Localization
of Structural Proteins in
African Swine Fever Virus
Particles by Immunoelectron Microscopy
JOSE L. CARRASCOSA,PEDRO GONZALEZ, ANGEL L. CARRASCOSA, BLANCAGARCIA-BARRENO,t LUIS ENJUANES, AND ELADIO VINUELA*
Centro deBiologia Molecular (CSIC-UAM), Facultad de Ciencias, UniversidadAut6noma de Madrid, Canto Blanco, 28049
Madrid,
Spain
Received 3 June 1985/Accepted 2 October 1985
Seven African swine fever virus structural proteins were localized in the virion by immunoelectron
microscopy. African swine fever virus-infected cells were incubated, before or after embedding and thin sectioning, withmonoclonalantibodiesspecific for different structural proteins,andafter labelingwith protein A-gold complexes,thesampleswereexamined in the electronmicroscope.Proteinsp14andp24werefoundin
theexternal regionof thevirion, proteins p12, p72, p17, andp37werefound in the intermediatelayers, and protein p150wasfoundin thenucleoid andinonevertex.Amonoclonal antibodythatrecognized protein p150 aswellasp220,avirus-induced,nonstructural protein,could also bindtoacomponent presentinthe nucleus ofboth uninfected and virus-infected cells.
African swine fever (ASF) virus is an icosahedral cyto-plasmic deoxyvirusthatmultipliesin members of the Suidae family and Ornithodoros (Argasidae: Acari) (reviewed in references 9,19,and20).Oneofthemoststrikingaspectsof ASF is that the ASF
virus-specific
antibodies, even those from recovered or chronically infected pigs or from ASF virus-resistant animal species inoculated with the virus, do notneutralizethevirus(6). Theinabilitytoproduce neutral-izing antibodies might beduetothenatureof thevirusrather than duetothehost,sincepigsrecovered from virulent ASF virus infections or chronically infected animals respond normally to foot-and-mouth disease virus, producingneu-tralizing antibodies (6).
ASF virusparticles consist ofa nucleoid of about 80nm
surrounded by a lipid membrane, probably associated with the morphological units ofthe capsid (4). The extracellular
virions
have an external membrane derived by budding through theplasmamembrane (1) and anexternal diameter of about200nm.Thevirionis built upbyabout 30 structural proteins,someof whichareofhostorigin(5, 17).Knowledge ofthe protein composition of the different virion regions, mainly that of the envelopes, is important because those proteins might have immunological significance.Localization of antigens in situ by the use of protein A-gold complexes is an accurate technique (14) which has been used to detect virus antigens in virions (7, 21) and tissues (2).
We show in this paper an analysis of ASF virus-infected cells by immunoelectron microscopy, using a collection of ASF virus-specific monoclonal antibodies (MAbs) (17) la-beled with protein A-gold complexes. This study showed thatproteinsp14and p24 were present in the external region of theovirion, proteins p12, p72, p17, and p37 were in the intermediate layers, and protein p150 was in the nucleoid andone vertex. An MAb that recognized protein p150and p220,avirus-induced,nonstructuralprotein, could also bind
* Correspondingauthor.
tPresent address: Centro Nacional deMicrobiologfa,Virologfae InmunologfaSanitarias, Majadahonda, Madrid, Spain.
to acomponentpresentin thenucleusofbothuninfectedand virus-infected cells.
MATERIALSANDMETHODS
Cellsand virus.Verocells(CCL81)wereobtainedfrom the American Type Culture Collection (Rockville, Md.). ASF virus adapted to grow in Vero cells and the conditions for plaque titration have been described previously (8). Extra-cellular ASF virionswerepurifiedasdescribedelsewhere(5). Immunoreagents. Sera against purified ASF virions were
obtained as describedby Carrascosa etal. (5). Hybridcells secreting MAbs specific for ASF virus were obtained by fusing mouse myeloma and spleen cells from ASF virus-immunized mice (17). Protein A-Sepharose-purified immu-noglobulin G from ascites was provided by J. Rey. The properties of the MAbs used in this work have been de-scribed previously (17).
Colloidal gold particles of about 5-nm diameter were
prepared bythemethoddescribed byHorisberger(10). The
coupling
of the colloidal gold to protein A fromStaphylo-coccus aureus
(Pharmacia,
Uppsala, Sweden)was done asindicated byRoth (14).
Virus-infected cells. Vero cells were cultured in plastic plates in Dulbecco modified Eagle mediumcontaining 10% calfserum to adensityof about105cells per cm2 and infected withASF virusat amultiplicity of infection of about 10 PFU percell.After 2 h at37°C, nonadsorbed viruswas removed, andfresh medium with 2% calfserumwasaddedfor further incubation.
Immunolabeling and low-temperature embedding. (i) La-beling before thin sectioning. ASF virus-infected Vero cells were collected 24to 36 hafter infection, when they showed
a strong cytopathic effect and manywere permeable to the antibodies. The cells were washed and suspended in phos-phate-buffered saline (PBS) containing 1% ovalbumin and different amounts of antibody. After 1 h at 25°C, the cells
were washed and resuspended in PBS containing 1% ovalbumin andprotein A-gold complexdiluted 1/20th froma
stock prepared as described by Roth (14). After 30 min at
25°C, the cellswere washedwithPBS, fixed withasolution
377
on November 10, 2019 by guest
http://jvi.asm.org/
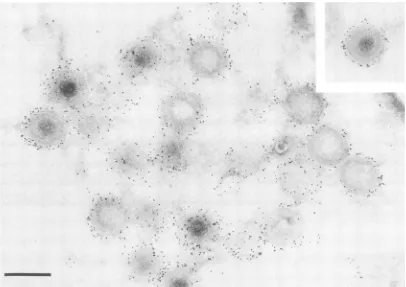
FIG. 1. Preembedding immunolabeling of ASF virus particles with aserumagainstpurifiedvirions. ASFvirus-infected Vero cells were treatedat40 hafter infection with an ASF virus antiserum and protein A-gold complexes. The cells were fixed, embedded in Lowicryl, and contrasted with uranylacetate.Bar, 200 nm.
containing 2% glutaraldehydeand2% tannic acidin PBS (13, 18) for 1 h at 25°C, and embedded in Lowicryl K4M (Chemische Werke Lowi, Waldraiburg, Federal Republic of Germany) asdescribed below.
(ii)Labeling after thin sectioning. ASFvirus-infected cells were collected at 16 to 20 h after infection, suspended in 0.5% glutaraldehyde in PBS and 2% tannic acid (13, 18),and incubated for 5 min at 25°C with gentle stirring. After low-speed centrifugation, the samplesweredehydratedwith ethanolandembedded inLowicrylK4Mbytheprocedure of Carlemalm et al. (3). The first steps of the dehydration process were carried out at4°C, and after the50%ethanol step, the samples were kept at -20°C. The resins were polymerized by irradiationwith 360-nm UVlight for48h at -20°C and 24 to 48 h at room temperature. Thin sections, obtained with a Sorvall Porter-Blum ultramicrotome, were adheredtonickelgrids, whichwerefloated on adrop of 1% ovalbumin in PBS for 15min at25°C. Afterthe
grids
weretransferred to a drop of the appropriate dilution of the antibody, they were incubated at 4°C for 12 to 16 h. The antibodywaswashedoffwith a streamofPBS,and thegrids were treated with 1% ovalbumin and protein A-gold com-plexes in PBS for 1 h at 25°C, washed with water, dried, stained with saturated uranyl acetate, and finally washed with water.
Electronmicroscopy. Samples were examined in aJEOL 100B electron microscope. Measurements were done on electronmicrographs of virus sections showingahexagonal outline. The radial distribution of the gold granules was
calculatedby determining,for each virussection,thecenter, the radius of the particle, and the distance of each gold
granule to the center, in a writing table connected to a PDP11-45 minicomputer. The radial distribution of each labeled component was thenexpressedasthe averageratio of the distances to the radii. The number of virus sec-tionsandgoldgranules counted are indicated in each exper-iment.
RESULTS
Labeling of ASFvirusparticles withserumagainstpurified virions. ASF virus-infected cells obtained at 24 to 36 h postinfection showed a strong cytopathic effect and were permeable to the immunoglobulins from an antiserum against purifiedASF virions. Afterthe unbound antibodies wereremovedby washing,theboundimmunoglobulinswere labeled with protein A-gold complexes, and the cells were fixed, embedded in resin,andthin sectioned. Figure1shows that theantibodies labeled the periphery ofthe virus parti-cles. Occasionally, gold particles were seen inside the virions, probablyowingto superimposition ofthe antibody-gold complexes on the surface of sliced virions present within the sections.
Todetectantigenicdeterminants inside the virusparticle, ASF virus-infected cells were fixed with 0.5% glutaralde-hyde and embedded under low-denaturation conditions to preserve as much aspossibletheantigenicpropertiesof the structural components of the virion. Thin-sectioned cells, which revealed the presence of ASF virus particles in different states ofmaturation,werelabeledwithseraagainst purified ASF virusparticles andtaggedwith proteinA-gold complexes. Aclearlabelingwas found with anASF
virus-1.
I f7fm-fil",,-,.j_,a
."i-t X
, t... ... .f..,..,
.
`'7f*F1
'..-,1.
-,
I ...:.on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.612.106.512.73.360.2]i.
Av
*.r* <..
b
FIG. 2. Postembeddingimmunolabeling of ASF virus particles with serum againstpurified virions. (a) ASF virus-infected Verocells,
collectedat24hafterinfection,werefixed,embedded inLowicryl,thinsectioned,labeled withanASF virus antiserum andprotein A-gold complexes,andcontrasted withuranylacetate.(b)Asacontrol, ASFvirus-infected cell sectionswerelabeled withapreimmuneserumand protein A-gold complexes. Bar, 200nm.
specificantiserum(Fig.2a) butnotwithapreimmuneserum
(Fig. 2b).
Labeling of structural proteins in ASF virus particles with
MAbs. The datadescribed above indicated thepossibilityof localizing the positions of the structural proteins of ASF virions byimmunolabeling with MAbs specific for different structural proteins.
(i) Proteins p14 and p24. ASF virus-infected cells were
incubatedwith several MAbsspecificfor different ASF virus proteins, tagged with protein A-gold complexes, fixed, thin sectioned, and processed for electron microscopy. Im-munolabeling ofthevirionwasonlyseenwith MAbs
specific
forproteins p14 andp24(Fig. 3). This indicated that these proteinswerepresentinthe virionperiphery. Inthecaseof theMAbspecific forprotein p24 (Fig. 3b), the labelwas seen
in many casesfollowing the side ofthe virion that was in
contactwith theouterpartof theviralfactory.This suggests
an accumulation of the proteinin thatinterface.
(ii) Proteins p72, p12, p17, and p37. Immunolabeling of thin sections of ASFvirus-infectedcells with MAb 19B.A2, specific for protein p72, a major component of the virion, labeledaregion neartheexternal partofthe virus
particles
(Fig. 4). Similar resultswereobtainedbyusingother MAbs specificfor otherprotein p72 epitopes (datanotshown). The
I
1-A .,. ..:
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.132.499.74.538.2]a
4
I.
it1
.... .A
...
....I
,- ..
I ...~..
/ o:.
w :.
r;iA
,
x.sts 1.:ws'2
A..-)
a.
.1. i,," t.. f...
.. ....'..4
a1
I.'
b
-.SO
-A, Abb
A
*
..so
..
'..;
A..9,
S
... _____ ....
%V
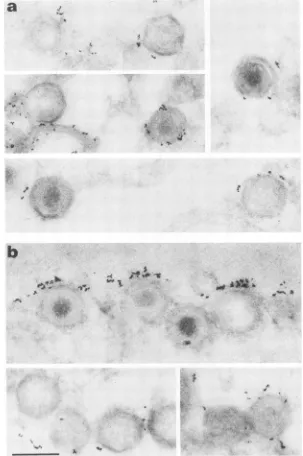
FIG. 3. Preembeddingimmunolabeling of ASF virusparticles witheitherMAb
specific
forprotein
p14
orMAbspecific
forp24.
ASF virus-infected Vero cellsweretreatedasdescribed in thelegendtoFig.2exceptthattheywerelabeled witheither MAbspecific
forprotein
p14(a)orMAbspecificforprotein p24(b). Bar,200nm.
"I
r
I
'04
-AW,
0# W%P%
a
0 .,
-10%
-:O.
*Sa '..
*..
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.148.451.159.615.2]I
c
d
1.B
-e
4... ...
1.
* .
FIG. 4. Postembeddingimmunolabeling of ASF virus particles with MAb19B.A2specific forprotein p72. ASF virus-infected Vero cells weretreated asdescribed in the legend to Fig. 2, except that they were labeled with MAb 19B.A2specific for protein p72. Bar, 200nm.
radialdistribution value of proteinp72,obtainedfrom 1,202 goldgranules counted in88virions, was0.80 + 0.18 (mean
value + standard deviation).
Labeling of this areaof thevirionwasalso obtained with MAbsspecific for other ASF structural proteins. The label-ing patterns obtained with the MAbs specific for proteins p12, p17, and p37 presented in the electron microscope a generalaspect
indistinguishable
from that shown for p72 in Fig. 4 (data not shown). The MAb 18B.B11, specific for proteinp12,
presenteda radial distribution value of0.82 + 0.17 (from 509 gold granules counted in 100 virions). For MAb17K.G12, specific
forproteinp17,
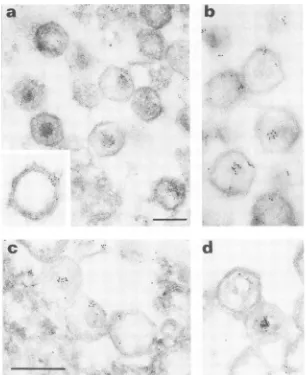
the radial distribu-tion value of the gold granules was 0.72 ± 0.21 (from 910 gold granules in 90 virions). A similar value was obtained from MAb 17F.E10, specific for protein p37 (0.71 ± 0.19 from1,300 gold granules in 140virions).(iii) Protein p150. Protein p150, one of the major virus structural proteins, is antigenically related to the virus-induced, nonstructural proteinp220 (17).Figure5shows that MAbs 17A.H2 and 17K.G6, whichrecognize both proteins p220 and
p150
(Fig. 5a and b)andonly proteinp150
(Fig. Sc andd),
respectively, labeled the nucleoid andone vertexof thevirion. From 187virions, 58contained gold granules in the nucleoid, 101 contained granules inone vertex, and 28 containedgranules in both.The MAb that recognized both proteins p220 and p150 labeledcell nucleusfrom infected (Fig. 6a and b) and from uninfected (datanotshown)cells. Incontrast, the MAb that recognized only protein
p150
did not label the nucleusfrom either cell(Fig. 6c).DISCUSSION
A study of ASF virus-infected cells by immunoelectron microscopy, using MAbs that bound protein A-gold
com-plexes, allowed us to localize seven structural proteins and todefinethree regions in the virions: the external region, the intermediatelayers, and thenucleoid.Both thenucleoidand theintermediate layers correspond to regions defined previ-ously, but it is uncertain whether the external region defined herecorresponds to the most externalvirionmembrane (4). Aserumagainst purified virions (Fig. 1) and MAbs specific for eitherprotein p14 or p24 (Fig. 3) labeled the external region in the virion in nonsectioned cells permeable to the antibodies. It islikelythatprotein p24 isahostproteinsince aMAbspecificforproteinp24binds touninfected and ASF virus-infected cells and immunoprecipitates a protein of relative molecularweight of 24,000from both kinds of cells (17). The location ofprotein p24 in the external region of ASFvirusparticlesisconsistent with thefindingthat a MAb specific for that protein reduces virus infectivity about 100-fold(L. Enjuanes, B. Garcia-Barreno, A. Sanz, M. L. Nogal, and E. Vinluela, unpublished data).
Thevirusantiserum labeled theexternalregion,the inter-mediatelayers,andthe nucleoidinthin-sectioned cells(Fig. 2). The labelingpattern of the externalregion showed gold granules closely following the external virus shape at dis-tances ranging from direct contact to 13 nm apart or even more. The labeling of the most external region was less intense in virions labeled after the infected cells were thin sectioned than in those labeled before thin sectioning. This could be due to apartial denaturation ofthe outerepitopes duringthe process offixation andembedding.
Theintermediate layers of ASF virusparticlescontained proteins p12, p17, p37, and p72. Antibodies against these proteinslabeled theareascorrespondingto the viruscapsid and the membrane layers situated below (4). The values obtained forthe radialdistribution of theseproteins ranged from 0.70 to 0.83with standard deviations around 20 to 30%
.7
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.106.512.77.332.2]a
c
b
d
FIG. 5. Postembedding immunolabeling of ASF virus particles with either MAb 17A.H2, specific for proteins p220 andp150, or MAb 17K.G6, specific for protein p150. ASF virus-infected Vero cells were treated as described in the legend to Fig. 2, except that they were labeled with either MAb 17A.H2 (a and b) or MAb 17K.G6 (c and d). The insert in panel a shows a detail of the labeling of avirion corner withMAb 17A.H2. Bar, 200 nm.
of thosevalues.Taking intoaccountthe actualdimensionsof ASFvirus particles(4), thevaluesofthestandarddeviations ranged between16and 20 nm. These values were similarto the average value for the probe (about 18 nm), which consisted of an immunoglobulin G molecule tagged with proteinAandgold granules withasize of around5nm(14). Thismeant that the deviationoftheexperimental datawas
due mainly to the size of the probe, indicating that the resolution wasaround 20 nm or less.
The inner nucleoid and one vertex of the virion were
labeled by two MAbs, 17A.H2 and 17K.G6; MAb 17A.H2 immunoprecipitatesthe nonstructural, virus-inducedprotein p220 and the structural protein p150 (17) and labeled the nucleus from both uninfected and virus-infected cells (Fig. 6). This result indicated the existence ofa structural rela-tionship betweenprotein
p150
anda nuclear component in the host-cell nucleus. Thatisnotsurprisingsince ASFvirus, like thepoxviruses, containsin thevirionmost,ifnotall, of the enzymesrequired forthesynthesis ofmRNA(11, 12, 15, 16). The second MAb (17K.G6), which recognizes onlyprotein p150, didnotlabel the host-cellnucleusfromeither uninfected or virus-infected cells. This indicated that the virus and the cell component recognized by MAb 17A.H2 were different.
The labeling oftwo different regions (nucleoid and one
vertex) with two MAbs specific for p150 raises a question about thelocationof this protein. Eitherp150 is located at
both sites or there is a common epitope in two different structural proteins located in those positions. The first hypothesisis consistent with the results obtained by treat-ment of ASF virus particles with nonionic detergents, in which
p150
seemstobe released undertwodifferent condi-tions,suggestingtwodifferentlocations for thispolypeptide
(A. L. Carrascosa, J. F. Santaren, and E.
Vinluela,
unpub-lisheddata). The secondpossibility has notbeensupported byimmunoprecipitation experiments, since MAbs thatrec-ognize
bothproteinsp150
andp220onlyprecipitated
protein
p150
fromdissociated virus (17).Figure 7 summarizes the localization in ASF virus
parti-clesof the proteinsdetectedbyimmunolabelingwith MAbs
-, il j.
.1
,roo
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.155.461.73.448.2]a
--
<;
c
* * '. '_
w} ~~.: ...:
4*
14
.4w- - *
FIG.6*
Postembeddig ig
oftFIG
6S Porstinembeddn
immuolabeling
ofrtheatdanuceuscrofeAFig
viruinexctedhaVhyeroellsbyeMAb wi7A eih2rMb172
specificforprtis20an
proteinsp220andp150 (aandb), orMAb17K.G6, specific forprotein p150 (c). Bar, 200nm.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.86.548.161.603.2]p150
'p14
p24
FIG. 7. Schematic representation of the structuralprotein distri-bution inASF virusparticlesasshownby immunolabelingof virions with MAbs. The virus model is a hexagonal section of the
icosahedron andshows the inner nucleoidandtheenvelopes. The localization ofthe proteins is basedonthe radialdistribution values
obtainedby immunolabelingof the virions with MAbs. The barsare
centered on the mean values for each protein, and their length includes the value ofthe 95% confidence interval values of the
mean.
andprotein A-gold complexes. The relative position of these proteins has been confirmed by immunolabeling with pairs of
MAbs tagged with gold granules of different size (data not shown).
ACKNOWLEDGMENTS
We are grateful to Martin Caballero for the computer data processing.
Thisinvestigationwasaided bygrantsfrom theComisi6nAsesora
para el Desarrollo de la Investigaci6n Cientffica y Tecnica to Ingenasa and Fondo de Investigaciones Sanitarias.
LITERATURE CITED
1. Breese, S. S., and C. J. DeBoer. 1966. Electron microscope observations ofAfrican swinefever virus in tissue culture cells. Virology 28:420-428.
2. Burrage,T.G., G.M.Tignor,and A. L.Smith. 1983. Immuno-electron microscope localization of rabies virus antigen in central nervous system and peripheral tissue using
low-temperatureembeddingandprotein A-gold. J. Virol. Methods 7:337-350.
3. Carlemalm, E., R. M. Garavito,and W. Villiger. 1981. Resin
developmentfor electronmicroscopyandananalysisof embed-dingatlow temperature.J. Microsc. 126:123-143.
4. Carrascosa, J. L., J.M.Carazo,A. L.Carrascosa, N. Garcia, A. Santisteban, and E. Vinuela. 1984. General morphology and capsid fine structure of African swine fever virus particles. Virology 132:160-172.
5. Carrascosa, A. L., M. del Val,J. F. Santaren, and E.Vinuela. 1985.Purification andproperties of African swine fever virus. J. Virol. 54:337-344.
6. DeBoer,C.J. 1967.Studiestodetermineneutralizing antibody in serafrom animals recovered from African swine fever and laboratory animals inoculated with African virus with adju-vants. Arch. Gesamte Virus forsch. 20:164-179.
7. Driedonks, R. A.,andJ. Caldentey. 1983.Gene 20product of bacteriophageT4.II.Itsstructuralorganization in prehead and bacteriophage. J. Mol. Biol. 166:341-360.
8. Enjuanes,L.,A.L.Carrascosa,M. A.Moreno, andE.Vinuela. 1976. Titration of African swine fever (ASF) virus. J. Gen. Virol. 32:471-477.
9. Hess,W. R. 1981. African swine fever: a reassessment. Adv. Vet.Sci. Comp. Med. 25:39-69.
10. Horisberger, M. 1979. Evaluation of colloidal gold as a cytochemical marker for transmission and scanning electron microscopy. Biol. Cell. 36:253-258.
11. Kuznar, J.,M. L.Salas,and E.Vinuela. 1980.DNA-dependent RNA polymerase in African swine fever virus. Virology 101:169-175.
12. Kuznar, J., M. L. Salas, and E. Vihuela. 1981. Nucleoside triphosphate phosphohydrolase activities in African swine fever virus. Arch. Virol. 96:307-320.
13. Mizuhira, V., andY.Futaesaki. 1972. New fixation for biologi-calmembranesusing tannic acids. Acta Histochem. Cytochem. 5:233-235.
14. Roth, J.1982.Theprotein A-gold technique-a qualitative and quantitative approach for antigen localization on thinsections, p.107-133. InG.R.Bullock and P. Petrusz (ed.),Techniquesin immunocytochemistry, vol. 1. Academic Press, Inc., New York.
15. Salas,M.L., J. Kuznar, andE.Vihruela.1981.Polyadenylation, methylation and capping of the RNA synthesized in vitro by Africanswine fever virus. Virology 113:484-491.
16. Salas, M. L., J. Kuznar, and E. Vinuela. 1983. Effect of rifamycin derivatives and coumermycin Al on in vitro RNA synthesis by African swine fever virus. Arch. Virol. 77:77-80. 17. Sanz, A.,B. GarciaBarreno,M. L.Nogal,E. Vifiuela, and L.
Enjuanes. 1985. Monoclonal antibodies specific for African swine fever virusproteins. J. Virol. 54:199-206.
18. Tsukita, S., S. Tsukita, and M. Ishikawa. 1980. Cytoskeletal network underlying the human erythrocyte membrane. Thin section electronmicroscopy. J.CellBiol. 85:567-576. 19. Vihuela,E. 1985. African swinefevervirus. Curr. Top.
Micro-biol. Immunol. 116:151-170.
20. Wardley,R. C.,C. de M.Andrade, D. N.Black, F. L. Castro Portugal, L. Enjuanes, W. R. Hess, C. Mebus, A. Ordis, D. Rutili, J.Sanchez Vizcaino, J. C.Vigario,P.J.Wilkinson, J.F. MouraNunes,andG. Thomson.1983.African swine fever virus. Arch.Virol. 76:73-90.
21. Weiland,F.1981.Ultrastructuralvisualization ofvirus-antibody binding by means of colloidal gold-protein A. Ann. Virol. 132E:549-556.