0022-538X/06/$08.00
⫹
0
doi:10.1128/JVI.80.5.2127–2140.2006
Copyright © 2006, American Society for Microbiology. All Rights Reserved.
Vaccinia Virus Proteome: Identification of Proteins in Vaccinia Virus
Intracellular Mature Virion Particles
Che-Sheng Chung,
1† Chein-Hung Chen,
2† Ming-Yi Ho,
2Cheng-Yen Huang,
1Chung-Lin Liao,
2* and Wen Chang
1*
Institute of Molecular Biology
1and The Genomics Research Center,
2Academia Sinica, Taipei, Taiwan, Republic of China
Received 25 October 2005/Accepted 5 December 2005
Vaccinia virus is a large enveloped poxvirus with more than 200 genes in its genome. Although many poxvirus
genomes have been sequenced, knowledge of the host and viral protein components of the virions remains
incom-plete. In this study, we used gel-free liquid chromatography and tandem mass spectroscopy to identify the viral and
host proteins in purified vaccinia intracellular mature virions (IMV). Analysis of the proteins in the IMV showed
that it contains 75 viral proteins, including structural proteins, enzymes, transcription factors, and predicted viral
proteins not known to be expressed or present in the IMV. We also determined the relative abundances of the
individual protein components in the IMV. Finally, 23 IMV-associated host proteins were also identified. This study
provides the first comprehensive structural analysis of the infectious vaccinia virus IMV.
Vaccinia virus is the prototype virus of the orthopoxvirus
genus in the family
Poxviridae
, which replicates in the
cyto-plasm of cells (57, 104) and encodes more than 200 open
reading frames (ORFs) in a 190-kb double-stranded DNA
genome. Vaccinia virus infection produces multiple forms of
infectious particles, namely, intracellular mature virions (IMV),
intracellular enveloped virions (IEV), cell-associated
envel-oped virions (CEV), and extracellular envelenvel-oped virions
(EEV). The IMV is the most abundant virion, with a single
membrane in cells; however, the origin of the membrane is
unknown (75, 77). A portion of the IMV is subsequently
wrapped with two layers of Golgi membrane to form an IEV,
which is transported through microtubules to the cell periphery
and loses one membrane during virion egress to become a
CEV (130, 140, 152, 153). The CEV remains associated with
the cell surface, where actin-containing microvilli are formed,
or can be released by host cell Src/Abl kinases into the medium
to become an EEV (41, 42, 61, 111, 156). The IMV is robust
and is known to be resistant to environmental and physical
changes, whereas the CEV and EEV are very fragile, and the
integrity of their outer membranes can be destroyed during
purification procedures (83).
Many of the poxvirus genomes, including those of different
strains of vaccinia virus, have been sequenced (http://www
.poxvirus.org/viruses.asp). The genome of the vaccinia virus
Western Reserve (WR) strain contains 218 potential ORFs;
however, the existence of an ORF does not necessarily reveal
the existence of a protein or the location of the protein. While
classical biochemical and genetic approaches have generated
significant knowledge of viral-gene functions, the physical
com-position of vaccinia virus IMV particles remains unknown.
Previous researchers have analyzed the protein composition
of the vaccinia virus IMV. Initial studies of the purified IMV
led to the identification on sodium dodecyl
sulfate-polyacryl-amide gel electrophoresis (SDS-PAGE) of 17 “regions”
con-taining 31 to 48 protein bands (103, 115, 139). Essani and
Dales increased the gel resolution and detected 56 polypeptide
bands on SDS-PAGE (54). Using different gel electrophoretic
conditions, Oie and Ichihashi found 84 protein spots that could
be assigned to 52 protein species in purified IMV (116).
Un-fortunately, the naming system for vaccinia virus proteins in
the above studies was based on the protein migration behavior
on gels, making it difficult to compare data generated in
dif-ferent gel electrophoresis systems. In addition, the majority of
viral genes encode proteins smaller than 50 kDa that tend to
cluster together and are difficult to resolve by SDS-PAGE.
Moreover, N-terminal sequencing of viral protein bands on
gels revealed that multiple protein bands can be derived from
a single viral gene as a result of posttranslational cleavage or
modification and that the 40 to 50 IMV bands seen on gels
were derived from only 12 viral genes (160). Similarly, Jensen
et al. found that the purified vaccinia virus core and membrane
contain 30 proteins encoded by 13 viral genes and 5 host genes;
additional, less abundant virion components were detected but
not identified (86).
Mass spectrometry (MS), in particular, tandem MS (MS/
MS), provides a powerful tool for proteome analysis because it
is much more sensitive than other methods, can deal with
protein mixtures, and offers a high throughput (120).
Wash-burn et al. identified 1,484 proteins in the yeast proteome using
gel-free liquid chromatography and tandem mass spectroscopy
(LC/MS/MS) (175). Similar analyses have been used to identify
virion proteins in human and murine cytomegalovirus,
Ep-stein-Barr virus, and Kaposi’s sarcoma-associated herpesvirus
(87, 92, 172, 191). The identification of virion proteins makes it
possible to start determining how the proteins interact, their
stoichiometry in the viral particle, and which cellular
compart-ments are involved in virion formation. In this study, we used
* Corresponding author. Mailing address for W. Chang: Institute
of Molecular Biology, Academia Sinica, Taipei, Taiwan, Republic of
China. Phone: 886-2-2789-9230. Fax: 886-2-2782-6085. E-mail: mbwen
@ccvax.sinica.edu.tw. Mailing address for C.-L. Liao: The Genomics
Research Center, Academia Sinica, Taipei, Taiwan, Republic of China.
Phone: 886-2-2652-3926. Fax: 886-2-2782-9143. E-mail: clliao@gate
.sinica.edu.tw.
† Che-Sheng Chung and Chein-Hung Chen contributed equally to
this work.
2127
on November 8, 2019 by guest
http://jvi.asm.org/
LC/MS/MS analysis to determine the viral and host proteins
that comprise each IMV particle. New components of vaccinia
virus IMV encoded by putative ORFs were discovered, and the
relative abundance of each component in the IMV was
deter-mined.
MATERIALS AND METHODS
Cells, virus, and reagents.HeLa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Vaccinia virus (Western Reserve strain) was propagated in HeLa cells and purified as described previously (80, 86). In brief, the infected cells were harvested when the cytotoxic
pathological effect was complete. All stages of purification were performed at 4°C. The cells were centrifuged at 850⫻gfor 15 min to remove the medium and washed three times with phosphate-buffered saline. The pelleted cells were resuspended in TM buffer (10 mM Tris, pH 7.4, 5 mM MgCl2), and the
suspen-sion was passed 20 times in a Dounce homogenizer to break the cells. Nuclei were removed by centrifugation at 850⫻gfor 10 min, and the supernatant, containing virions, was laid on top of 36% sucrose solution and centrifuged at 45,000⫻gfor 80 min in a Beckman SW28 rotor. The virus pellet was resus-pended in TM buffer, sonicated, and further purified by centrifugation through a continuous 25 to 40% sucrose gradient at 27,500⫻gfor 40 min in a Beckman SW28 rotor. Fractions were taken, and the virion infectivity in each fraction was analyzed by a plaque formation assay. Fractions containing virions were diluted fourfold in TM buffer and centrifuged to pellet the IMV. The purity of the IMV
FIG. 1. (A) Purified IMV. Electron microscopy of purified IMV particles using negative uranyl acetate staining (left) and silver staining of IMV
proteins, 340 ng (lane 1) and 170 ng (lane 2), on 12% SDS-PAGE (right). (B) MS/MS spectrum of one tryptic peptide with a sequence identified
as HAFDAPTLYVK and with a Mascot score of 72. (C) Amino acid sequence of the putative E6R protein. Tryptic peptides detected by MS,
including the peptide in panel B, are underlined and give 53% sequence coverage. (D) MS/MS spectrum of one tryptic peptide with a sequence
identified as ADEDDNEETLK and with a Mascot score of 80. (E) Amino acid sequence of A27L envelope protein. Tryptic peptides detected by
MS, including the peptide in panel D, are underlined and give 71% sequence coverage.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:2.585.136.449.66.530.2]was confirmed by electron microscopy of negatively stained preparations. No detectable cellular debris was found in these preparations.
Tryptic and lysine C digestion of IMV particles.Purified IMV (9 to 50g) were incubated at 25°C overnight with 0.5 N cyanogen bromide (Fluka) in 90% formic acid (Fluka) to cleave the proteins into smaller polypeptides, and excess reagents were removed by vacuum drying. The polypeptides were denatured and reduced at 37°C for 1 h in 6 M urea (Sigma), 2 M thiourea (Riedel-de Hae¨n), and 10 mM dithiothreitol (Pharmacia Biotech), and then iodoacetamide (Sigma) was added to a final concentration of 20 mM and the sample was incubated at 37°C for 1 h in the dark. The peptide mixture was then diluted eightfold with 100 mM ammonium bicarbonate (Sigma), and sequencing-grade porcine trypsin (Pro-mega) or Lys-C (Wako) was added at a substrate-to-enzyme ratio of 20:1 (wt/wt), and the mixture was incubated at 37°C overnight. The peptides formed were desalted using a C18trap, lyophilized on a SpeedVac, and stored at⫺80°C.
Two-dimensional chromatography.The peptide mixture from 50g of IMV was fractionated by two-dimensional chromatography (strong cation-exchange chromatography [SCX]-reverse-phase liquid chromatography). The first dimen-sion, SCX, was eluted with a linear gradient of 0 to 300 mM KCl in 5 mM ammonium formate, pH 3.0. The peptides eluted from the SCX column were trapped in two C18reverse-phase traps operating alternately; the bound peptides
were eluted at 2-min intervals and collected on a fraction collector; a total of 22 fractions were collected. The amount of peptide in each fraction was estimated from the UV absorption at 214 nm.
Mass spectrometry. Mass spectrometric analysis was performed on a nanoscale LC-tandem mass spectrometry (quadrupole time-of-flight mass spec-trometer; QStar XL; Applied Biosystems). The instrument setup was as follows. The flow (150l/min) from the binary pump (Agilent 1100, with solvent A [100% deionized water] and solvent B [90% acetonitrile] [J. T. Baker], both solvents containing 0.1% formic acid) was split with two T-shaped connectors connected to a self-packed precolumn with an appropriate flow restrictor to give a column flow rate of 10l/min for sample loading and 200 to 300 nl/min for sample elution from the analytical column. The sample of 2 to 5g was injected onto the precolumn (15 mm long; 150-m internal diameter; C18) via a 20-l sample loop.
The analytical column (C18; 15 cm long; 75-m internal diameter) was connected
to a 15-mm electrospray emitter (10-m tip opening) by a 1-cm Teflon sleeve. The chromatographic separation was performed with a 120-min gradient profile as follows: 2% B (0 to 4.5 min), linear gradient of 2 to 10% B (4.5 to 5 min), 10 to 40% B (5 to 80 min), 40 to 50% B (80 to 100 min), 50 to 80% B (100 to 105 min), 80 to 2% B (105 to 106 min), and 2% B (106 to 120 min). The spectra of the eluted peptides were acquired in data-dependent mode by first acquiring a full MS scan fromm/z400 to 1900 for 1 second to determine the three most
intense peptide peaks with charge states above 2, and then three MS/MS scans betweenm/z100 and 2000 (1.5 s each) were performed for the MS-scanned parent ions with a threshold above 20 counts. Once sampled, each MS/MS precursor mass was excluded from further tandem experiments for 2 min.
The data files completed from the LC-MS runs were converted to Mascot generic-format files using the Mascot.dll script supplied with the Analyst QS software. The Mascot software package (Matrix Science) was used for database searching and protein identification using the modified vaccinia virus protein database. Peptide mass tolerance and fragment mass tolerance were set at 100 ppm and 0.25 Da, respectively, for the initial search. An alternative calibration algorithm based on Mascot protein identifications was applied to the raw data file to give mass accuracies within 20 ppm.
Proteomic analysis of IMV-derived peptides and database generation. Indi-vidual MS/MS spectra were submitted to analysis using MASCOT version 2.0 (Matrix Science Inc.) and searched against the Human International Protein Index protein sequence database (version 3.10; 57,478 protein sequences; Euro-pean Bioinformatics Institute [http://www.ebi.ac.uk/IPI/]) or against a modified vaccinia virus protein database. The modified viral-protein database combines 218 protein sequences of the WR strain of vaccinia virus obtained from http: //poxvirus.org, 64 nonredundant Copenhagen strain orthologs, and 12 additional peptide sequences predicted from WR viral genome sequences using two pro-grams, GeneMarkS (http://opal.biology.gatech.edu/GeneMark/) and fgenesV (http://softberry.com; 17). These databases allowed the identification of novel vaccinia virus proteins by an exhaustive search of all possible virus-derived peptide sequences. Proteins were scored using a probability-based MOWSE algorithm, and the scores were reported in the form⫺10⫻log(P), wherePis the probability that the observed match is a random event (123). Matches with scores higher than the 95% confidence level were regarded as significant. In all searches, phosphorylation, methionine oxidation, homoserine lactone, and car-bamidomethylation of cysteine residues were considered as possible modifica-tions. In order to increase the data confidence, the same data sets were submitted for a search against a “reverse database,” in which all peptide sequences were put in reverse order in silico; the percentage of peptides identified in the reverse database was less than 3.5%, confirming the specificity of the data search. Individual peptide interpretations were classed as significant if the MASCOT MOWSE score for the MS/MS spectra was greater than the cutoff value of 25.
[image:3.585.137.449.74.330.2]Relative quantification of MS.Quantification of protein abundance in IMV was performed based on the criteria and equations defined previously (85). In brief, the relative abundances of the proteins were estimated using an exponen-tially modified protein abundance index (emPAI) approach, which is based on the correlation of peptides identified in MS/MS experiments with observable
FIG. 1—
Continued.
on November 8, 2019 by guest
http://jvi.asm.org/
TABLE 1. Vaccinia virus IMV proteins identified by LC/MS/MS
aVACV-WR ORF
Mass (Da)
VACV-CP
ORF Description
CNBr-trypsin digestion CNBr-LysC digestion
Reference(s) No. of
unipeptides
Sequence coverage (%)
No. of unipeptides
Sequence coverage (%)
VACV-WR_022 17,311 C6L Unknown 1 7
VACV-WR_035 48,842 K4L Phospholipase D-like 5 14 3 8 21, 24
VACV-WR_047 7,846 F8L Protein with iActA-like proline repeats not required for actin tail formation
3 46 76
VACV-WR_048 23,776 F9L S-S bond formation pathway thiol substrate 5 27 2 12 146
VACV-WR_049 52,098 F10L Ser/Thr kinase 12 31 7 22 98
VACV-WR_056 11,329 F17R Putative DNA-binding phosphoprotein in virus core
8 56 5 54 86, 90, 91, 121,
182, 190 VACV-WR_057 55,496 E1L Poly(A) polymerase catalytic subunit VP55 10 20 2 4 65 VACV-WR_060 29,808 E4L DNA-dependent RNA polymerase subunit rpo30,
intermediate-gene transcription factor VITF1 TFIIS-like
8 28 3 15 1, 31
VACV-WR_062 66,685 E6R Unknown 30 53 12 27
VACV-WR_064 31,868 E8R Membrane protein may help wrap virosome associated with IV/IMV and core F10L kinase substrate
11 52 3 13 51
VACV-WR_066 10,844 E10R S-S bond formation pathway sulfhydryl oxidase, substrates L1R/F9L
3 32 145
VACV-WR_067 14,890 E11L Virion core protein 2 20 1 10 174
VACV-WR_069 12,347 O2L Nonessential glutaredoxin not part of E10R-G4L S-S bond formation pathway
6 47 2 24 4, 88, 178
VACV-WR_070 35,819 I1L DNA binding core protein 6 18 3 11 148
VACV-WR_071 8,493 I2L IMV membrane protein required for membrane fusion
2 36 1 20 112
VACV-WR_072 29,978 I3L ssDNA-binding phosphoprotein 5 22 1 4 47, 168, 177
VACV-WR_074 8,738 I5L IMV protein VP13 1 12 1 12 84, 160
VACV-WR_076 48,979 I7L Viral core cysteine proteinase 12 24 5 9 10, 32, 33, 89
VACV-WR_077 77,550 I8R RNA-helicase, DExH-NPH-II DNA-helicase aided by L4R
16 29 7 14 13, 56, 93, 150
VACV-WR_078 67,997 G1L Insulin metalloproteinase-like has ENE and HLLEH inverse of HXXEX sites
16 29 3 7 74
VACV-WR_079 12,795 G3L Unknown 2 19 1 19
VACV-WR_081 13,978 G4L S-S bond formation pathway thioredoxin-like 3 29 2 12 72, 86, 178, 179
VACV-WR_083 7,283 G5.5R DNA-dependent RNA polymerase subunit rpo7 3 47 8, 106
VACV-WR_085 41,924 G7L Virion structural protein 16 38 9 27 107, 157, 158, 160
VACV-WR_087 38,761 G9R Myristylprotein 2 7 2 7 102
VACV-WR_088 27,262 L1R IMV membrane protein target of neutralizing antibody S-S bond formation pathway thiol substrate; myristylprotein
6 26 3 10 59, 86, 102, 126,
127, 146, 184
VACV-WR_090 40,595 L3L Unknown 11 29 4 10
VACV-WR_091 28,439 L4R Core protein vp8 microtubule-associated, ssRNA/ ssDNA-binding protein
21 66 9 31 86, 121, 124, 160,
181, 186 VACV-WR_092 15,034 L5R IMV membrane protein required for membrane
fusion
2 25 1 6 165
VACV-WR_093 17,910 J1R Virion protein required for morphogenesis 7 34 4 24 37, 38 VACV-WR_095 38,863 J3R Multifunctional poly-A polymerase subunit, cap
methyltransferase, and transcription elongation factor
13 48 5 21 65, 141
VACV-WR_096 21,328 J4R DNA-dependent RNA polymerase subunit rpo22 6 33 1 4 29, 79, 164 VACV-WR_098 146,740 J6R DNA-dependent RNA polymerase subunit rpo147 47 39 15 13 29, 53, 79, 164
VACV-WR_099 19,713 H1L Tyr/ser protein phosphatase 8 49 4 26 69, 100, 110
VACV-WR_100 21,529 H2R IMV membrane protein required for membrane fusion
1 6 1 3 142
VACV-WR_101 37,422 H3L IMV heparin binding surface protein involved in IMV maturation
20 54 10 40 44–46, 86, 97, 160
VACV-WR_102 93,574 H4L RAP94 tightly associated with DNA-dependent RNA polymerase, aids early-stage transcription, preinitiation, and termination
27 38 9 13 2, 5, 189
VACV-WR_103 22,287 H5R Morphogenesis-related, substrate of B1R kinase late gene transcription factor VLTF-4
4 28 2 15 14, 15, 16, 27, 94
VACV-WR_104 36,642 H6R Topoisomerase type IB 11 46 2 7 149,151
VACV-WR_106 96,673 D1R Large subunit of mRNA capping enzyme transcription termination factor VTF
30 39 16 24 109, 173
VACV-WR_107 16,935 D2L Virion core protein 5 36 3 21 52
VACV-WR_108 27,973 D3R Virion core protein 7 41 1 3 52
VACV-WR_111 73,784 D6R 70kDa small subunit of early gene transcription factor VETF
14 27 4 9 28, 66
VACV-WR_112 17,900 D7R DNA-dependent RNA polymerase subunit rpo18 1 6 1 8 3, 125 VACV-WR_113 35,424 D8L IMV membrane protein binds cell surface
chondroitin may effect viral entry
11 51 5 20 80, 86, 114
VACV-WR_116 72,264 D11L ATPase, nucleoside triphosphate phosphohydrolase-I, NPH-I transcription elongation, termination, release factor
14 25 6 14 30, 93, 131
VACV-WR_117 33,330 D12L Small subunit of mRNA capping enzyme transcription termination factor VTF
11 41 6 33 113, 173
Continued on facing page
on November 8, 2019 by guest
http://jvi.asm.org/
peptides obtained by in silico digestion, which allows the relative abundances of proteins in a complex protein mixture to be determined. The abundance of each protein is presented as the molar and weight percentages of the total protein molecules in the IMV particles.
RESULTS
Identification of IMV proteins.
Vaccinia IMV particles were
purified from the lysates of HeLa cells infected with the
vac-cinia virus WR strain, using two consecutive sucrose
purifica-tion procedures to achieve maximal purity. The purified IMV
particles were examined by electron microscopy to ensure that
the virions had normal virion morphology and that cellular
organelles and debris were undetectable. Proteins in purified
virions were separated on 12% SDS-PAGE and silver stained
(Fig. 1A). Gel electrophoresis of the purified virions followed
[image:5.585.47.539.80.504.2]by in-gel digestion often causes loss of proteolytic fragments
generated from low-abundance proteins, and the gel matrix is
inherently not optimal for resolving proteins of low molecular
weight. Thus, a gel-free scheme with two-stage proteolysis was
adopted to facilitate the efficient extraction of peptides from
purified IMV particles. The purified IMV were completely
dissolved in 90% formic acid and subjected to cyanogen
bro-mide cleavage, followed by tryptic digestion in solution to
sequentially yield a mixture of peptides. Alternatively,
proteo-lytic digestion with Lys-C was performed in order to preserve
longer peptides for better quality in data acquisition. These
peptides were then analyzed by MS/MS using a modified
vac-cinia virus (WR) protein database constructed (see Materials
and Methods) to include all predicted vaccinia virus translation
products, as the commonly used vaccinia virus (WR) strain
TABLE 1—
Continued
VACV-WR ORF
Mass (Da)
VACV-CP
ORF Description
CNBr-trypsin digestion CNBr-LysC digestion
Reference(s) No. of
unipeptides
Sequence coverage (%)
No. of unipeptides
Sequence coverage (%) VACV-WR_118 61,852 D13L Rifampicin target associates with inner surface
immature virus membrane
1 2 2 3 11, 108, 161, 162
VACV-WR_121 8,922 A2.5L S-S bond formation pathway CxxxC links SH-oxidase E10R and thioredoxin G4L
4 36 3 42 147
VACV-WR_122 72,578 A3L p4b precursor of core protein 4b 46 65 15 34 121, 135, 160
VACV-WR_123 30,908 A4L 39-kDa core protein complexes with core protein p4a/4a
14 55 5 13 49, 86, 121
VACV-WR_124 18,984 A5R DNA-dependent RNA Polymerase subunit rpo19 5 45 2 12 6
VACV-WR_125 43,147 A6L unknown 5 14 6 23
VACV-WR_126 82,220 A7L 82-kDa large subunit of early gene transcription factor VETF
23 33 14 22 66
VACV-WR_128 12,102 A9L IMV membrane protein required for morphogenesis
1 11 187
VACV-WR_129 102,209 A10L Precursor p4a of core protein 4a, complexes with A4L
69 62 20 23 86, 121, 160, 171
VACV-WR_132 7,691 A13L IMV membrane protein 5 55 2 42 86, 160, 169
VACV-WR_133 9,987 A14L Phosphorylated IMV membrane protein required for morphogenesis
3 25 1 8 18, 86, 133, 134,
160, 167
VACV-WR_135 10,944 A15L Unknown 2 32 1 14
VACV-WR_136 43,396 A16L Soluble myristylprotein 7 25 1 5 102
VACV-WR_137 22,984 A17L IMV membrane protein undergoes phosphorylation and proteolytic processing; required for morphogenesis
5 21 3 17 50, 86, 95, 133,
160, 183 VACV-WR_138 56,679 A18R DNA helicase effects elongation and termination
of postreplicative viral transcription
8 17 1 4 93, 188
VACV-WR_140 13,636 A21L IMV membrane protein required for membrane fusion
1 10 166
VACV-WR_142 21,865 A22R Palmitylprotein; Holliday junction endonuclease; resolves viral DNA concatemers into unit length genomes
1 5 63, 67
VACV-WR_144 133,279 A24R DNA-dependent RNA polymerase subunit rpo132 35 34 14 13 9, 78 VACV-WR_148 84,299 A25L Gene fragment, cowpox A-type inclusion protein 34 44 13 19 62 VACV-WR_149 57,957 A26L p4c protein; IMV membrane protein required for
directing IMV into A-type inclusion body
23 49 10 23 105
VACV-WR_150 12,564 A27L IMV surface protein roles in IMV-cell attachment, fusion, and microtubule transport
10 71 3 24 39, 86, 101, 132,
138, 160 VACV-WR_151 16,319 A28L IMV membrane protein required for membrane
fusion
3 23 1 8 143, 144
VACV-WR_152 35,370 A29L DNA-dependent RNA polymerase rpo35 6 21 2 9 9
VACV-WR_153 8,740 A30L IMV protein for association of dense viroplasm with viral membranes during morphogenesis
2 54 107, 157, 158, 159
VACV-WR_154 14,200 A31R Unknown - 1 13
VACV-WR_167 15,043 A42R Profilin-like 8 57 2 12 20
VACV-WR_171 13,654 A45R Inactive Cu-Zn superoxide dismutase-like in virion
4 25 1 7 7
VACV-WR_172 27,618 A46R Toll/IL1-receptor [TIR]-like suppresses TIR-dependent signal transduction host defense modulator
2 7 25, 155
a
The data presented in Table 1 are compiled from three different IMV preparations. Tryptic digestions of different IMV preparations revealed 92% reproducibility, and Lys-C digestions revealed 87% reproducibility. Peptides generated by both trypsin and Lys-C are required to obtain a total of 75 viral proteins: 65 proteins (86.7%) were detected in peptides generated by either trypsin or Lys-C digestions, 9 proteins (12%) were detected only in trypsin-digested peptides, and 1 protein (1.3%) was detected only in Lys-C-digested peptides. VACV, vaccinia virus; CP, Copenhagen strain; ssDNA, single-stranded DNA; ssRNA, single-stranded RNA.
on November 8, 2019 by guest
http://jvi.asm.org/
protein database does not include all predicted viral proteins
or small proteins with fewer than 50 amino acids. As shown in
Fig. 1B, the MS/MS spectrum of a tryptic peptide was
inter-preted as HAFDAPTLYVK, one of 30 unique peptides
iden-tified in a putative protein, WR062/E6R, resulting in 53%
sequence coverage (Fig. 1C). Another tryptic peptide, ADED
DNEETLK, was identified in an envelope protein, A27L (Fig.
1D), that contains 71% sequence coverage, as shown in Fig.
1E. A total of three different IMV preparations were used in
five independent mass spectrometry analyses. Combining the
peptide data generated from trypsin and Lys-C digests, we
identified 75 viral proteins in the IMV (Table 1). Because the
gene names in the vaccinia virus WR strain are not as widely
used as their orthologs in the Copenhagen strain, the latter
gene names are used to refer to the viral translation products
we identified in this work. Of these, 69 were identified by at
least two trypsin- or Lys-C-generated peptides, while the other
6 (C6L, I5L, A9L, A21L, A22R, and A31R) were identified by
single peptide hits in the trypsin- or Lys-C-digested samples. A
further study using two-dimensional chromatographic
fraction-ation to separate peptides prior to MS/MS was performed
(SCX-LC/MS/MS), and at least one extra peptide (each) was
identified for C6L, A9L, A21L, and A31R (Table 2). All the
extra peptides identified in the SCX-LC/MS/MS study had
MASCOT scores of at least
⬎
28. No extra peptide was
iden-tified for I5L and A22R by SCX-LC/MS/MS, although the
same peptides as in one-dimensional LC/MS/MS were
repro-ducibly detected. Both proteins are included in Table 1,
be-cause they were previously detected in the IMV (63, 84, 160).
Of the 75 viral proteins identified, 65 were consistent with
previous reports of their presence in IMV particles (references
in Table 1); these were 22 membrane proteins (H3L, D8L,
H2L, L1R, L5R, A2.5, A9L, A13L, A14L, A17L, A21L, A25L,
A26L, A27L, A28L, E8R, E10R, F9L, I2L, I5L, J1R, and
D13L), 12 core proteins (A3L, A4L, A10L, A30L, F17R,
E11L, I1L, I3L, G7L, L4R, D2L, and D3R), 24 enzymes
[ki-nase (F10L), phosphatase (H1L), glutaredoxins (G4L and O2L),
topoisomerase (H6R), proteinases (G1L and I7L), ATPase
(D11L), poly(A) polymerase subunits (E1L and J3R), capping
enzymes (D1R and D12L), DNA-dependent RNA polymerase
complexes (A5R, A24R, A29L, J4R, J6R, H4L, E4L, G5.5R,
and D7R), helicases (I8R and A18R), and endonuclease
(A22R)], 3 viral transcription factors (A7L, H5R, and D6R),
and 4 proteins (A42R, A45R, A46R, and F8L) interacting with
host proteins. This shows that our proteomic data are reliable
and consistent with previous results. More importantly, the
proteomic data revealed 10 new viral proteins present in IMV,
consisting of the products of seven former putative ORFs
(E6R, G3L, L3L, A6L, A15L, A31R, and C6L) and three
proteins (K4L, G9R, and A16L) previously with unknown
lo-cations and functions. K4L shows homology with
phospho-lipase D but is nonessential for viral growth in cell cultures (21,
24, 122), while both G9R and A16L have been identified as
myristylated proteins in virus-infected cells (102).
Six viral proteins, I6L, G5R, A12L, A14.5, A32L, and B1R,
have been cited in the literature as being IMV associated but
were not identified in our analyses (Table 3) (19, 34, 43, 48, 68,
99, 160). All six proteins were not detected in LC/MS/MS using
different IMV preparations, although three proteins (A12L,
A32L, and B1R) were detected in SCX-LC/MS/MS, suggesting
that the abundances of these three proteins were below the
threshold of LC/MS/MS and that SCX chromatography was
necessary to reduce the sample complexity in order to enhance
the detection sensitivity (Table 3). Consistent with our
inter-pretation, A32L was previously shown to be difficult to detect
in purified IMV (34). The other three proteins (I6L, G5R, and
A14.5L) were not identified in either LC/MS/MS or SCX-LC/
MS/MS analyses. Several possible reasons may explain why
they were not detected in both MS/MS analyses. First, MS is
biased against small and hydrophobic proteins. A14.5L
con-tains 53 amino acids, producing at most two tryptic peptides
(19). It is also very hydrophobic, with a grand average of
hydropathicity index of 1.438 (96). These features of A14.5L
may have jeopardized its detection in MS analyses. Second,
MS, despite great sensitivity, can only detect peptides at
fem-tomole range. Third, peptides that are too close to be resolved
in MS will also be ignored by data acquisition software. We
speculate that these technical reasons might explain the lack of
detection of G5R and I6L.
[image:6.585.45.543.79.224.2]Relative abundances of viral proteins in the IMV.
The
quan-tity of each viral component within the IMV particle is an
important issue, but it has been difficult to address in the past.
Crude estimation of the quantity of each protein by gel staining
intensity is not suitable for complex structure analysis. While
TABLE 2. Viral proteins identified by LC/MS/MS by a single tryptic peptide and confirmed by SCX-LC/MS/MS
VACVa -WR ORF
Reverse-phase LC/MS/MS SCX followed by reverse-phase LC/MS/MS
Reference for IMV association No. of
total hits
No. of unique peptides
Mascot score
Peptide sequence (CNBr-trypsin)b
No. of total
hits
No. of unique peptides
Mascot score
Peptide sequence (CNBr-LysC)
VACV-WR_022(C6L) 1 1 30 YYDGNIYELAK 3 2 66 ADSFSLESDSIK
32 IDAVRYYDGNIYELAK
VACV-WR_074(I5L) 2 1 39 VISGAALIVK 3 1 72 VISGAALIVK 160
VACV-WR_128(A9L) 2 1 35 LRPNSFWFVVVR 4 2 46 SCYTAILK 187
28 RNESSINSNSSPK
VACV-WR_140(A21L) 5 1 42 LFSYNFTTSGIK 6 4 40 NVPIPCSK 166
49 INEVNNNK 40 DVDTLYCDK
31 NFICVDDRLFSYNFTTSGIK
VACV-WR_142(A22R) 1 1 32 DNSVRVLDI(p)SK 1 1 34 DNSVRVLDI(p)SK 63
VACV-WR_154(A31R) 1 1 33 YLSGGGIYHDDLVVLGK 4 2 31 VTINNLK
33 YLSGGGIYHDDLVVLGK a
VACV, vaccinia virus. b
(p), predicted phosphorylated site.
on November 8, 2019 by guest
http://jvi.asm.org/
recent advances in differential protein analyses have provided
a means for comparing relative protein expression between
two populations, there is a need for a method giving the
rela-tive quantification of a single protein in a complex population.
Although quantification of particular proteins of interest using
isotope-labeled synthetic peptides, termed Protein-AQUA, is
in principle applicable to comprehensive analyses (12, 64), it is
hampered by the high cost of isotope-labeled peptides and the
difficulty of quantitative digestion of proteins in gel (73).
Recently, Ishihama et al. demonstrated that it is feasible to
quantify label-free protein components using an emPAI
ap-proach (85), which fully utilizes the advantages of the wide
dynamic detection range provided by LC/MS analysis and
re-liable protein search algorithms. This approach to determining
the relative abundances of proteins in a complex protein
mix-ture is based on the correlation of peptides identified by
tan-dem MS with observable peptides obtained by in silico
diges-tion. The abundance of each protein determined in this study
is presented as the molar and weight percentages of the total
protein molecules in IMV particles (Table 4). The most
abun-dant viral-protein group (weight percentage,
⬎
5) consisted of
four core proteins (A4L, A10L, F17R, and A3L). The relatively
abundant protein group (5
⬎
weight percentage
⬎
1) consisted of
seven envelope proteins (A27L, A25L, A14L, H3L, A26L, D8L,
and A13L,), two core proteins (L4R and G7L), four proteins
involved in transcription (D11L, J6R, A24R, and D1R), and
one novel protein (E6R). Eleven of the above 18 abundant
proteins have been previously identified as abundant proteins
in IMV particles (86, 160). The intermediate-abundance
pro-tein group (1%
⬎
weight percentage
⬎
0.5%) contained 15
viral proteins, and the less abundant group (weight percentage,
⬍
0.5%) contained 41 viral proteins. The distribution of viral
proteins based on different molar or weight abundances in
IMV revealed a similar distribution (Fig. 2); the low
abun-dances of many proteins explain why they have escaped
previ-ous proteomic detection.
Identification of host proteins associated with the IMV.
The
host protein content of vaccinia virus IMV was determined by
comparing the detected peptides with a Human International
Protein Index protein sequence database, and 23 host proteins
associated with vaccinia virus IMV particles were identified
(Table 5). All were present at low abundance (intermediate
and less abundant categories) in virions and could be divided
into several categories, namely, calcium binding proteins
(an-nexin A1 and A2), chaperon proteins (cyclophilin A, Hsc71,
and Hsp90), cytoskeleton proteins (actin, tubulin, and myosin),
chromosome architecture proteins (histone and HMG1),
transla-tion components (eIF4A-1, eIF1
␣
-1-1, and 60S ribosomal
pro-tein), protein transport/vesicular-trafficking proteins (ADP
ribo-sylation factors1/3 and 4, Rab7, Rab10, and ubiquitin), and
proteins involved in redox regulation (thioredoxin and
peroxire-doxin 1). Of these host proteins, cyclophilin A,

-actin, and
ubiquitin have been previously reported as components of
vac-cinia virus IMV particles (35, 86, 176), and another 13 have
been reported in other viruses (Table 5) (22, 87, 92, 172, 191).
Thus, seven host proteins (60S acidic ribosomal proteins,
66-kDa protein, ADP ribosylation factor 4, HMG1, peroxiredoxin
1, Rab10, and thioredoxin) were found to be associated with
vaccinia virus IMV and have not yet been detected in other
viruses.
DISCUSSION
[image:7.585.46.541.80.241.2]Proteomic techniques are widely used to identify protein
components of large complexes that participate in different
biological processes. MS remains the most suitable tool, as it is
fast, sensitive, and widely applicable. Current high-sensitivity
methods using MS for protein identification have greatly
low-ered detection limits, making it easy to analyze proteins with
low cell copy numbers. In addition, the quality of
bioinformat-ics continues to improve, making the prediction of gene
prod-ucts deduced from genome information increasingly accurate
when MS data are matched to known protein sequences. The
use of nano-LC for the high-resolution separation of digested
peptides and nano-electrospray ionization/MS analysis allows
protein detection in the low femtomole range in routine
anal-ysis. This approach can also be used to complement traditional
biochemical methods of elucidating viral structures and
func-tions and determining the number and nature of proteins
within a virus. In this study, we identified 75 viral and 23 host
proteins associated with purified vaccinia virus IMV particles,
a significant increase from previous studies (86, 160). Ten
TABLE 3. Virion proteins cited in literature but not identified by LC/MS/MS
VACVa -WR ORF
LC/MS/MS SCX-LC/MS/MS Reference
showing IMV association No. of
unique peptides
No. of total
hits
No. of unique peptides
Mascot score
Peptide sequence (CNBr-LysC)
VACV-WR_075(I6L)
0
0
0
48, 68
VACV-WR_082(G5R)
0
0
0
43
VACV-WR_131(A12L)
0
8
5
57
SSSSSTSASK
160, 180
41
NLLAQIGGDAAVK
56
DGQIVQAVTNAGK
40
VGEINHDLLGIDSVNAGK
25
NLAVRSSYDDYIETVNK
VACV-WR_134(A14.5L)
0
0
0
19
VACV-WR_155(A32L)
0
1
1
29
NCFQEK
34
VACV-WR_183(B1R)
0
4
3
41
NQWVVGPLIGK
99, 128
29
ASNIVLDQIDK
53
LYLVDYGLVSK
a
VACV, vaccinia virus.
on November 8, 2019 by guest
http://jvi.asm.org/
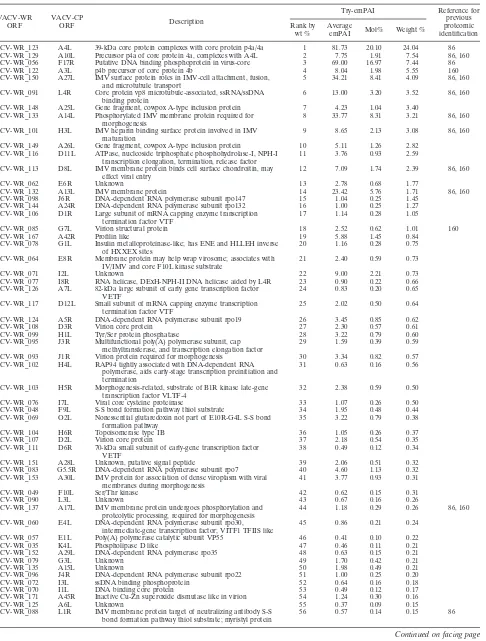
TABLE 4. Relative viral protein abundances in vaccinia virus IMV
aVACV-WR ORF
VACV-CP
ORF Description
Try-emPAI Reference for
previous proteomic identification Rank by
wt %
Average
emPAI Mol% Weight % VACV-WR_123 A4L 39-kDa core protein complexes with core protein p4a/4a 1 81.73 20.10 24.04 86 VACV-WR_129 A10L Precursor p4a of core protein 4a, complexes with A4L 2 7.75 1.91 7.54 86, 160 VACV-WR_056 F17R Putative DNA binding phosphoprotein in virus-core 3 69.00 16.97 7.44 86
VACV-WR_122 A3L p4b precursor of core protein 4b 4 8.04 1.98 5.55 160
VACV-WR_150 A27L IMV surface protein roles in IMV-cell attachment, fusion, and microtubule transport
5 34.21 8.41 4.09 86, 160
VACV-WR_091 L4R Core protein vp8 microtubule-associated, ssRNA/ssDNA binding protein
6 13.00 3.20 3.52 86, 160
VACV-WR_148 A25L Gene fragment, cowpox A-type inclusion protein 7 4.23 1.04 3.40 VACV-WR_133 A14L Phosphorylated IMV membrane protein required for
morphogenesis
8 33.77 8.31 3.21 86, 160
VACV-WR_101 H3L IMV heparin binding surface protein involved in IMV maturation
9 8.65 2.13 3.08 86, 160
VACV-WR_149 A26L Gene fragment, cowpox A-type inclusion protein 10 5.11 1.26 2.82 VACV-WR_116 D11L ATPase, nucleoside triphosphate phosphohydrolase-I, NPH-I
transcription elongation, termination, release factor
11 3.76 0.93 2.59
VACV-WR_113 D8L IMV membrane protein binds cell surface chondroitin, may effect viral entry
12 7.09 1.74 2.39 86, 160
VACV-WR_062 E6R Unknown 13 2.78 0.68 1.77
VACV-WR_132 A13L IMV membrane protein 14 23.42 5.76 1.71 86, 160
VACV-WR_098 J6R DNA-dependent RNA polymerase subunit rpo147 15 1.04 0.25 1.45 VACV-WR_144 A24R DNA-dependent RNA polymerase subunit rpo132 16 1.00 0.25 1.27 VACV-WR_106 D1R Large subunit of mRNA capping enzyme transcription
termination factor VTF
17 1.14 0.28 1.05
VACV-WR_085 G7L Virion structural protein 18 2.52 0.62 1.01 160
VACV-WR_167 A42R Profilin like 19 5.88 1.45 0.84
VACV-WR_078 G1L Insulin metalloproteinase-like; has ENE and HLLEH inverse of HXXEX sites
20 1.16 0.28 0.75
VACV-WR_064 E8R Membrane protein may help wrap virosome; associates with IV/IMV and core F10L kinase substrate
21 2.40 0.59 0.73
VACV-WR_071 I2L Unknown 22 9.00 2.21 0.73
VACV-WR_077 I8R RNA helicase, DExH-NPH-II DNA helicase aided by L4R 23 0.90 0.22 0.66 VACV-WR_126 A7L 82-kDa large subunit of early gene transcription factor
VETF
24 0.83 0.20 0.65
VACV-WR_117 D12L Small subunit of mRNA capping enzyme transcription termination factor VTF
25 2.02 0.50 0.64
VACV-WR_124 A5R DNA-dependent RNA polymerase subunit rpo19 26 3.45 0.85 0.62
VACV-WR_108 D3R Virion core protein 27 2.30 0.57 0.61
VACV-WR_099 H1L Tyr/Ser protein phosphatase 28 3.22 0.79 0.60
VACV-WR_095 J3R Multifunctional poly(A) polymerase subunit, cap methyltransferase, and transcription elongation factor
29 1.59 0.39 0.59
VACV-WR_093 J1R Virion protein required for morphogenesis 30 3.34 0.82 0.57 VACV-WR_102 H4L RAP94 tightly associated with DNA-dependent RNA
polymerase, aids early-stage transcription preinitiation and termination
31 0.63 0.16 0.56
VACV-WR_103 H5R Morphogenesis-related, substrate of B1R kinase late-gene transcription factor VLTF-4
32 2.38 0.59 0.50
VACV-WR_076 I7L Viral core cysteine proteinase 33 1.07 0.26 0.50
VACV-WR_048 F9L S-S bond formation pathway thiol substrate 34 1.95 0.48 0.44 VACV-WR_069 O2L Nonessential glutaredoxin not part of E10R-G4L S-S bond
formation pathway
35 3.22 0.79 0.38
VACV-WR_104 H6R Topoisomerase type IB 36 1.05 0.26 0.37
VACV-WR_107 D2L Virion core protein 37 2.18 0.54 0.35
VACV-WR_111 D6R 70-kDa small subunit of early-gene transcription factor VETF
38 0.49 0.12 0.34
VACV-WR_151 A28L Unknown, putative signal peptide 39 2.06 0.51 0.32
VACV-WR_083 G5.5R DNA-dependent RNA polymerase subunit rpo7 40 4.60 1.13 0.32 VACV-WR_153 A30L IMV protein for association of dense viroplasm with viral
membranes during morphogenesis
41 3.77 0.93 0.31
VACV-WR_049 F10L Ser/Thr kinase 42 0.62 0.15 0.31
VACV-WR_090 L3L Unknown 43 0.67 0.16 0.26
VACV-WR_137 A17L IMV membrane protein undergoes phosphorylation and proteolytic processing; required for morphogenesis
44 1.18 0.29 0.26 86, 160
VACV-WR_060 E4L DNA-dependent RNA polymerase subunit rpo30, intermediate-gene transcription factor; VITF1 TFIIS like
45 0.86 0.21 0.24
VACV-WR_057 E1L Poly(A) polymerase catalytic subunit VP55 46 0.41 0.10 0.22
VACV-WR_035 K4L Phospholipase D like 47 0.46 0.11 0.21
VACV-WR_152 A29L DNA-dependent RNA polymerase rpo35 48 0.63 0.15 0.21
VACV-WR_079 G3L Unknown 49 1.70 0.42 0.21
VACV-WR_135 A15L Unknown 50 1.98 0.49 0.21
VACV-WR_096 J4R DNA-dependent RNA polymerase subunit rpo22 51 1.00 0.25 0.20
VACV-WR_072 I3L ssDNA binding phosphoprotein 52 0.64 0.16 0.18
VACV-WR_070 I1L DNA binding core protein 53 0.49 0.12 0.17
VACV-WR_171 A45R Inactive Cu-Zn superoxide dismutase like in virion 54 1.24 0.30 0.16
VACV-WR_125 A6L Unknown 55 0.37 0.09 0.15
VACV-WR_088 L1R IMV membrane protein target of neutralizing antibody S-S bond formation pathway thiol substrate; myristyl protein
56 0.57 0.14 0.15 86
Continued on facing page
on November 8, 2019 by guest
http://jvi.asm.org/
novel viral proteins were identified in IMV (Table 1).
EEV-specific viral proteins, such as F13L, A33R, A34R, A36R, and
B5R, and viral nonstructural proteins, such as A11R, were not
detected in our analyses, supporting the idea that the 10 novel
viral proteins are not just copurified lysate contaminants (129,
137). If we include the additional six virion proteins cited from
the literature (Table 3), there are a total of 81 viral proteins in
IMV.
Furthermore, the relative abundance of each viral and host
protein in IMV was determined by calculating the emPAI
value, providing important quantification information for
pro-teomic experiments. The most abundant viral proteins were in
general agreement with previous reports (86), validating our
quantification methods. In addition, our analysis also revealed
that a significant proportion of virion proteins were present at
low abundance. It is worth noting that the quantification
method based on the emPAI value is fairly new, and the
rel-ative abundance would be better utilized to compare proteins
of different abundance groups (Fig. 2) rather than to compare
proteins within the same low-abundance group.
In addition to the viral proteins, 23 IMV-associated host
proteins were identified. Compared with the data
reproduc-ibility of 75 viral proteins obtained from different IMV
prep-arations, the host proteins indeed revealed somewhat more
variation from one preparation to another. Several, such as
ARF1/3, ARF 4, Rab-7, and Rab-10, are involved in transport
and vesicle trafficking, and their association with IMV particles
may be due to the intracellular route taken by the IMV during
virion egress. Some host proteins, such as tubulin, actin,
an-nexin A2, cyclophilin A, Hsc71, and Hsp90, have been
identi-fied in other virions, such as human cytomegalovirus, murine
cytomegalovirus, and adenovirus (Table 3). It is worth noting
[image:9.585.44.544.80.325.2]that our previous study (82) showed a transient association of
Hsp90 with viral factories in cells, while no Hsp90 was detected
by immunoblot analysis in purified IMV. Since MS/MS is much
more sensitive than immunoblot detection, this discrepancy
could be due to the low abundance of Hsp90 associated with
viral particles. Although we cannot rule out the possibility that
some of the cytoskeleton and chaperon proteins could be
for-tuitously associated with viral particles during IMV
prepara-tion, as previously shown by Franke and Hruby (58), other
[image:9.585.341.501.502.668.2]FIG. 2. Distribution of viral proteins with different abundances in
IMV. All the viral proteins identified in this study were quantified as
described in Materials and Methods and divided into four categories
based on the protein contents in IMV, as shown below the graph. The
black columns show the molar percentages of the total proteins in
IMVs, while the white columns show the weight percentages.
TABLE 4—
Continued
VACV-WR ORF
VACV-CP
ORF Description
Try-emPAI Reference for
previous proteomic identification Rank by
wt %
Average
emPAI Mol% Weight %
VACV-WR_136 A16L Soluble myristyl protein 57 0.32 0.08 0.13
VACV-WR_081 G4L S-S bond formation pathway; thioredoxin like 58 0.93 0.23 0.12 86
VACV-WR_066 E10R S-S bond formation pathway sulfhydryl oxidase, substrates L1R/F9L
59 1.03 0.25 0.11
VACV-WR_121 A2.5L S-S bond formation pathway CXXXC links SH oxidase E10R and thioredoxin G4L
60 1.20 0.30 0.10
VACV-WR_112 D7R DNA-dependent RNA polymerase subunit rpo18 61 0.58 0.14 0.10 VACV-WR_138 A18L DNA helicase effects elongation and termination of
postreplicative viral transcription
62 0.18 0.05 0.10
VACV-WR_074 I5L IMV protein VP13 63 1.15 0.28 0.10 160
VACV-WR_047 F8L Protein with iActA-like proline repeats not required for actin tail formation
64 1.24 0.30 0.09
VACV-WR_087 G9R Myristyl protein 65 0.23 0.06 0.08
VACV-WR_100 H2R Unknown 66 0.38 0.09 0.08
VACV-WR_092 L5R Putative membrane protein 67 0.39 0.10 0.06
VACV-WR_142 A22R Palmityl protein; Holliday junction endonuclease; resolves viral DNA concatemers into unit-length genomes
68 0.26 0.06 0.05
VACV-WR_128 A9L IMV membrane protein required for morphogenesis 69 0.46 0.11 0.05
VACV-WR_067 E11L Virion core protein 70 0.33 0.08 0.05
VACV-WR_118 D13L Rifampin target associates with inner surface immature virus membrane
71 0.08 0.02 0.05
VACV-WR_140 A21L Unknown 72 0.33 0.08 0.04
VACV-WR_022 C6L Unknown 73 0.26 0.04
VACV-WR_172 A46R Toll/IL1-receptor (TIR) likesuppresses TIR-dependent signal transduction host defense modulator
74 0.15 0.04 0.04
VACV-WR_154 A31R Unknown 75
aData presented in Table 4 are compiled from three different IMV preparations. VACV, vaccinia virus; CP, Copenhagen strain; ssRNA, single-stranded RNA; ssDNA, single-stranded DNA.
on November 8, 2019 by guest
http://jvi.asm.org/
TABLE
5.
Host
proteins
identified
in
vaccinia
virus
IMV
a Accession no. Mass (Da) Description CNBr-trypsin digestion CNBr-LysC digestion Association with known virion particles b Reference(s) No. of unipeptides Sequence coverage (%) emPAIProtein content (mol%)
No. of unipeptides Sequence coverage (%) emPAI
Protein content (mol%)
IPI00008530 34,252 60S acidic ribosomal protein P0 2 6 0.52 0.13 IPI00333428 66,265 66-kDa protein 1 1 0.06 0.02 1 1 0.06 0.07 IPI00215914 20,684 ADP ribosylation factor 1 and/or 3 1 5 0.29 0.07 1 9 0.58 0.68 HCMV 172 IPI00215918 20,367 ADP ribosylation factor 4 2 9 0.67 0.16 IPI00218918 38,559 Annexin A1 2 8 0.33 0.08 HCMV, MCMV 92, 172 IPI00455315 38,449 Annexin A2 7 26 0.51 0.13 HCMV, KSHV 172, 185, 191 IPI00021439 41,710 Actin, cytoplasmic 1 8 30 0.57 0.14 2 5 0.12 0.14 ASFV, HCMV, HIV, KSHV, MCMV, VV 55, 86, 92, 119, 172, 191 IPI00639885 50,109 Elongation factor 1-alpha 1 9 21 0.63 0.16 2 5 0.12 0.14 HCMV, HIV, MCMV 40, 92, 172 IPI00025491 46,125 Eukaryotic initiation factor 4A-I 2 4 0.23 0.06 3 8 0.27 0.31 HCMV 172 IPI00003865 70,854 Heat shock cognate 71-kDa protein 16 28 0.90 0.22 5 11 0.45 0.52 HCMV, HIV 71, 172 IPI00382470 98,052 Heat shock protein HSP 90-alpha 2 3 10 0.22 0.05 2 9 0.25 0.30 DHBV, HCMV, KSHV, 81, 172, 191 IPI00334775 84,790 Heat shock protein HSP 90-beta 11 16 0.86 0.21 6 9 0.32 0.37 DHBV, HCMV, KSHV, 81, 172, 191 IPI00419258 24,747 High-mobility group protein 1 3 20 1.51 0.37 IPI00003935 13,781 Histone H2B.q 1 7 0.47 0.12 1 6 0.58 0.68 MCMV, SV40 36, 92 IPI00019502 226,392 Myosin-9 (myosin heavy chain, nonmuscle IIa) 7 5 0.22 0.05 1 1 0.02 0.03 HCMV, KSHV 172, 191 IPI00419585 17,870 Peptidyl-prolyl cis-trans isomerase A (cyclophilin A) 3 21 0.78 0.19 2 13 0.67 0.78 HCMV, HIV, KSHV, SIV, VSV, VV 23, 26, 35, 60, 163, 172, 191 IPI0000874 22,096 Peroxiredoxin 1 5 23 0.76 0.19 IPI00016513 22,527 Ras-related protein Rab-10 2 11 0.52 0.13 1 6 0.23 0.27 IPI00016342 23,475 Ras-related protein Rab-7 3 16 0.70 0.17 1 8 0.33 0.39 KSHV 191 IPI00216298 11,599 Thioredoxin 1 7 0.47 0.12 1 7 0.47 0.55 IPI00387144 50,120 Tubulin alpha-ubiquitous chain 9 26 0.63 0.16 5 13 0.36 0.42 ASFV, HCMV 55, 172 IPI00011654 49,727 Tubulin-beta-2 chain 7 19 0.48 0.12 5 13 0.61 0.71 ASFV 55 P62988 (Swiss- Prot ID) 8,565 Ubiquitin 5 30 4.44 1.09 3 29 2.70 3.15 ASFV, baculovirus, HIV, HSV, MuLV, SIV, VV 55, 70, 86, 117, 118, 176 aTwenty-three host proteins are compiled from both trypsin-digested and Lys-C-digested virion preparations. The data reproducibility among dif fe rent virus preparations reached 67% for trypsin digestions and 87% for Lys-C digestions. bASFV, African swine fever virus; DHBV, duck hepatitis virus; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; HSV, herpes simplex vi rus; KSHV, Kaposi’s sarcoma-associated herpesvirus; MCMV, murine cytomegalovirus; MuLV, murine leukemia virus; SIV, simian immunodeficiency virus; SV40, simian virus 40; VSV, vesicular stomatitis vi rus; VV, vaccinia virus.
on November 8, 2019 by guest
http://jvi.asm.org/
abundant host proteins, such as myosin and vimentin, were not
detected in purified IMV. Also, we did not detect host proteins
that are known to be on vaccinia virus EEV, i.e., CD46, CD59,
CD29, CD71, CD81, and MHC-1 (170). We also did not detect
CD55, moesin, and cofilin, which were frequently identified in
other viruses, such as human T-cell leukemia/lymphoma virus
type 1, human immunodeficiency virus, and human
cytomega-lovirus, implying that host protein association with vaccinia
virus IMV has some selectivity (92, 119, 136, 154, 172).
Inter-estingly, six host proteins were uniquely identified in vaccinia
virus, and their roles in vaccinia virus biology need to be
stud-ied in the future.
ACKNOWLEDGMENTS
This work was supported by grants from the Academia Sinica and
the National Science Council (NSC94-2627-M-001-005 and
NSC91-3112-P-001-057-Y), Taiwan, Republic of China.
REFERENCES
1.Ahn, B. Y., P. D. Gershon, E. V. Jones, and B. Moss.1990. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol. Cell. Biol.10:5433–5441. 2.Ahn, B. Y., P. D. Gershon, and B. Moss.1994. RNA polymerase-associated
protein Rap94 confers promoter specificity for initiating transcription of vaccinia virus early stage genes. J. Biol. Chem.269:7552–7557.
3.Ahn, B. Y., E. V. Jones, and B. Moss.1990. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5⬘poly(A) leader on its early transcript. J. Virol.64:
3019–3024.
4.Ahn, B. Y., and B. Moss.1992. Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascor-bate reductase activities. Proc. Natl. Acad. Sci. USA89:7060–7064. 5.Ahn, B. Y., and B. Moss.1992. RNA polymerase-associated transcription
specificity factor encoded by vaccinia virus. Proc. Natl. Acad. Sci. USA
89:3536–3540.
6.Ahn, B. Y., J. Rosel, N. B. Cole, and B. Moss.1992. Identification and expression of rpo19, a vaccinia virus gene encoding a 19-kilodalton DNA-dependent RNA polymerase subunit. J. Virol.66:971–982.
7.Almazan, F., D. C. Tscharke, and G. L. Smith.2001. The vaccinia virus superoxide dismutase-like protein (A45R) is a virion component that is nonessential for virus replication. J. Virol.75:7018–7029.
8.Amegadzie, B. Y., B. Y. Ahn, and B. Moss.1992. Characterization of a 7-kilodalton subunit of vaccinia virus DNA-dependent RNA polymerase with structural similarities to the smallest subunit of eukaryotic RNA poly-merase II. J. Virol.66:3003–3010.
9.Amegadzie, B. Y., M. H. Holmes, N. B. Cole, E. V. Jones, P. L. Earl, and B. Moss.1991. Identification, sequence, and expression of the gene encoding the second-largest subunit of the vaccinia virus DNA-dependent RNA polymerase. Virology180:88–98.
10.Ansarah-Sobrinho, C., and B. Moss.2004. Role of the I7 protein in pro-teolytic processing of vaccinia virus membrane and core components. J. Vi-rol.78:6335–6343.
11.Baldick, C. J., Jr., and B. Moss. 1987. Resistance of vaccinia virus to rifampicin conferred by a single nucleotide substitution near the predicted NH2 terminus of a gene encoding an Mr 62,000 polypeptide. Virology
156:138–145.
12.Barr, J. R., V. L. Maggio, D. G. Patterson, Jr., G. R. Cooper, L. O. Hen-derson, W. E. Turner, S. J. Smith, W. H. Hannon, L. L. Needham, and E. J. Sampson.1996. Isotope dilution—mass spectrometric quantification of spe-cific proteins: model application with apolipoprotein A-I. Clin. Chem.42:
1676–1682.
13.Bayliss, C. D., and G. L. Smith.1996. Vaccinia virion protein I8R has both DNA and RNA helicase activities: implications for vaccinia virus transcrip-tion. J. Virol.70:794–800.
14.Beaud, G., and R. Beaud.1997. Preferential virosomal location of under-phosphorylated H5R protein synthesized in vaccinia virus-infected cells. J. Gen. Virol.78:3297–3302.
15.Beaud, G., and R. Beaud.2000. Temperature-dependent phosphorylation state of the H5R protein synthesised at the early stage of infection in cells infected with vaccinia virus ts mutants of the B1R and F10L protein kinases. Intervirology43:67–70.
16.Beaud, G., R. Beaud, and D. P. Leader.1995. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase. J. Virol.69:1819–1826.
17.Besemer, J., A. Lomsadze, and M. Borodovsky.2001. GeneMarkS: a
self-training method for prediction of gene starts in microbial genomes. Impli-cations for finding sequence motifs in regulatory regions. Nucleic Acids Res.29:2607–2618.
18.Betakova, T., E. J. Wolffe, and B. Moss.1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane pro-teins and C-terminal truncation of the A17L protein are dependent on the F10L kinase. J. Virol.73:3534–3543.
19.Betakova, T., E. J. Wolffe, and B. Moss.2000. The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses. J. Virol.74:4085–4092.
20.Blasco, R., N. B. Cole, and B. Moss.1991. Sequence analysis, expression, and deletion of a vaccinia virus gene encoding a homolog of profilin, a eukaryotic actin-binding protein. J. Virol.65:4598–4608.
21.Blasco, R., and B. Moss.1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encod-ing the 37,000-dalton outer envelope protein. J. Virol.65:5910–5920. 22.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and
R. Sun.2003. Identification of proteins associated with murine gammaher-pesvirus 68 virions. J. Virol.77:13425–13432.
23.Bose, S., M. Mathur, P. Bates, N. Joshi, and A. K. Banerjee.2003. Re-quirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol.84:1687–1699.
24.Boursnell, M. E., I. J. Foulds, J. I. Campbell, and M. M. Binns.1988. Non-essential genes in the vaccinia virus HindIII K fragment: a gene re-lated to serine protease inhibitors and a gene rere-lated to the 37K vaccinia virus major envelope antigen. J. Gen. Virol.69:2995–3003.
25.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O’Neill.2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA97:10162– 10167.
26.Braaten, D., E. K. Franke, and J. Luban.1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV(CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol.70:4220–4227. 27.Brown, N. G., D. Nick Morrice, G. Beaud, G. Hardie, and D. P. Leader.
2000. Identification of sites phosphorylated by the vaccinia virus B1R kinase in viral protein H5R. BMC Biochem.1:2.
28.Broyles, S. S., and B. S. Fesler.1990. Vaccinia virus gene encoding a component of the viral early transcription factor. J. Virol.64:1523–1529. 29.Broyles, S. S., and B. Moss.1986. Homology between RNA polymerases of
poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and tran-scriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc. Natl. Acad. Sci. USA83:3141–3145.
30.Broyles, S. S., and B. Moss.1987. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. J. Virol.61:1738–1742.
31.Broyles, S. S., and M. J. Pennington.1990. Vaccinia virus gene encoding a 30-kilodalton subunit of the viral DNA-dependent RNA polymerase. J. Vi-rol.64:5376–5382.
32.Byrd, C. M., T. C. Bolken, and D. E. Hruby.2002. The vaccinia virus I7L gene product is the core protein proteinase. J. Virol.76:8973–8976. 33.Byrd, C. M., and D. E. Hruby.2005. A conditional-lethal vaccinia virus
mutant demonstrates that the I7L gene product is required for virion morphogenesis. Virol. J.2:4.
34.Cassetti, M. C., M. Merchlinsky, E. J. Wolffe, A. S. Weisberg, and B. Moss.
1998. DNA packaging mutant: repression of the vaccinia virus A32 gene results in noninfectious, DNA-deficient, spherical, enveloped particles. J. Virol.72:5769–5780.
35.Castro, A. P., T. M. Carvalho, N. Moussatche, and C. R. Damaso.2003. Redistribution of cyclophilin A to viral factories during vaccinia virus in-fection and its incorporation into mature particles. J. Virol.77:9052–9068. 36.Chen, Y. H., J. P. MacGregor, D. A. Goldstein, and M. R. Hall.1979. Histone modifications in simian virus 40 and in nucleoprotein complexes containing supercoiled viral DNA. J. Virol.30:218–224.
37.Chiu, W. L., and W. Chang. 2002. Vaccinia virus J1R protein: a viral membrane protein that is essential for virion morphogenesis. J. Virol.
76:9575–9587.
38.Chiu, W. L., P. Szajner, B. Moss, and W. Chang.2005. Effects of a tem-perature sensitivity mutation in the J1R protein component of a complex required for vaccinia virus assembly. J. Virol.79:8046–8056.
39.Chung, C. S., J. C. Hsiao, Y. S. Chang, and W. Chang.1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Vi-rol.72:1577–1585.
40.Cimarelli, A., and J. Luban.1999. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol.73:5388–5401.
41.Cudmore, S., P. Cossart, G. Griffiths, and M. Way. 1995. Actin-based motility of vaccinia virus. Nature378:636–638.
42.Cudmore, S., I. Reckmann, G. Griffiths, and M. Way.1996. Vaccinia virus: a model sustem for actin-membrane interactions. J. Cell Sci.109:1739– 1747.
on November 8, 2019 by guest
http://jvi.asm.org/
43.da Fonseca, F. G., A. S. Weisberg, M. F. Caeiro, and B. Moss. 2004. Vaccinia virus mutants with alanine substitutions in the conserved G5R gene fail to initiate morphogenesis at the nonpermissive temperature. J. Vi-rol.78:10238–10248.
44.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss.2000. Charac-terization of the vaccinia virus H3L envelope protein: topology and post-translational membrane insertion via the C-terminal hydrophobic tail. J. Vi-rol.74:7508–7517.
45.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss.2000. Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J. Virol.74:7518–7528.
46.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty.2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol.
79:11724–11733.
47.Davis, R. E., and C. K. Mathews.1993. Acidic C terminus of vaccinia virus DNA-binding protein interacts with ribonucleotide reductase. Proc. Natl. Acad. Sci. USA90:745–749.
48.DeMasi, J., S. Du, D. Lennon, and P. Traktman.2001. Vaccinia virus telomeres: interaction with the viral I1, I6, and K4 proteins. J. Virol.
75:10090–10105.
49.Demkowicz, W. E., J. S. Maa, and M. Esteban.1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Vi-rol.66:386–398.
50.Derrien, M., A. Punjabi, M. Khanna, O. Grubisha, and P. Traktman.1999. Tyrosine phosphorylation of A17 during vaccinia virus infection: involve-ment of the H1 phosphatase and the F10 kinase. J. Virol.73:7287–7296. 51.Doglio, L., A. De Marco, S. Schleich, N. Roos, and J. Krijnse Locker.2002.
The vaccinia virus E8R gene product: a viral membrane protein that is made early in infection and packaged into the virions’ core. J. Virol.76:
9773–9786.
52.Dyster, L. M., and E. G. Niles.1991. Genetic and biochemical character-ization of vaccinia virus genes D2L and D3R which encode virion structural proteins. Virology182:455–467.
53.Ensinger, M. J.1987. Phenotypic characterization of temperature-sensitive mutants of vaccinia virus with mutations in a 135,000-Mrsubunit of the virion-associated DNA-dependent RNA polymerase. J. Virol.61:1842–1850. 54.Essani, K., and S. Dales.1979. Biogenesis of vaccinia: evidence for more
than 100 polypeptides in the virion. Virology95:385–394.
55.Esteves, A., M. I. Marques, and J. V. Costa.1986. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology152:192–206.
56.Fathi, Z., and R. C. Condit.1991. Genetic and molecular biological char-acterization of a vaccinia virus temperature-sensitive complementation group affecting a virion component. Virology181:258–272.
57.Fenner, F.1990. Poxviruses, p. 2113–2133.InB. Fields and D. M. Knipe (ed.), Virology. Raven Press, New York, N.Y.
58.Franke, C. A., and D. E. Hruby.1987. Association of non-viral proteins with recombinant vaccinia virus virions. Arch. Virol.94:347–351.
59.Franke, C. A., E. M. Wilson, and D. E. Hruby.1990. Use of a cell-free system to identify the vaccinia virus L1R gene product as the major late myristylated virion protein M25. J. Virol.64:5988–5996.
60.Franke, E. K., H. E. Yuan, and J. Luban.1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature372:359–362.
61.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way.1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature401:926–929. 62.Funahashi, S., T. Sato, and H. Shida.1988. Cloning and characterization of
the gene encoding the major protein of the A-type inclusion body of cowpox virus. J. Gen. Virol.69:35–47.
63.Garcia, A. D., and B. Moss.2001. Repression of vaccinia virus Holliday junction resolvase inhibits processing of viral DNA into unit-length ge-nomes. J. Virol.75:6460–6471.
64.Gerber, S. A., J. Rush, O. Stemman, M. W. Kirschner, and S. P. Gygi.2003. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. USA100:6940–6945.
65.Gershon, P. D., B. Y. Ahn, M. Garfield, and B. Moss. 1991. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell66:1269–1278.
66.Gershon, P. D., and B. Moss.1990. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc. Natl. Acad. Sci. USA87:4401– 4405.
67.Grosenbach, D. W., S. G. Hansen, and D. E. Hruby.2000. Identification and analysis of vaccinia virus palmitylproteins. Virology275:193–206. 68.Grubisha, O., and P. Traktman.2003. Genetic analysis of the vaccinia virus
I6 telomere-binding protein uncovers a key role in genome encapsidation. J. Virol.77:10929–10942.
69.Guan, K. L., S. S. Broyles, and J. E. Dixon. 1991. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature350:359–362.
70.Guarino, L. A., G. Smith, and W. Dong.1995. Ubiquitin is attached to
membranes of baculovirus particles by a novel type of phospholipid anchor. Cell80:301–309.
71.Gurer, C., A. Cimarelli, and J. Luban.2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol.
76:4666–4670.
72.Gvakharia, B. O., E. K. Koonin, and C. K. Mathews.1996. Vaccinia virus G4L gene encodes a second glutaredoxin. Virology226:408–411. 73.Havlis, J., and A. Shevchenko.2004. Absolute quantification of proteins in
solutions and in polyacrylamide gels by mass spectrometry. Anal. Chem.
76:3029–3036.
74.Hedengren-Olcott, M., C. M. Byrd, J. Watson, and D. E. Hruby.2004. The vaccinia virus G1L putative metalloproteinase is essential for viral replica-tion in vivo. J. Virol.78:9947–9953.
75.Heuser, J.2005. Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic “honeycomb” surface coat. J. Cell Biol.169:269–283.
76.Higley, S., and M. Way.1997. Characterization of the vaccinia virus F8L protein. J. Gen. Virol.78:2633–2637.
77.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux.1999. Vaccinia virus intracellular mature virions contain only one lipid mem-brane. J. Virol.73:1503–1517.
78.Hooda-Dhingra, U., D. D. Patel, D. J. Pickup, and R. C. Condit.1990. Fine structure mapping and phenotypic analysis of five temperature-sensitive mutations in the second largest subunit of vaccinia virus DNA-dependent RNA polymerase. Virology174:60–69.
79.Hooda-Dhingra, U., C. L. Thompson, and R. C. Condit.1989. Detailed phenotypic characterization of five temperature-sensitive mutants in the 22-and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA poly-merase. J. Virol.63:714–729.
80.Hsiao, J. C., C. S. Chung, and W. Chang.1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol.73:8750–8761. 81.Hu, J., D. O. Toft, and C. Seeger.1997. Hepadnavirus assembly and reverse
transcription require a multi-component chaperone complex which is in-corporated into nucleocapsids. EMBO J.16:59–68.
82.Hung, J. J., C. S. Chung, and W. Chang.2002. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J. Virol.76:1379–1390. 83.Ichihashi, Y., and M. Oie.1996. Neutralizing epitope on penetration
pro-tein of vaccinia virus. Virology220:491–494.
84.Ichihashi, Y., M. Oie, and T. Tsuruhara.1984. Location of DNA-binding proteins and disulfide-linked proteins in vaccinia virus structural elements. J. Virol.50:929–938.
85.Ishihama, Y., Y. Oda, T. Tabata, T. Sato, T. Nagasu, J. Rappsilber, and M. Mann.2005. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteomics.4:1265–1272. 86.Jensen, O. N., T. Houthaeve, A. Shevchenko, S. Cudmore, T. Ashford, M.
Mann, G. Griffiths, and J. Krijnse Locker.1996. Identification of the major membrane and core proteins of vaccinia virus by two-dimensional electro-phoresis. J. Virol.70:7485–7497.
87.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff.2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA101:16286–16291.
88.Johnson, G. P., S. J. Goebel, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti.1991. Vaccinia virus encodes a protein with similarity to glu-taredoxins. Virology181:378–381.
89.Kane, E. M., and S. Shuman. 1993. Vaccinia virus morphogenesis is blocked by a temperature-sensitive mutation in the I7 gene that encodes a virion component. J. Virol.67:2689–2698.
90.Kao, S. Y., and W. R. Bauer.1987. Biosynthesis and phosphorylation of vaccinia virus structural protein VP11. Virology159:399–407.
91.Kao, S. Y., E. Ressner, J. Kates, and W. R. Bauer.1981. Purification and characterization of a superhelix binding protein from vaccinia virus. Virol-ogy111:500–508.
92.Kattenhorn, L. M., R. Mills, M. Wagner, A. Lomsadze, V. Makeev, M. Borodovsky, H. L. Ploegh, and B. M. Kessler.2004. Identification of pro-teins associated with murine cytomegalovirus virions. J. Virol.78:11187– 11197.
93.Koonin, E. V., and T. G. Senkevich. 1992. Vaccinia virus encodes four putative DNA and/or RNA helicases distantly related to each other. J. Gen. Virol.73:989–993.
94.Kovacs, G. R., and B. Moss.1996. The vaccinia virus H5R gene encodes late gene transcription factor 4: purification, cloning, and overexpression. J. Virol.70:6796–6802.
95.Krijnse-Locker, J., S. Schleich, D. Rodriguez, B. Goud, E. J. Snijder, and G. Griffiths.1996. The role of a 21-kDa viral membrane protein in the assem-bly of vaccinia virus from the intermediate compartment. J. Biol. Chem.
271:14950–14958.
96.Kyte, J., and R. F. Doolittle.1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol.157:105–132.
97.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang.2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important
on November 8, 2019 by guest
http://jvi.asm.org/
for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol.74:3353–3365.
98.Lin, S., and S. S. Broyles.1994. Vaccinia protein kinase 2: a se