0022-538X/86/050671-05$02.00/0
Copyright © 1986,American SocietyforMicrobiology
Epstein-Barr Virus-Specific DNA Polymerase
in
Virus-Nonproducer Raji Cells
T. OOKA,l* G. M. LENOIR,2 G.
DECAUSSIN,'
G. W. BORNKAMM,3 AND J.DAILLIE'
Laboratoired'Epidemiologie et Immunovirologie des tumeurs, Centre National de la Recherche Scientifique UM 380030,
Faculte de Medecine Alexis Carrel, 69372 Lyon Cedex
08,1
CentreInternational de Recherche sur le Cancer, 69008 Lyon,2 France; and Institut fur Virologie, Zentrum fur Hygiene, 7800 Freiburg, West Germany3Received11 September 1985/Accepted31December1985
Virus-nonproducer Raji cells, when induced to early antigen synthesis by 12-O-tetradecanoyl-phorbol-13-acetate and sodium butyrate, showed an increase in DNA polymerase activity. This enzyme has the characteristics ofatypical Epstein-Barrvirus DNApolymerase withregardtochromatographicalpatternand biological properties: it is eluted fromDEAE-cellulose at 0.08 M NaCl, has ahighsaltresistance, issensitive tophosphonoacetic acid andphosphonoformate, and showsasubstratepreferencefor
poly(dC)-oligo(dGj2.18).
Theresistanceof Epstein-Barr virus polymerase activity to aphidicolinis aproperty distinctfrom that of HSV DNA polymerase. Viral DNA polymerase activity increases in the absence of Epstein-Barr virus DNA replication,indicating that this enzyme is an early viral protein.Epstein-Barr virus (EBV), like other herpes viruses, is knowntoinducea numberofvirus-specified enzymes such as DNA polymerase (6, 10, 23), DNase (4, 5, 22), and
thymidine kinase (3, 17, 20, 21, 26). Theinduction ofDNA
polymerase and DNase was reported in Raji cells
superinfected byEBV (4, 8) and EBV producer P3HR-1 or B95-8 cells treated with chemical inducers such as 12-0-tetradecanoyl-phorbol-13-acetate (TPA) (6, 10, 22) or
iododeoxyuridine (18, 23). In all cases, the induction of
enzyme activity occurs during viral replication.
EBV-nonproducer Raji cells containing multiple copies of the EBV genomearenotabletoproduceviralparticles(12, 29),
andonly earlyeventsofthelytic viralcycle(14; G. Lenoir,
T. Ooka, M. Tovey, M. de Turenne, and J. Daillie, Abstr. 4th Cold Spring Harbor Meeting on Herpesviruses, Cold Spring Harbor,N.Y., p.178,1979) could beinducedin these
cellsby chemical inducers (1, 15). Therefore, this celllineis
agood toolto studytheearly events oftheviral cycle. We
recently describedthe presenceof EBV-specificDNase(22)
andthymidine kinase(6a, 20, 21)activitiesinactivatedRaji cells. These enzymes are present also in EBV producer
P3HR-1 cells simultaneously treated with TPA, sodium butyrate (SB)andarabinofuranosylthymine(Ara-T) inwhich only early antigens (EA) are expressed. This indicates that these enzymesbelongtotheearly viralproteins. We do not know whether EBV-DNA polymerase is one ofthe early
polypeptides. Some authors (1, 27) recently reported that
induced or uninduced Raji cells do not contain any DNA
polymerase activity and concluded that the lack of this enzyme is the reason for the incapacity of Raji cells to
produce virus. Because we expected a close link between
thymidinekinase, DNase, and DNA polymerase,wewanted to reexamine this question. In this report, we demonstrate the presenceofatypicalEBV DNA polymeraseactivity in induced Raji cells.
The Burkitt's lymphoma-derived cell lines Raji with its TK+ (25) and TK- (11) variants and P3HR-1 TK- variants were grown in RPMI 1640 medium containing 10%
heat-*Correspondingauthor.
inactivated fetal calf serum supplemented with 100 U of
penicillinand 250 ,ug of streptomycin per ml.
Cells were induced with TPA and SB as previously described(20). Briefly,all cells were grown toadensity ofat least 2 x 106cells per ml anddilutedto afinalconcentration of5 x 105 cells per ml. TPA and SB were added at final
concentrations of20ng/ml and 2mM, respectively.
Atfirst,we studiedthe effectofsaltonDNA polymerase
activity of the total cell extractspreparedfrom induced and uninduced Raji cells. The enzyme activity extracted from
induced Raji cells was stimulated by 50 to 100 mM (NH4)2SO4by about180to200%;80 and 20% oftheactivity remainedwhen thesalt concentrations were increased to 150 and 200 mM,respectively. In contrast,the DNApolymerase activity from uninduced cellswasprogressively inhibited by increasing the saltconcentration andwascompletely inhib-ited at 150 mM (data not shown). The characteristics of stimulation and inhibition ofthe enzyme activity by salt is
similar to EBV DNA polymerase activity extracted from
EBVproducercells (6, 10, 18, 23).
The DNApolymerase ofRaji cells was thenanalyzed by DEAE-cellulose column chromatography (Fig. 1). Extracts fromRaji cells treated with TPA-SB(whichexpressed 41% ofEA-positive cells) and fromuntreated control cells were
applied to a column and eluted with a linear salt gradient
from 0to0.3 MNaCl. Under theseconditions,thecellular 3
polymerasewasnot retainedonthis column andwaseluted
during washing.The DNApolymerase activityelutedat0.12 MNaClrepresentedthe cellular a DNApolymeraseandwas
foundin both uninduced andinducedRaji cells. Inextracts
from induced cells a second DNA polymerase activity was observed whichwaselutedfrom the columnat0.08MNaCl. Extracts from untreated control cells contained only one
peakof cellularaDNApolymerase.Wheneachfractionwas tested in the presenceof 150 mM(NH4)2SO4 only apeakat 0.08 M NaCl was found in extracts ofinduced cells with a reduced activity, whereas DNA polymerase a activity was
completely inhibitedby this saltconcentration. We charac-terizedfurther the DNA polymerase eluted at 0.08M NaCl toconfirmthatitwastheviral DNApolymerase (Table1).(i)
671
on November 10, 2019 by guest
http://jvi.asm.org/
672 NOTES
FIG. 1. TPA-SB-treated Raji cells were centrifugedat600 x g
for 6 min and washed twice in phosphate-buffered saline. After suspensionataconcentration of2 x108 cells per ml in TKMD buffer (50 mM Trishydrochloride [pH 7.5], 10 mMKCl, 1 mM MgCl2, 1 mMdithiothreitol containing 20% glycerol), the cells were disrupted by sonication for four30-speriodsatlevel4settingwitha Branson sonicator. The suspension of disrupted cells was centrifuged at 105,000 x g for 60 min. After centrifugation the supernatant fluid was applied directly onto a column ofDEAE-cellulose (Whatman Ltd., Kent, United Kingdom) in TKMD buffer containing 20% glycerol.Thecolumnwaseluted with an NaCl lineargradient from 0 to 0.3 M.Samples (20jl)of eachcolumn fraction wereassayed for DNApolymeraseactivity. Assays for cellular a and , polymerase X containedthe following: 50 mM Trishydrochloride buffer (pH 7.0 fora polymerase and pH 8.5 forPpolymerase),4mMMgCl2;0.5 * mMdithiothreitol;34p.Mactivated calf thymus DNA (predigested with pancreatic DNase I until20% was solubilized); 50 ,uM each dCTP, dTTP, dATP, and dGTP containing 1 jj.Ci of [3H]dTTP (specific activity, 20 Ci/mmol; Radiochemical Centre, Amersham, United Kingdom); and 50 ,ug ofbovine serum albumin in a final volume of0.125 ml. After1h of incubation at 37°C, the reaction was placed onice,and theincorporation of acid-insolubleradioactivity was determined as described previously (23). Assays for EBV
polymerase contained the following: 100 mM Tris hydrochloride
(pH 8.5);4mMMgCl2; 150 mM(NH4)2SO4; 0.5 mM dithiothreitol; 34 ,uM activated calfthymus DNA; 50 F.M each dCTP, dTTP, dATP, anddGTP; 1 ,uCi of[3H]dTTP, and50p.gofbovineserum albumin. Theenzymeactivitywasmeasuredby a and1Bpolymerase assays(0)and EBVpolymerase assays(0).Abbreviations: control, uninduced Raji cells; V, EBV-specific DNA polymerase; TPA-SB, inducedRaji cells; a,a DNApolymerase.
Thisactivitywasstimulatedby 50to100mM
(NH4)2SO4
and was resistant to 150 mM. (ii) The enzyme efficiently usedpoly(dC)-oligo(dG12.18) and poly(dA)-oligo(dT12.18) as tem-plates, 20-fold and 5-fold more efficiently than activated
DNA, whereas DNA polymerase a was stimulated only
2.5-fold and 0.5-fold by these templates, respectively. (iii) The sulfhydryl inhibitor N-ethylmaleimide (0.5 mM) had little effectupon polymerase but inhibited the viral poly-merase by 85%. (iv) The enzyme was sensitive to phos-phonoacetic acid and phosphonoformate. The 50% inhibi-tory doses ofphosphonoacetic acid and phosphonoformate
were 0.8 and 0.4 ,ug/ml for the enzyme eluting at 0.08 M NaCl, but20 and 10 p.g/ml for polymerase atand 150 and 180
,ug/ml for polymerase 1, respectively. (v) The enzyme is
inhibited by Ara-TTP; 50% inhibition wasobserved at ap-proximately 10 mMforthe viral polymerase and at50 and 250 ,uMforpolymerase a and 13, respectively.
Aphidicolin is known as a specific inhibitor of cellular DNApolymeraseaactivity, whereas polymerase is
insen-sitivetothisdrug. This drugis alsoan efficient inhibitor of HSV DNA polymerase. To further characterize the viral activity, the effect ofaphidicolin was studied on a, 1, and
viral DNA polymerase activities. By comparison with a
polymerase, the viral activity was not sensitive to aphidicolinasthe polymerase (Fig. 2A).Thiswasthecase
for both P3HR-1 andRajiextractedviralenzymes.The50% inhibitory dose of aphidicolinwas8to10 ,uM for polymerase
[image:2.612.66.303.73.359.2](xandmore than 100 ,uMforpolymerase and theenzyme
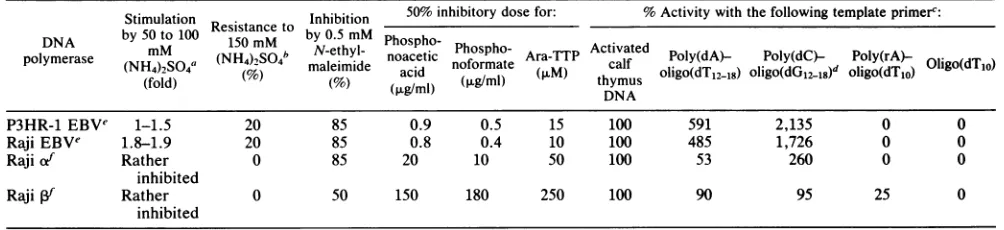
TABLE 1. Analysis of DNApolymeraseactivities
Stimulation Resit t Inhibition 50%inhibitory dose for: %Activity with the following templateprimerc:
DA by50to 100
150stnc
mM by0.5 mm PopopoAymerase
polymrAsm(NH4)2SO4a
mM
b N-ethyl-nhoacetic
Phospho-AaTPActivatedPoyd-
Poyd) Plyr-Oig(T) (NH4)2S 4 maleimide noacetic noformate Ara calfil(dA)
oly(dC)- P )(fold) (%)
ac.imd
(pLg/mI)(L)
thymusoligo(dT12..18)
oligo(dG12..18)d oligo(dT1O)
(~Lg/ml) DNA
P3HR-1 EBVe 1-1.5 20 85 0.9 0.5 15 100 591 2,135 0 0
RajiEBVe 1.8-1.9 20 85 0.8 0.4 10 100 485 1,726 0 0
Rajiiof Rather 0 85 20 10 50 100 53 260 0 0
inhibited
Raji
Vf
Rather 0 50 150 180 250 100 90 95 25 0inhibited
aIncreasedactivity comparedwith that without salt.
bResidualactivity.
c[3H]dTTPwasusedas alabeled substrate foramixture ofnucleosidetriphosphates.Theactivitywith activated calfthymusDNAwastakenas100%.
d[3H]dGTPwasusedinsteadof[3H]dTTPasthelabeledsubstrate.
eViral DNApolymeraseactivitiesofphosphocellulosecolumn fractions(23)from P3HR-1andRajicellswerepooledanddesignatedP3HR-1andRajiEBV DNApolymerase, respectively.
fBothRajicellular a andPDNApolymeraseswerepreparedfromphosphocellulosecolumnchromatography.
01
0
a
I-0
0
I-z
IL
20
FRACTION
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.612.62.564.553.668.2]
I0-
I-44
0
*4.
0
2
g
0
a
0 24 48 72
HOURS
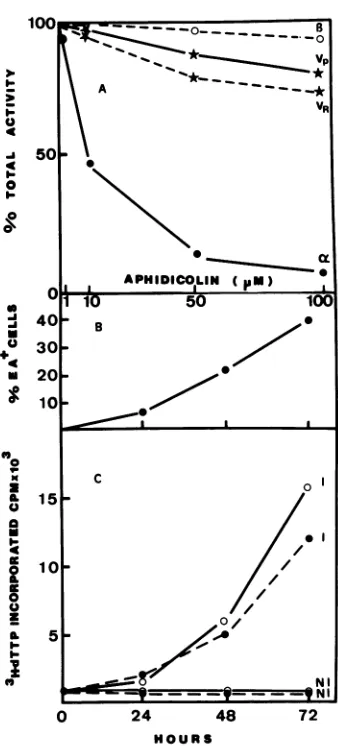
FIG. 2. (A) Effect ofaphidicolin ontheactivity of cellular and EBV-induced DNApolymerase activity. The DEAE-cellulosepeak ofEBVDNApolymerase activitywasassayedforDNApolymerase activity. The a and ,B polymerase activities were prepared from
phosphocellulose column elution. The DNA polymerase activity
was measured using activated calf thymus DNA as a
template-primer. The results are expressed as relative activity, with the activity obtained inthe absence ofaphidicolinas 100%. Symbols: (@-S)apolymerase,(0-0) , polymerase, (*-*)P3HR-1 EBV
polymerase, (*- -*) Raji EBV polymerase. (B) Kinetics of EA positive cells (B) and (C) EBV-induced DNA polymerase activity in TPA-SB-treatedRaji cells. At the indicated times after induction of TPA-SB at zero time, cells were harvested for assaying either
EBV-specific EA by indirect immunofluorescenceorDNA polymer-aseactivity in thepresenceof 100FMaphidicolin (0-0)or100F.M
aphidicolin plus 100 mM (NH4)2SO4 (@- -0). Abbreviations: I,
inducedcellextract; NI, noninduced cellextract.
eluting at 0.08 M NaCl. At 100 ,uM aphidicolin, the a
polymerase activitywas almostcompletely inhibited. The substrate specificity on poly(dC)-oligo(dG12.18) and
poly(dA)-oligo(dT12.18)templates and the resistanceto high saltandaphidicolin thus allowedustodistinguish clearly the viralpolymerase from DNA polymerase a and,B. Inregard
to the high salt resistance and template specificity of the inducedactivity, EBVDNApolymerasewassimilartoHSV DNA polymerase. The 50% inhibitory doses ofaphidicolin
were 0.5 p.M for HSV-DNA polymerase and 0.22 p.M for DNA polymerase a (16). In contrast, the EBV polymerase
TABLE 2. Induction of EBV-specific DNA polymerase activity and thenumberofEBVgenomeequivalentspercell in human
lymphoblastoid cellsupon treatmentwithTPAandSB EBVDNA
% of cells polymerase NofEB
Cell line
Cnductinine_Chemical_
Chemicalinduction"incrpratd!equivalent
positiveforb: activityc (pmolof dTTP nomegenomeincorporated/ per celld EA VCA mgof protein
perh)
Raji TK+ Control 0.1 Negative 0 60
TPA-SB 19.5 Negative 902 60
RajiTK- Control 0.1 Negative 2 39
TPA-SB 17 Negative 570 40
P3HR-1 TK- Control 0.1 0.1 0 63
TPA-SB 25 9 1,255 1,000
aControl, Noninduced cells;TPA-SB, TPA-SB-treated cells.
IThe percentageof EA-orviralcapsid antigen (VCA)-positive cellswas determinedby indirect immunofluorescence.
cDNA polymerase activity wasdetermined in the presence of150 mM
(NH4)2SO4.
dTheamount of viral DNA percell was quantitated by measuring the
renaturation kinetics ofa3H-labeled EBV DNA in thepresenceof cellular DNA aspreviouslydescribed(14).
fromRaji cells was much more resistantto
aphidicolin
and lostonly 30% of its activityat100 ,uM. Asimilar resistancewas found in EBV-specific DNA polymerase from
virus-producer P3HR-1 cells. This indicates that identical
poly-merasesareinducedinbothEBVproducer andnonproducer cells. Thedifferencein the sensitivity ofthe DNA
polymer-ase ofEBVand HSV was also observed by Allaudeen and
Rani (1).
When the extracted enzyme from TPA-SB-treated Raji cells was examined in the presence of 100 p.M
aphidicolin
aloneorin thepresence of100 ,uMaphidicolin
plus 150 mM(NH4)2SO4 at different times after chemical induction
(Fig.
2C),
anincreasein thepolymerase activity wasobservedinboth assays with a peak at 72 h after induction. The
increasingcurveofenzymeactivity correlated wellwith the
increase ofEA-positivecellsafterinduction(Fig. 2B). Since
100,uMaphidicolinand 150 mM(NH4)2SO4 inhibitedalmost
completely the enzyme
activity
of cellular DNApolymer-ases a and
3,
theobservedactivity resistant tobothinhibi-tors isa specific virus-inducedDNApolymerase.
Arapid estimation ofthe EBV-inducedDNApolymerase
is thuseasily possibleinanassayin which both inhibitorsare present. The appearance of this enzyme activity is an
indicator ofthe activation ofthe viral genome like DNase
(22) andthymidine kinase(6a, 20, 21).
EBV-nonproducer Raji cells are known to be unable to
synthesize viral capside antigen after chemical induction. Only EA was shown to be induced by the chemical treat-ment. A viral polymerase induced in EA-positive cells has thus tobeconsidered as anearly viral protein.
The treatment ofthese cellswith TPA and SB led to EA
synthesis and the appearance of viral polymerase activity,
whereas viral capsid antigen synthesis did not occur (Table 2). In the uninduced control cells, no viral polymerase
activity was detected in the absence of EA synthesis. The number of EBV genome copies per cell was measured by DNA-DNA reassociation kinetics. In both Raji cells (TK+ andTK- variants), noincrease in the numberofEBVviral
copies was observed. Under similar conditions, the EBV-producer P3HR-1 TK- cells showed an increase in EBV genomesfrom 63 toabout 1,000copies concomitantly with
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.103.272.65.438.2]674 NOTES
the rise in viral capsid antigen-positive cells. The synthesis of viral DNA polymerase can thus take place in the absence of viral DNA synthesis and is not necessarily linked to viral DNA replication.
Similar results have been obtained with EBV-producer P3HR-1 cells treated simultaneously with TPA, SB, and Ara-T in which the viral DNA synthesis is specifically inhibited by Ara-T and the synthesis of EBV antigens is limited only to EA (20, 21).
Raji cells are unable to produce virus particles unless they are superinfected with P3HR-1 virus. Superinfection by P3HR-1 virus leads to the production of transforming EBV particles by a recombination between the viralgenomes of Raji and P3HR-1 cells (9, 28). Recent work demonstrated deletions in the viral genome of Raji cells (2, 24) anda lack of some viral polypeptides with high molecular weight (15, 27; F. Angel, G. Decaussin, J. Daillie,and T. Ooka, submit-ted for publication). Someinvestigations did not succeed in detecting EBV polymerase in Raji cells (1, 27) and concluded that the incapacity to produce virus isdue toa lack in viral DNA polymerase. However, our results demonstrate the presence of an EBV-like DNA polymerase activity in Raji cells expressing 40% of EAs. It is possible that the authors previously failed to identify viral activity because oflow EA induction by TPA. In fact, the induction of nonproducer cells by TPA alone gives very low EA induction, generally about 0.5 to 10%(19). We alsofailed to identify viral DNA polymerase unequivocally when only afewpercent of cells were induced toEA synthesis. In this regard, our induction system based on a combined action ofTPA andSB isa good tool to study induced viral proteins (7; Angelet al., submit-ted for publication).
The detection of the viral polymerase in Raji cells indi-cates that the incapacity of the cells to produce virus is not due to a lack of viral DNA polymerase. The sequence
homology with herpes simplex virus DNA polymerase (2) suggests apossible localization of the EBV DNA polymer-asegene onthe left-hand side of B95-8 BamHI fragment A. This region is indeed notdefective in Raji viralgenome(24). The region deleted in the Raji virus genome could code the early genes necessary for the functional activity of viral DNA polymerase in vivo.
We thank A.H. Todd (Imperial ChemicalIndustries, Pharmaceu-tical Division, England), B. Oberg (Astra Lakemedel A.B., Swe-den), and G. A. Gentry (The University of Mississippi Medical Center)for the gift ofaphidicolin,trisodiumphosphonoformate, and Ara-TTP,respectively. We thank C. Molacek Weiler for secretarial work.
LITERATURE CITED
1. Allaudeen, H. S.,and G. Rani. 1982. Cellular and Epstein-Barr virus-specific DNA polymerase in virus-producing Burkitt's lymphomacelllines. Nucleic Acids Res. 10:2453-2465. 2. Baer, B., A. Banker, M. Biggin, P. Deininger, P. Farrell, T.
Gibson, G. Hatfull, G. Hudson, S. Satchwell, C. Seguin, P. Tuffnell,and B. Barrell. 1984. DNA sequence andexpressionof the B95-8 Epstein-Barr virus genome. Nature (London) 310:207-211.
3. Chen, S. T., J. E. Estes, E. S. Huang, and J. S. Pagano. 1978. Epstein-Barr virus-associated thymidine kinase. J. Virol. 26:203-208.
4. Cheng,Y.K., J. Y. Chang, P. J.Hoffmann,and R. Glaser. 1980. Studies on the activity of DNaseassociated withthereplication ofthe Epstein-Barr virus. Virology 100:334-338.
5. Clough, W. 1979. Deoxyribonuclease activity found in Ep-stein-Barr virus producing lymphoblastoid cells. Biochemistry
18:4517-4521.
6. Datta,A. K., R. J. Feighny, and J. S. Pagano. 1980. Induction of Epstein-Barr virus associated DNA polymerase by 12-0-tetradecanoyl-phorbol-13-acetate. Purification and characteriza-tion. J. Biol. Chem.255:5120-5125.
6a.DeTurenne-Tessier,M., T.Ooka,G. deThe,andJ.Daille.1986. Characterization of an Epstein-Barr virus-induced thymidine kinase. J.Virol. 57:1105-1112.
7. Epstein, A. 1984. Immunobiochemical characterization with monoclonal antibodies of Epstein-Barr virus-associated early antigensinchemically induced cells. J. Virol. 50:372-379. 8. Feighny, R. J., I. B. E.Henry, A. K. Datta, andJ.S.Pagano.
1980. Induction ofDNApolymerase activity after superinfec-tion of Raji cells with Epstein-Barr virus. Virology 107:415-425.
9. Fresen, K. O., M. S. Cho, L. Gissmann, and H. ZurHausen. 1980. Recombination between Epstein-Barr virus genomes. Viruses in naturally occuring cancers. Cold Spring Harbor Conf.Cell Proliferation 7:35-44.
10. Goodman, S. R., C. Prezyna, and W. Clough. 1978. Two Epstein-Barr virus-associated DNA polymerase activities. J. Biol. Chem. 254:8617-8628.
11. Hampar, B., J. G. Derge, L. M.Martos,andJ. L. Walker.1972. Synthesis of Epstein-Barr virus after activation of the viral genomeina"virusnegative"humanlymphoblastoid cell(Raji) maderesistantto5'-bromodeoxyuridine. Proc.Natl. Acad.Sci. USA 69:78-82.
12. Hampar, B.,J.G.Derge, M.Nonoyama,S. Y.Chang,and S. D. Showalter. 1974. Programming ofeventsin Epstein-Barr virus-activated cells induced by 5'-iododeoxyuridine. Virology 62:71-89.
13. Henle, G., and W. Henle. 1966. Immunofluorescence in cells derived from Burkitt's lymphoma.J. Bacteriol. 91:1248-1256. 14. Hudewentz, J., G. M. Bornkamm, and H. Zur Hausen. 1980.
Effectofthediterpeneester TPAonEpstein-Barr virusantigen and DNA synthesis in producer and nonproducer cell line. Virology 100:175-178.
15. Kallin, B., and G. Klein. 1983. Epstein-Barr virus carried by Rajicells: a mutantinearly functions? Intervirology 18:47-51. 16. Kroban, H., P.Schaffer,and M.L. Depamphilis.1979. Involve-ment of eucaryotic deoxyribonucleic acid polymerase in the replication of cellularandviraldeoxyribonucleic acid. Biochem-istry 18:4431-4443.
17. McGabhann, P., K. Sugawara, and Y. Ito. 1984. Characteriza-tion ofEpstein-Barr virus-related thymidine kinase induced in nonproducer cells by superinfection or chemical treatment.
Intervirology21:104-109.
18. Miller, R. L., R. Glaser, and F. Rapp. 1977. Studies of an
Epstein-Barr virus-induced DNA polymerase. Virology 76: 494-502.
19. Ooka, T. 1983. EBV DNA polymerase (areview). In Koikeet al. (ed.), DNA synthesis in eucaryotes, vol. 28, p. 271-281. Kyoritsu Shuppan,Tokyo.
20. Ooka, T., and A. Calender. 1980. Effects of arabinofu-ranosylthymine on Epstein-Barr virus replication. Virology
104:218-223.
21. Ooka, T., A. Calender, M. de Turenne, and J. Daillie. 1983. Effects of arabinofuranosylthymine on the replication of
Ep-stein-Barrvirus andrelationshipwitha new inducedthymidine
kinaseactivity. J.Virol. 46:187-195.
22. Ooka, T., M. de Turenne, G. de The, and J. Daillie. 1984. Epstein-Barrvirus-specificDNase activity innonproducerRaji
cells aftertreatmentwith12-0-tetradecanoylphorbol-13-acetate
and sodiumbutyrate. J. Virol. 49:626-628.
23. Ooka,T., G.Lenoir, andJ.Daillie. 1979.Characterization ofan
Epstein-Barr virus-induced DNA polymerase. J. Virol. 46: 187-195.
24. Polack, A., H. Delius, U.Zimber,andG. W. Bornkamm. 1984. Twodeletionsin theEpstein-Barrvirusgenomeof theBurkitt's lymphomanonproducerline Raji. Virology 133:146-157. 25. Pulvertaft, R.J.V. 1965. AstudyofmalignanttumorsinNigeria
by short-term tissue culture. J. Clin. Pathol. 18:261-273. 26. Roubal, J., and G. Klein. 1981. Synthesis ofthymidine kinase
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
(TK)inEpstein-Barrvirus-superinfected RajiTKnegative cells. 28. Yajima, Y.,B.Marcynska,and M.Nonoyama. 1978. Transform-Intervirology 15:43-48. ing activity of Epstein-Barr virus obtained by superinfection of 27. Sugawara, K., M. Kawanishi, and Y. Ito. 1981. Epstein-Barr Raji cells.Proc. Natl. Acad.Sci. USA 75:2008-2010.
virus-induced early polypeptides in Raji and NC 37 cells acti- 29. ZurHausen, H., and H. Schulte-Holtausen. 1970. Presence of vated by diterpene ester TPA in combination withn-butyrate. Epstein-Barr virus nucleic acid homology ina"virus-free" line Virology 115:406-408. of Burkitttumorcells. Nature(London) 227:245-248.