pr65sag
(spots 1, 10,and 29 and one spot at the top center of the
Pr80ag map). The facts that some p30tryptic
peptidesweremissinginPr80gaG but were
pres-* 29
9 10 9
34
26
*
j t.
0
FIG. 3. Two-dimensionaltryptic peptide map of[3H]tyrosine-labeled Pr8O"'9.R-MuLV-infectedJLS-V16
cellswerepulsed for15minwith 15 mlof[3H]tyrosine(1501LCi/ml)inHanks balanced salt solution at 37°C. Thecytoplasmicextractwastreatedwithanti-p30, and theimmunoprecipitatewasanalyzedbySDS-PAGE.
Pr80w'waseluted anddigestedwithtrypsin,and the[3H]tyrosine-labeledtryptic peptides were resolved as
described inthelegendtoFig.2.Approximately 70,000 cpm were applied, with a 5-week exposure time.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
PEPTIDE MAPS OF gag-pol GENE PRODUCTS
28$
5
94
93
25 2
Q
0
FIG. 4. Two-dimensional tryptic peptide map of[3H]tyrosine-labeledPr65g. PrO5ag was isolated, and thetryptic peptides were resolved as described in the legend to Fig. 3. Approximately 50,000 cpm were applied, witha4-week exposure time.
ent in Pr659'9 and that Pr65Vg contained
an-other tyrosine-containing tryptic peptide (spot
33) not found in Pr80g9g suggest that Pr809'9
may notbe theprecursor to
Pr659'9,
aconclusionthat is indisagreementwith our previous
inter-pretations (1, 2, 10). However, further work is
neededonthispoint.
Peptide
mapsofPr2OO9"0-"1
and theinter-mediate
RTprecursors.Pr20(gYg-P"
sharesan-tigenic determinants withthe group-specific
an-tigens p30, p15, p12, and plO and the virion
RNA-directed DNApolymerase (Fig. 1 and
ref-erence 10).
Pr200gag9P"
has also been shown toshare methionine-containing tryptic peptides
withthe major viral structural protein p30 (2). In the present study we further characterized the
tryptic
peptide sequences present inPr2009ag-P°',
placing particular emphasis onde-termining whether all four viral core proteins
are present in
Pr200ga"'.
The results (Fig. 5) showedthatp30spots 2, 4, 5, and 9 are presentin Pgr200gagP. Viralp15spot 6, p12 spot 7, and
plO spot 3 werealso present inPr200gag-Po.There
aremanyotherspots in
Pr200gag°"'
that are notrelated to thosepresent in Pr809ag (Fig. 3) and inPr659ag (Fig. 4). Some of these spots, i.e., spots
10, 13 to 20, 29, and 36, were found in
cell-associated RT
(Pr80PO')
(compareFig.
5and6).
Thus, these results show that
M009ag-P°
con-tainssequencesfoundin all four viral core
po-teins,aswellas
peptide
sequences found in theRT; the latter is inagreement withour
previous
findings with
methionine-containing tryptic
pep-tides(13). We noted that Pr80MYo didnotcontain
any of the
peptide
spots characteristic of p15,p12,or plO (compare
Fig.
4 and6).
Pr8O""'
didcontain one peptide which is characteristic of
p30, i.e., spot 4. However, the other five p30
tyrosine-containing peptides
are not found inPr8OP°I.
It remains to be determined whether spot4inPr8MOI°
isabona fidep30peptide.Anotherinteresting fact about
Pr200Og"P°
isspots 10and29(Fig. 5).These
tyrosine-contain-ing peptides are presentin all RT-related
pro-teins (Fig. 5 through 7)
(Pr2009ag-1°1,
Pr125Po',
andPr80IP°),
aswellasinthe gag geneproduct
Pr80Vg (Fig. 3). Theyare,however,absent from
Pr65'9andallof the viral coreproteins.These
resultssuggestan
overlap
betweenPr809`9andPr80M°o
and also indicate that the gag andpol genes are adjacent onthe viral genome. How-ever,further work is neededtofirmly
establishVOL. 30, 1979 615
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.497.103.395.69.346.2]616 KOPCHICK, KARSHIN, AND ARLINGHAUS
26
3..14
S.
I
"P
*24
.35
16 28
36
2
32
23 22
3-,
i8
FIG. 5. Two-dimensionaltryptic peptidemapof [3H]tyrosine-labeledPr20099P°.Amixtureof the b andc
componentsofPr2OO'90P" wasisolated,andthetryptic peptideswereresolvedasdescribed inthelegendto
Fig.3.Approximately 100,000cpm wereapplied,witha12-weekexposuretime.
the presence of shared peptide sequences
be-tweenPr809'9andRT-relatedproteins.
The intermediate RT precursors (Prl45Po-Pr125POI) were also examined by
two-dimen-sional peptide mapping (Fig. 7). Pr125PI
con-tained the tyrosine-labeled RT-specific tryptic peptides 10, 11, 13to21, 24, 29,and 36 (compare Fig. 6 and 7). Pr125Pol also contained spots 12
and21.Spot21waspresentin
Pr2009'9-1°;
how-ever, spots 11 and 12 were not (Fig. 5). The
tyrosine-labeled peptide maps of Pr145PoI and
Pr135PY were verysimilartothemapof Pr125Po/
(data not shown). One cannot readily observe
spots4and36inFig.7;however,ontheoriginal
fluorograph, one candetect these peptides. We
emphasize that Pr145Pol-Pr125Po didnotcontain
anypeptide spots, except spot 4, characteristic ofthe viral core proteins. They did, however,
containspots 12and21notfound inanyof the
viralcoreproteins, RT,orthegaggeneproducts
ofPr80Vg and Pr659as. The possible nature of
thesesequences will be discussed below. These
results indicate that Pr145Po0-Pr125PoI did not ariseby partial removal of thegaggeneproducts orbyaprematureterminationevent. If
prema-ture termination were the cause of
Pr145PoI-Pr125p°' production, thenallof thecoreproteins
would beexpected in
Pr145P°'-Pr125PI'
andpos-sibly only part of the RT sequences. This is
clearlynotthecase.
Anotherimportant point is that the complex-ity of the peptidemapsof
Pr200g-P°,
Pr125PI,andPr801P° decreased with the decrease in
ap-parentmolecularweight.
Pr20099fgPoI
had about30 major tyrosine-labeled tryptic peptidesthat can beresolved;
Pr125PO'
had 16 peptide spots; Pr80Pol had 14 tyrosine-containing tryptic pep-tide spots. Thus,PrQ00939aPOI
and the intermedi-ateRTprecursorsarenotaggregatesofPr80PI. Noncore and non-RTtryptic peptidesas-sociated with
Pr2009-1"°.
Pr2009a9P°1
is theprimarygeneproductthat leadstothe formation
of the RT (10, 13). Our previous published
ex-periments have indicated that
Pr2009gaPOI,
aswellasthe intermedite RTprecursors, contains
methionine-labeledtryptic peptidesthatarenot found ingagorpolgene products (13). Inthis
paper,analyses of two-dimensional [3H]tyrosine-labeled tryptic peptide maps of
Pr2009ag-P",
aswellasthe intermediate RTprecursors
(Pr125P"I,
Pr135Pol, and Pr145POI) and the viral core pro-teins, gave results that are consistent with a20
*f 17
3
0
1I
J. VIROL.
I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.497.116.399.70.351.2]PEPTIDE MAPS OF gag-pol GENE PRODUCTS
20 29
10
ii
,13
1424 15 24
is 17
4
36 18
19
[image:8.497.109.396.68.336.2]0
FIG. 6. Two-dimensionaltrypticpeptide mapof[3H]tyrosine-labeled Pr80". Pr8OP"'wasisolated from R-MuLV-infectedJLS-V16 cells. The cellswerepulsedfor15minwith
[3H]tyrosine
(150,uCi/ml) in25mlof Hanks balanced salt solution at37°C. After pulsing,the cellsheetwasrinsed with Hanks balanced salt solution, and completegrowthmediumwasaddedfor1h.Thecytoplasmicextract wastreatedwith absorbed anti-RT serum, and theimmunoprecipitatewasprocessedasdescribed in thelegendtoFig.1.Digestionwith trypsin and two-dimensionalmappingwere asdescribed in thelegendtoFig.2.Approximately20,000 cpmwereapplied,witha4-week exposuretime.
similar interpretation. Thus, tyrosine-labeled
peptide 21 was found in
Pr200eaggP"
and in theintermediate RT precursors, but it was absent
in
Pr80PO'
and in all of the four core proteins(Table 1). This is the only tyrosine-containing
peptide shared bypr2000'9agPo andPr125POl that
isnotfoundineither
Pr80oI
or thecore proteins.Also, spot 12is ofinterest since itwas not seen
in
Pr200g'g-P"
but appeared inPr145POI, Pr135Pol,
andPr125POI butnotinPr80OP°. Spot 11was not
present in Pr20ffas9Po butwasfound in
cell-as-sociated RT, aswell as intheintermediate RT
precursors.
Inadditiontotrypticpeptides thatareshared
by
Pr200g'g-P"
and theintermediate RTprecur-sors, Pr200g'g-P° has tyrosine-labeled tryptic
peptidesthat arefound onlyin
Pr2)g(a5g-PO
(Ta-ble 1). None of the known viral gene products contains these tryptic peptides (see composite shown inFig. 8). Spots 22, 23, 30 to 32, 35, and 37 are
examples
of suchtryptic peptides, spots35and37beingminor spots (Table1).
Methionine-containing
tryptic
peptides
of
Pr200"-P°.
Figure9showsa[35S]methionine
profileobtainedbyfractionatingatrypticdigest
of
Pr2009'9-P'1
(the b component) on acation-exchange column, asdescribed previously (13).
A similar profile was obtained from band a,
except that one major methionine-containing
peptide was found atthe position indicated by
the arrow in Fig. 9. Peaks 8 and 10 in Fig. 9
comigrate with p30 tryptic peptides (13). The
other
methionine-containing
coreprotein,
p12, has an acidic tryptic peptide that elutes intheflow-through
of the column(1).
Although
themajority of the methionine-containing tryptic
peptidesin
Pr200g'g-P"
areRT related (13),sevenpeptides (peaks 2, 4 5, 12 to 14, and 16) are
neithercoreproteinnorRTrelated(Table1and
reference13). Three of thepeptides(peaks 5, 14, and 16) are found in
Pr2009oa9PO
but not in any ofthe core or lower-molecular-weight RT pre-cursors. Peaks 2, 4, 12, and 13 are found in Pr200gagYPo and thelower-molecular-weight RT precursors Pr145P°',Pr135P01,
andPr125Pol
butnot in Pr80°OP or in any of the core proteins
(Table 1 and reference 13). These latter
se-quences may be derived from the same
se-VOL. 30, 1979 617
on November 10, 2019 by guest
http://jvi.asm.org/
618 KOPCHICK, KARSHIN, AND ARLINGHAUS
20
29
.. 10
; 11 13
12 14
15 24
16
17 4
36
18
19
0
FIG. 7. Two-dimensionaltrypticpeptidemapof[3H]tyrosine-labeled Pr125PoI. Pr125PoIwasisolated and mappedasdescribed in thelegendtoFig. 6.Approximately20,000 cpmwereapplied; the exposuretimewas
Imonth.
TABLE 1. Radioactivetyrosine-andmethionine-containing trypticpeptidesassociated withgag andpol geneproducts and proposed A, B, and C viralproteins
Viralpro- gag A pol B C
tein tyro' methb tyro meth tyro meth tyro meth tyro meth
Mr20ag-P"' 1-7,8?, 8,10 10,29 4,10, 13-20, 1, 3, 6,7, 9, 21 2, 4, 22, 23, 5, 14,
9, 27, 24, 29,36 10,11, 15 12, 30, 31, 16
28 13 32
Pr125P"' 10,29 4,10, 11,13- 1, 3, 6,7, 9, 12,21 2,4, 20,24, 29, 10, 11, 15 12,
36 13
Pr80P"' 10,29 4,10, 11,13- 1,3, 6,7, 9, 20,24, 29, 10, 11, 15 36
Pr8o""5 1-7,8?, 8,10 10,29 9,25,
26,34 Pr65"-' 2-7,8?, 8,10
9, 25-28, 33, 34
p30 2-5,9, 8,10 27,28
p15 6, 8 None
p12 7,25
plO 3 None
aTyrosine(tyro)-labeled trypticpeptidesarenumberedasshown inFig.5and 8.
Methionine(meth)-labeled trypticpeptidesarenumbered as shown inFig.9.Thepeptidemapsrelating to the methionine-containingtryptic peptides havebeenpublishedelsewhere(13).
quences thatyieldtyrosine-containing peptides
12 and 21. It is possible that they represent a
protein which is cleaved from Pr125po'toyield
Pr801J'.Theputative proteinwould beexpected
tohaveamolecularweight of about40,000.
Themethionine-containingtrypticpeptides5,
14,and16and the fivemajortyrosine-containing
tryptic peptides (spots 22, 23, 30 to 32) found
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.497.113.398.68.323.2] [image:9.497.61.453.385.588.2]PEPTIDE MAPS OF gag-pol GENE PRODUCTS 619
29
270 70
704
35 16%
28U
2
0
IC) FIG. 8. Schematic ofthe tide map ofPr200`9P°. TI
shown in Fig. 5have been gene related (0), polgene i
protein ((). Spots 10 and 2,
related.
0 20 40 60 80
Fractij
FIG. 9. Cation-exchange I component of [3H]methion [3H]methionine-labeled tryl tionsareidentifiedby numb thelocation ofapeak founc
Pr200ga9-°' but missing from
only in Pr2()gagaPol could viralgeneproduct. Two of
(butnotall) could be jun cussedbelow.
DISCUS' The results presented previous fmdings that PI
genic determinants and
quences with the core
Pr2009'9-P°l is comprised labeled "a", "b", and "c'
20 antigenically and
structurally
related based onimmunoprecipitation experiments with mono-specific sera (Fig. 1) andmethionine-containing
32 tryptic peptide analyses on ion-exchange
col-10 umns (2, 13). When oxidized
methionine-con-4M ,13
tainig
tryptic
peptides
werecompared
(2),
the"a" polypeptide was found to be closely related,
V24
%W15
but not identical, to the "b" polypeptide. Underreducing conditions, the methionine-labeled
6D 22
tryptic peptide profiles
of al threepolypeptides
d4
p~
UP were similar(G.
A.Jamjoom
and R. B.Arling-(5
haus,
unpublished
data).
TreatmentofceUs
with4
41
4I37
theprotease
inhibitor TPCK caused the "a" polypeptide to accumulate at the expense of the 9§18 "b" and "c"polypeptides
(10).
Similar results3Q
were obtained when the amino acid analogcan-avanine was substituted for arginine during
pulse-labeling experiments (10). Thus, the a
o0
119
polypeptide
appears to be the precursor to theb and c polypeptides. In this regard, we note
that Evans et al. (6) reported that 220,000- and
3Hetyrosine-labeled
pep- 230,000-dalton polypeptides containing corepro-ie
tryptic peptide
spots teins bind to lectincolumns.Wehave found thatidentified
as eithergag one of the three bands ofPr2009`9-P°
becomesrelated (0), or as extra labeled
with radioactive
mannose(J.
J.Kop-9 are bothgagandpoi chick, V. Ng, and R. B.Arlinghaus,unpublished data).
Pr80P') has been shown to be formed by
syn-thesis and cleavage of a high-molecular-weight
lo precursor polyprotein,
Pr2009g-P°
(13).Antise-rumpreparedagainst RT specifically recognized
Pr200919-Po1
and three intermediate polypeptides,Pr145P°1,
Pr135POl, and Pr125PIo. Anotherpoly-89 11 121314 1516 peptide,
Pr80P°1,
identical in size to the maturelI
viral enzymep80P01,
is found in infectedcells
(13).
The twoproteins
appearidentical relativeto their methionine-containing tryptic peptide
profiles, with the exception of one peak (13).
Theresults reportedherefurther emphasizethe
100 120 140 160 180 relationship between
Pr80OP°
andPr2Wag-P°l.
All [image:10.497.48.234.62.275.2]on Number except one (spot 11) of the tyrosine-containing
elution profile of the b tryptic peptidesfound inPr80(P°'arealsoseenin
ine-labeled Pr2009ag-P°l.
Pr2009ag-P°l.
All ofthese findings, together withDtic peptide peak frac- pulse-chase experiments done in the presence of
ers. The arrowindicates cycloheximide (13), indicate that
Pr80°°'
is de-i in the acomponent Of rived by cleavage ofPr2009agcP°'
through inter-the b component. mediatepol
precursors(Pr145P°I,
Pr135Pol,
andbe another unknown
Pr125Io').
'thesetrypticpeptides Our previous work showedthat one of the RT
Lctionpeptides, asdis- methionine-containing tryptic peptides comi-grated with one of thetwo p30
methionine-con-SION
taining
tryptic
peptides
(13).
Inthisreport
oneof the six tyrosine-containing p30 tryptic pep-in this study confirm tides migrated with one of the Pr80PoI
tyrosine-r200g9g-POI
shares anti- containingtryptic
peptides, i.e., spot 4 (Fig. 2 tryptic peptide se- and 6). Pr80P°, immunoprecipitated with anti-and RT proteins. RT sera from Moloney MuLV-infected cells,ofthree polypeptides shares no tyrosine-containing tryptic peptides
in Fig. 1. They are withp30,p15,p12, orplO,asdeterminedby ion-VOL. 30, 1979
E
-C
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.497.44.241.351.482.2]620 KOPCHICK, KARSHIN, AND ARLINGHAUS
exchangechromatography (D. Lyons and R. B.
Arlinghaus, unpublished data). This
observa-tion,along with resultsobtainedwithantiserato all four core proteins (Fig. 1 andreference 10), establishes that RT does not share peptide se-quenceswith any of thefourcoreproteins.
Regarding
Pr200ga1P"
and the core proteins,tyrosine-containing tryptic peptides
character-istic ofp30, p15, phosphoprotein p12, and plO
canbereadilyresolved in
M009',""
by peptidemapping(Table 1).
Concerning
thetwotyrosine-containing p15 tryptic peptides, spot 6 is
defi-nitely present in
M009`'-P"',
but there is somedoubt about spot8. There isuncertaintyabout
spot8 in Pr809'l- and
Pr659"',
as well. We haveshownpreviouslythatonlyoneofthetwomajor
tyrosine-containing tryptic peptides of p15 is
present in Pr809` and Pr65('Lg (1). It is clear,
however, that
Pr2009a9P°'
does contain p15an-tigenicdeterminantssinceitcanbeprecipitated
byanti-p15sera(Fig. 1,laneB).
Thedata presented here furthersubstantiate
the results of Arcement et al. (1, 2), in that
Pr80Y'- and Pr65gag showantigenicdeterminants
andtrypticpeptidesequenceswithp30, p15,p12,
and plO and that Pr809ag contains additional peptide sequences relative to pMY5"g'. One
sur-prisingfindingwasthat twop30tryptic peptides
(no. 27and 28)foundinPr65-ga were notfound
in Pr809'-, which couldmean that Pr809` may
not be cleaved to yield Pr65g'l. An additional
tyrosine peptide (no. 33) was also found in Pr65-ag and notinPr809'9.Our previous studies with amino acidanalogsand proteaseinhibitors (10) suggested that Pr80g"'l was the direct
pre-cursor to
Pr65-a*.
However, anotherinterpreta-tion,which is also consistent withourpastdata,
is that Pr659ag gives rise to core proteins,
whereasPr80ga is modified for another purpose
and never gives rise to mature core proteins.
Pr809'gmight be similar to theglycosylated
sur-face gag gene polyproteins first observed by Tungetal. (30) in 1976. Inagreementwith this
conclusion, we have observed recently that
Pr80Y'- became radioactive during pulse-chase
experiments, using [3H]mannose, whereas
nei-ther Pr65-a- nor p30 was labeled with mannose (Kopchick and Arlinghaus, unpublished data).
In this regard, Evans et al. (6) have shown recently that in Friend MuLV-infected
cells
a 75,000-dalton core polyprotein is slowly proc-essed to form a glycoprotein with an apparent molecular weight of 93,000.Regarding theorigin of the core protein
pre-cursor
Pr659'l,
itispossible that Pr659'9 may be generated by nascent chain cleavage and that Pr80"' is the first genuine termination productofthe gaggene. Thisinterpretation is consistent
with our
previous findings (10)
and those ofPhilipson
etal.(22),whofound thatyeast amber suppressor tRNA increased the frequency ofMoloney-MuLV
Pr180Ya"P°I synthesisatthe ex-pense ofa PR789a. Theamount ofPr65-ga syn-thesized was not affected by the suppressor tRNA. Inadditiona 135,000-daltonproteinwas alsoincreased inamount.Thelatterwasthought
to be similar to Pr135Y''' and could be the re-mainder ofPr200,g-l"'afternascentchain cleav-age ofPr65,9'9.
Anotherpointthat bearsmentioningpertains
to
spliced
mRNA's. The splicing mechanism is believed to generate the envelope mRNA in avian andmurinetype C viruses (16, 23). Such a mechanism couldreadily explain theabsence ofsome p30 sequences inPr809't9,
those same sequences,however, beingpart ofPr659a.Thus, Pr809a9 would arise from one class of 35S viral RNA,whereasPr65-' would be translated from another 35S class. However, it is difficult toreconcile the results previously obtained with protease inhibitors and amino acidanalogs with
suchasplicingmechanism (10).
Another interesting point concerns Pr80gag and the RT precursors. Wefound that twomajor tyrosine-containing tryptic peptides (spots 10 and 29) are shared by Pr80g'l and all the RT-related proteins, yet spots 10 and 29are notin Pr65gagorin any of the viral coreproteins. One interpretation of these results is thatPr809ag is terminated within thepolgene. Thefunction of
this putative shared polypeptide is
unknown,
butaprotease would be a possiblecandidate for suchapolypeptide fortworeasons.First, the C-terminalportion of avian sarcoma virus
Pr76-"(I
appearstocontain a protease in the form of viral protein p15 (34; K. Von der Helm, W. Wile, and K. Willeck, Hoppe Seylers Z. Physiol. Chem. 358:1216-1217, 1977). Second, MuLV RT prep-arationsundergoproteolysisduring purification (18). Thus, the possible shared polypeptide within Pr80g9g and Pr80OP) could be a protease. It is of interest that Pr2009g',P° contains six majortyrosine-containing tryptic peptides andseven methionine-containing tryptic peptides
notfound in the core structural proteins and the virionpolymerase (Table 1). One of the tyrosine-containing peptides and four of the
methionine-containing peptides can be found in the
inter-mediate pol precursors Prl45Po/, Pr135Po/, and Pr125Pol, but not in Pr80O°o (Table 1). These peptide sequences may represent a new gene productasyetunidentified.
Pr2009'9-°Ialso containstrypticpeptides not
found in the core structuralproteins,pol,orthe intermediate RT precursor (Table 1). In the methionine map, there arethree such peptides
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
PEPTIDE MAPS OF gag-pol GENE PRODUCTS 621
(no. 5, 14,and 16in Fig.9). The tyrosine
finger-printshows five such major peptides(no.22, 23,
30,31,and 32),aswellasminorspots (no. 35 to
37),whichare neithergag,pol,orintermediate
RT precursor related. Two of these peptides may representsequencesatthe junctures of the
proteolytic cleavage sites which, when mapped
in themature protein, migrate differently. The
remaining tryptic peptides couldrepresent still
another geneproduct, but this requires further
study(seebelow).
The results presented above have prompted
the formulation of a working model (Fig. 10)
thatdescribes the gene content ofR-MuLV
ge-nomic RNA,withparticular emphasis given to
thegagand polgeneproducts. The main point
of the working modelis notonlythat
Pr2009'9"P"
is theprecursor to RT, butalso thatitleadsto
the synthesis of at least two other viral gene
products thatwehave identifiedasB andC.We
emphasize that additional proteinsequences in
Pr2009ag-P"
are not env gene-related products,since
M009ag-P"
does not cross-reactwith anti-gp69/71 serum, nordoes it contain themethio-nine-containingtrypticpeptidescharacteristicof
p15(E) (2, 13) (see Fig. 1). The natureofthese
viral gene products is unknown, but at least
three possible virion enzymes are good
candi-dates. It iswellknown thattypeC viruses
con-tainaprotein kinase (8,27). AviantypeC viruses
also contain an endonuclease (7, 24). A third
possible virus-coded enzyme is a protease (34,
35; Von derHelmet
al.,
HoppeSeylers
Z.Phys-iol. Chem. 358:1216-1217, 1977). Otherenzymes
are also found in the mature particle (17, 29).
Noneof theseenzymeshasasyet beenfoundto
T
R-MuLVGenomicRNA LA. Pr200ga9-Pd
,gag'
Pr80gag
' Pr6598
--__________
pl5,p12,p30,plO
A
beviruscoded, except for recent evidenceonthe
avian32,000-dalton endonuclease(24).
The evidence supporting this hypothetical model is of two kinds.First,themolecularweight
of
Pr2009'9-P"
isestimated between200,000and220,000, based on its comigration in SDS gels with myosin (2, 19); since the core proteins
(65,000 daltons) and the polymerase(80,000
dal-tons)onlyaccountfor about150,000daltons,one
is left with about 50,000 to 70,000 daltons of unidentified protein sequences. Second, addi-tional protein material is detected in. peptide
maps of Pr2009'9-I° and the intermediate RT
precursors(e.g.,
Pr125P1I)
that are not gagorpol generelated.Because of the recentfindingof an endonucle-asein the avian RT
,8
subunit (24), it ispossible that the non-pol sequences inPr145P'1-Pr125PoI
that wehave identified as B in Table 1 andFig. 10 are in fact the Rauscher analog of a viralendonuclease. We haveplaced the B protein C
terminal to pol for two reasons. One is that Pr809ag andPr8MYPI share two tyrosine-contain-ing tryptic peptides, which places gag andpol adjacent to each other. Second, in the avian system, the endonuclease sequences in the ,B-polymerase subunit are C terminal to the a-polymerase sequences(24, 26).
The hypothetical C region of the genome is
generated from the datapresented abovewhich show that
Pr200919-P°
contains threemethio-nine-containing tryptic peptides (no.5, 14, and
16) and five major tyrosine-containing tryptic peptides (no. 22,23, 30 to 32) that are not gag, pol, or B related (Table 1). The C-protein se-quences areplaced C terminaltotheB-protein
T
'pol'
Pr135 RT
Alt---RT
_
1__
-4
B
B
B C
C
'env'
Viral Precursor Polyproteins
Viral Enzymes
Viral Core Proteins
FIG. 10. Modeldescribingthe translationproductsofR-MuLVgenomicRNA.I,Initiation; T,termination.
The genes and geneproductsdesignated A, B,andCareunidentified.
VOL. 30, 1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:12.497.107.392.443.627.2]622 KOPCHICK, KARSHIN, AND ARLINGHAUS
sequences. TheCprotein would be generatedby
theprocessing of
Mr0#'"".
Some of these apparently extra amino acid
sequences in
pM2009ag9Po
could be explainedbywayofcleavage
junctions
between thegag-pol
genes and the B-C genes. Assuming that these
two junctions do occur in
pg200gag-Po
and arelabeled with methionine and
tyrosine,
onewouldpredict the appearanceoftwo new
polypeptides
inthetryptic mapsof
Pr145Pol-Pr125Po'
relativeto
M009'9-P°l.
Two newtyrosine-containing
tryptic
peptides
are seen in Pr125PoI(spots
11and12) whicharenotfound in
PrMgagP°l.
Spot
11 is also found in
Pr801°o,
whereas spot 12 isfoundonlyinPr145POI-Pr125Pol.
Although
twoofthe extra
tyrosine-containing tryptic
peptides
could be explained by
junction peptides,
threeextra
tyrosine-containing
peptides
still remaininPr2009'"°. In termsof the
methionine-contain-ing tryptic peptides, one does not see unique
peptidesinPr145Pol-Pr125POI thatare notfound
in Pr2009ag-P° (Table 1 and reference 13). A
simple explanation for this occurrence is that
the amino acid methionine is not located in
thejunction region. Although two ofthe
tyro-sine-containing tryptic peptides could be
ex-plained by this locationatcleavage junctions in
Mr0gag-Pl,
at least three majortyrosine-con-taining tryptic peptides and three
methionine-containing tryptic peptidesareobserved thatare
notgag, pol, or Brelated. Thus,wehave labeled
theseextrasequencesC (Fig. 10).
This model also includes a gene for a viral
protease.Theprotease gene(termed"A" inthe
model) isplacedatthe 5'end of thepolgene or
Nterminalin
Pr8O!)o.
Theevidencefor this is asfollows. Protease activity has been detectedin
highly purified preparations of Friend MuLV
RT (18). In this regard, since avian retrovirus
p15 has been showntobe associated with
pro-tease activity (34; Von der Helmet al., Hoppe
Seylers Z.
Physiol.
Chem. 358:1216-1217, 1977)and it is the C-terminalprotein in the aviancore
proteinprecursor Pr769ag (33), ourfindingthat
Pr809agand
Pr801°'
may have apartial overlapcould have some bearing on the location of a
protease in the murine retrovirus system.
Spe-cifically, protease in virions of R-MuLV
de-scribed by Yoshinaka and Luftig (35) could be
generated by cleavageofeither
Pr80°°'
(RT) orPrW0ag.
ACKNOWLEDGMENTS
Wethank J.Syrewicz forexcellenttechnical assistance. This research was supported in part by Public Health Service contractNO1-CP-6-1017from the VirusCancer Pro-gram ofthe National Cancer Institute and by grant G-429 fromTheRobert A. Welch Foundation. J.J.K. is supported byafellowship from the Rosalie B. Hite Foundation.
LITERATURE CITED
1. Arcement, L. J., W. L. Karshin, R. B. Naso, and R. B. Arlinghaus. 1977. gag polyprotein precursors of Rauscher murine leukemia virus. Virology 81:284-297. 2. Arcement, L. J., W. L. Karshin, R. B. Naso, G. A. Jamjoom, and R. B. Arlinghaus.1976.Biosynthesis of Rauscher leukemia viral proteins: presence ofp30 and envelopep15 sequences in precursor polyproteins. Virology69:763-774.
3. Arlinghaus, R. B., R. B. Naso, G. A. Jamjoom, L. J. Arcement, and W. L. Karshin. 1976. Biosynthesis andprocessing ofRauscher leukemia viral precursor polyproteins, p. 689-716. In D. Baltimore,A.S. Huang, and C. F. Fox(ed.), AnimalVirology ICN-UCLA Sym-posia on Molecular and CellularBiology, Vol.4. Aca-demic PressInc., New York.
4. Baltimore, D. 1974.Tumor viruses: 1974.Cold Spring HarborSymp. Quant. Biol. 39:1187-1200.
5. Bonner, W.M.,and R. A.Laskey.1974.Afilm detection method for tritium-labeled proteins and nucleic acids in polyacrylamidegels. Eur. J. Biochem.46:83-88. 6. Evans, L. H., S.Dresler,and D. Kabat.1977.Synthesis
andglycosylation ofpolyprotein precursorstothe in-ternal proteins of Friend murine leukemia virus. J. Virol. 24:865-974.
7. Grandgenett,D.P., A. C.Vora,and R. D.Schiff.1978. A32,000-dalton nucleic acid-binding protein from avian retrovirus cores possesses DNA endonucleaseactivity. Virology 89:119-132.
8. Houts, G. E., M.Miyagi, C. Ellis, D.Beard, K. F. Watson, and J. W. Beard.1978.Protein kinase from avianmyeloblastosisvirus. J. Virol.25:546-552. 9. Ikeda, H., W.Hardy, Jr., E.Tress,and E. Fleissner.
1975.Chromatographic separation and antigenic anal-ysis of proteins of theoncornaviruses.V.Identification ofa newviralprotein,p15(E).J.Virol.16:53-61. 10.Jamjoom,G.A., R. B.Naso,and R. B.Arlinghaus.
1977.Further characterization ofintracellularprecursor polypeptides of Rauscher leukemia virus.Virology78: 11-34.
11. Karshin, W.L.,L. J.Arcement, R. B. Naso, and R.B. Arlinghaus. 1977. Common precursor for Rauscher leukemia virus gp69/71, p15(E),andp12(E). J. Virol. 23:787-798.
12.Kessler, S. W.1975.Rapidisolation ofantigensfromcells with a staphylococcal protein A-antibody absorbent; parametersof the interaction ofantibody-antigen com-plexes with proteinA.J. Immunol. 115:1617-1624. 13.Kopehick,J.J.,G. A.Jamjoom,K. F.Watson, and
R. B.Arlinghaus.1978.Biosynthesis of reverse tran-scriptase from Rauscher murine leukemia virus by syn-thesis and cleavage of agag-pol read-through viral precursorpolyprotein.Proc.Natl. Acad. Sci. U.S.A.75: 2016-2020.
14. Laemmli, U. K. 1970. Cleavage ofstructural proteins duringassemblyof thehead ofbacteriophageT4. Na-ture(London)227:680-685.
15. Laskey,R.A., andA. D. Mills. 1975.Quantitativefilm detection of3Hand14Cinpolyacrylamide gels by fluo-rography. Eur.J.Biochem. 56:335-341.
16.Mellon, P., and P. H. Duesberg. 1977. Subgenomic, cellularRous sarcoma virusRNAs contain oligonucle-otides fromthe3' half and the5' terminusof virion RNA. Nature(London)240:631-634.
17. Mizutani, S., and H. M. Temin. 1971. Enzymes and nucleotides in virionsofRous sarcoma virus. J. Virol. 8:409-416.
18.Moelling,K. 1976. Furthercharacterizationof theFriend murineleukemia virusreversetranscriptase-RNaseH complex.J.Virol.18:418-425.
19.Murphy,E.C.,J. J.Kopchick,K.F.Watson, and R.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
PEPTIDE MAPS OF gag-pol GENE PRODUCTS 623
B.Arlinghaus. 1978. Cell-free synthesis ofaprecursor
polyprotein containing both 'gag' and 'pol'gene
prod-uctsby Rauscher murine leukemia virus35S RNA. Cell 13:359-369.
20. Naso, R.B., L. J. Arcement, and R. B. Arlinghaus. 1975.Biosynthesisof Rauscher leukemia viral proteins. Cell4:31-36.
21. Naso, R. B., L. J. Arcement,W.L.Karshin, G. A. Jamjoom, and R. B. Arlinghaus. 1976. A fucose-deficient glycoproteinprecursortoRauscherleukemia virusgp69/71.Proc. Natl. Acad. Sci. U.S.A. 73:2325-2330.
22. Philipson, L.,P.Anderson,V.Olshevsky, R. Wein-berg, andD.Baltimore. 1978.Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte
ex-tracts: enhancement of the gag-pol polypeptide with
yeastsuppressortRNA. Cell 13:189-199.
23. Rothenberg, E., D. J. Donoghue, and D. Baltimore. 1978. Analysis ofa5'leadersequence onmurine
leu-kemia virus21S RNA: heteroduplex mapping with long
reversetranscriptase products. Cell13:435-451. 24. Schiff, R.D.,and D. P.Grandgenett.1978.Virus-coded
originofa32,000-dalton protein from avianretrovirus cores: structural relatedness ofp32 and the,B
poly-peptide of the avian retrovirus RNA polymerase. J. Virol.28:279-291.
25. Shapiro,S.Z.,M.Strand,and T.August.1976.High molecularweightprecursorpolypeptidestostructural proteinsofRauscher murine leukemiavirus. J. Mol. Biol. 107:459-477.
26. Shealy,D.J., and R. R. Rueckert. 1978. Proteinsof Rous-associated virus61,anavianretrovirus:common precursorforglycoproteinsgp85 andgp35 anduseof
pactamycin tomaptranslational order of proteins in
thegag,pol, andenvgenes.J. Virol.26:380-388. 27. Strand, M., and J. T.August.1971.Protein kinase and
phosphateacceptorproteinsinRauschermurine leu-kemia virus. Nature(London)New Biol.233:137-140. 28. Syrewicz, J. J., R.B.Naso, C. S.Wang, and R. B. Arlinghaus. 1972. Purification of large amounts of murineribonucleicacidtumorvirusesproducedinroller bottle cultures.Appl. Microbiol.24:488-498.
29. Temin, H. M., and D. Baltimore.1972.RNA-directed DNAsynthesis and RNAtumorviruses. Adv. Virus Res. 17:129-159.
30. Tung, J.-S., T.Yoshiki,and E. Fleissner. 1976. Acore
polyprotein of murineleukemia virusonthe surface of mouseleukemia cells.Cell 9:573-578.
31. Van Zaane, D.,A.L. J.Gielkens,M. J. A. Dekker-Michielson,and H. P. J. Bloemers. 1975. Virus-spe-cific precursor polypeptides in cells infected with Rauscher leukemia virus.Virology67:544-552. 32. Vogt, V.M., and R. Eisenman.1973.Identification ofa
large polypeptideprecursorof avian oncornavirus
pro-teins. Proc. Natl. Acad.Sci. U.S.A.70:1934-1938. 33. Vogt,V.M.,R.Eisenman,and H.Diggelman. 1975.
Generation of avianmyeloblastosisvirus structural
pro-teinsby proteolyticcleavageofaprecursorpolypeptide.
J. Mol.Biol. 96:471-493.
34. Von derHelm,K. 1977.Cleavageof Roussarcomaviral
polypeptide precursorinto internalstructuralproteins in vitro involves viralprotein p15.Proc. Natl. Acad. Sci. U.S.A. 74:911-915.
35. Yoshinaka, Y.,and R. B.Luftig.1977.Propertiesofa
p70 proteolyticfactor of murineleukemia viruses. Cell 12:709-719.
VOL. 30, 1979
![FIG. 2.Approximatelyfollowedride-agarosetotrypsinlabeled preflashed Two-dimensional tryptic peptide maps of [:H]tyrosine-labeled viral core proteins](https://thumb-us.123doks.com/thumbv2/123dok_us/1513906.104013/4.497.48.447.73.447/approximatelyfollowedride-agarosetotrypsinlabeled-preflashed-dimensional-tryptic-peptide-tyrosine-proteins.webp)
![FIG. 4.withthe Two-dimensional tryptic peptide map of [3H]tyrosine-labeled Pr65g. PrO5ag was isolated, and trypticpeptides were resolved as described in the legend to Fig](https://thumb-us.123doks.com/thumbv2/123dok_us/1513906.104013/6.497.103.395.69.346/dimensional-tryptic-peptide-tyrosine-isolated-trypticpeptides-resolved-described.webp)
![FIG. 5.componentsFig. Two-dimensional tryptic peptide map of[3H]tyrosine-labeled Pr20099P°](https://thumb-us.123doks.com/thumbv2/123dok_us/1513906.104013/7.497.116.399.70.351/fig-componentsfig-two-dimensional-tryptic-peptide-tyrosine-labeled.webp)
![FIG. 6.wereanti-RTtrypsinsolution,MuLHanks Two-dimensional tryptic peptide map of[3H]tyrosine-labeled Pr80"](https://thumb-us.123doks.com/thumbv2/123dok_us/1513906.104013/8.497.109.396.68.336/wereanti-rttrypsinsolution-mulhanks-dimensional-tryptic-peptide-tyrosine-labeled.webp)
![FIG. 7.mappedI Two-dimensional tryptic peptide map of [3H]tyrosine-labeled Pr125PoI. Pr125PoI was isolated and as described in the legend to Fig](https://thumb-us.123doks.com/thumbv2/123dok_us/1513906.104013/9.497.61.453.385.588/mappedi-dimensional-tryptic-peptide-tyrosine-labeled-isolated-described.webp)

