JOURNAL July 43,No. 0022-538X/82/070332-05$02.00/0
Inhibitory
Effect of
E-5-(2-Bromovinyl)-l-Ip-D-Arabinofuranosyluracil
on
Herpes Simplex
Virus
Replication
and
DNA
Synthesis
J. DESCAMPS,1 R. K.SEHGAL,1 E. DECLERCQ,2ANDH. S.ALLAUDEEN1*
DepartmentofPharmacology, YaleUniversitySchool of Medicine, New Haven, Connecticut06S10,1and RegaInstitute,Katholieke Universiteit Leuven, B-3000 Leuven,
Belgium2
Received23October1981/Accepted2March1982
The effect of E-5-(2-bromovinyl)-1-13-D-arabinofuranosyluracil (BVaraU) on
herpessimplex virus (HSV) replicationwasexamined and compared with thatof
E-5-(2-bromovinyl)-2'-deoxyuridine
(BVdUrd). The50%
inhibitory dose againstHSVtype1 (HSV-1)was0.1 ,ug/mlcomparedwith 0.008,ug/mlfor BVdUrd; the
antimetabolic 50% inhibitory dose of BVaraU ranged from 20 to 95 ,ug/ml. The
addition of 50 gxg of BVaraUperml toHSV-1-infected Vero cells decreasedthe
synthesis of viral and cellular DNA by 37 and 28%, respectively. The
5'-triphosphate(BVaraUTP) competed withdTTPin DNAsynthesis by the
herpes-viral and cellular DNA polymerases; the apparent K, values of HSV-1 DNA
polymerase, DNApolymerase a,and DNApolymerase 1Bwere0.14, 0.32, and5
,uM, respectively. Thus, BVaraUwas aless effective
antiherpesvirus
agentthanBVdUrd; unlike BVdUrd, it did not appear to be
internally
incorporated
intoreplicatingDNA in virus-infected cells.
Among thenewly developed analogs,
E-5-(2-bromovinyl)-2'-deoxyuridine (BVdUrd)
emerged as one of the most promising agents
against herpes simplex virus type 1 (HSV-1)
replicationboth in cell culture and in
experimen-tal animals (5, 6). More recently, anarabinose analog of BVdUrd,
E-5-(2-bromovinyl)-1-13-D-arabinofuranosyluracil (BVaraU), was
synthe-sized by Sakata et al. (12) and at the Rega
Institute, Leuven, Belgium. Inits initial in vitro
trials, it showed promising selective antiviral
action. (E. De Clercq, R. Busson, L. Colla, J.
Descamps, J. Balzarini, and H. Vanderhaeghe,
Program Abstr. Int. Congr. Chemother., 12th,
Florence, Italy, abstr. no. 316, 1981). To
eluci-date the mode of selective antiherpesviral action of BVaraU, we investigated its antiviral and
antimetabolic propertiesandexamineditseffect onDNAreplication in virus-infectedcells. The
effect of chemically synthesized 5'-triphosphate
on the activities of purified viral and cellular DNApolymerasesis alsodescribedin this
com-munication.
The antiviral assays were carried out with
primaryrabbitkidney(PRK) cellcultures
infect-ed with HSV (4). The results are expressed as
ID50(50%inhibitorydose), the concentrationof
compound required to reduce viral cytopatho-genic effect (CPE) by 50% when virus-infected control PRK cells reached complete CPE. The data presented are anaverage of at least three separate experiments. The ID50 value for
BVaraUagainst three strains ofHSV-1was0.1
,ug/ml compared with 0.007 to 0.009 p.g/ml for
BVdUrd (Table 1). BVaraU was less effective against HSV-2 replication;theID50rangedfrom
15to70 ,ug/mlcomparedwith 1 to1.5,ug/mlfor
BVdUrd. Neither BVaraU nor BVdUrd was
active against an HSV-1 TK- (B2006) mutant, indicating that both nucleoside analogs needto
be phosphorylated by the virus-induced
thymi-dine kinase to exert their antiviral activity. BVaraUwasnotactiveagainst vaccinia virusor
vesicularstomatitisvirus. The antimetabolic
ac-tivity ofBVaraUwas monitored by the
inhibi-tion ofdThdordUrdincorporation intoDNAof
PRKcells (4);[1',2'-3H]2'-deoxyuridine
(specif-icactivity, 42 Ci/mmol), and
[methyl-3H]thymi-dine(specific activity 12 Ci/mmol) were used as
thelabeled DNA precursors. BVaraU inhibited
cellular DNA synthesis only at doses much higher than that required to inhibit the viral-induced CPE (Table 1).
To confirm that the inhibitory effect of BVaraUonCPE isdue toits abilitytoinhibit the
virusmultiplication, we tested its effect on the replication of HSV-1 (KOS) in PRK cells. Both BVaraU and BVdUrd inhibited the viral multi-plication, albeit to adifferent extent. For exam-ple,at100,ug/ml after 24 h, BVaraU reduced the virus replicationby 3.9 log'0, whereas BVdUrd reduced it by 5.9 log10;after 48 h,BVaraUat 100
,ug/ml reduced virus replication by 3.4 log10, whereas BVdUrd reduced it by 5.4
logl0.
332
on November 10, 2019 by guest
http://jvi.asm.org/
NOTES 333
TABLE 1. Antiviral and antimetabolic activities of BVaraU and BVdUrd in PRK cell culturesa
Antiviralor-metabolicactivity D qgMb
BVaraU BVdUrd Virus
HSV-1 (KOS) 0.1 0.007
HSV-1 (F) 0.1 0.007
HSV-1 (Mac Intyre) 0.1 0.009
HSV-1TK-(B2006) >200 100
HSV-2 (G) 15 1
HSV-2(Lyons) 20 1
HSV-2 (1%) 70 1.5
Vaccinia >200 7
Vesicular stomatitis virus >200 >200
Antimetabolicactivityc
[methyl-3H]dThd 20 70
incorporation
[1',2'-3H]dUrd 95 20
incorporation
a PRK cells were inoculated with 100 cell culture
infectingdoses of virus for 1 h at 37°C and exposed to various concentrations of the test compound (in Eagle minimal essential medium plus 3% fetal bovine se-rum). CPE was recorded daily. In virus-infected but untreated cell culture controls, the viral CPE was generally complete after3daysfollowing viral inocula-tion.
bResultsareexpressedasID50,i.e., the concentra-tionrequired to inhibit viral CPE or incorporation of labeled precursors by 50%compared with untreated
controls;0.1p.g/mlisequivalentto0.29and 0.3 I1M of BVaraU and BVdUrd,respectively.
c[1'2'-3H]2'-deoxyuridine (specific activity, 42 Ci/ mmol)and
[methyl-3H]thymidine
(specific activity,12Ci/mmol)wereusedasthelabeled DNA precursors in
experiments todeterminetheantimetabolicactivity.
The effectof the 5'-triphosphate(BVaraUTP)
onthe utilization ofdTTPbyviral and cellular
DNA polymerases was determined. The
BVaraUwasconvertedtoits5'-triphosphateby
procedures described previously (3). The DNA
polymerases used in these experiments were
purified by successive chromatography, using
phosphocellulose
and DNA-cellulose columns.These enzymes were free of
cross-contamina-tion;forexample,HSV-1 DNApolymerasewas
free of DNA polymerase a and vice versa. In
their
properties
suchaselutionpositionsinion-exchangecolumns,primer-template preference, effectsofmono-anddivalentcations,sensitivity
to
N-ethylmaleimide
and aphidicolin, theseen-zymesresembledtherespectiveviral and
cellu-lar DNA polymerases. The polymerase assays were
performed
under conditions optimum fortheindividualenzymes with the concentration of
[3H]dTTP
maintainedattwo tothree times theKm value for the respective polymerase (1 LM
for HSV-1 DNA polymerase, 10 ,uM for DNA
polymerasea, and30 ,uM for DNApolymerase
,B and Epstein-Barrvirus [EBV] DNA
polymer-ase). HSV-1 DNA polymerase was the most
sensitive among the polymerases tested; DNA
polymerase a was the next sensitive enzyme.
However, DNA polymerase M and EBV DNA
polymerase were relatively insensitive. At 1
FM, BVaraUTPinhibited the activities of HSV-1 DNApolymerase, DNApolymerase a, DNA
polymerase
1,
and EBV DNA polymerase by60, 48, 15, and 10%, respectively; at 10 FM, it
inhibitedtheiractivitiesby 90, 82, 34, and 23%,
respectively.
Todeterminethe nature ofBVaraUTP inhibi-tion, further experiments were performedwith
increasing concentrations of dTTP. Results
show that the inhibition was competitive with
dTTP(8); the mode ofinhibition appears to be
thesameforall ofthe enzymes examined(Fig.
1).TheKmvalues for dTTP and the
Ki
value forBVaraUTP are depicted in Table 2. Although
theKivalues forHSV-1 DNApolymerase(0.14
,uM BVaraUTP) and DNA polymerase a (0.32
TABLE 2. Kinetic analysis of BVaraUTP
inhibitiona
Km Kj
Enzyme (dTTP) (BVaraUTP) K/K, (>M) j(M)
HSV-1 DNA polymerase 0.35 0.14 2.5 Human DNApolymerase a 5.16 0.32 16.12
Human DNApolymerase,1 14.8 52.5
aDNApolymeraseaactivitywasassayed ina50-,ul
reaction mixture consisting of 50 mM Tris-hydrochlo-ride (pH 8.0), 2 mMdithiothreitol, 8mMMgCl2,100 FM each of dATP, dCTP, and dGTP, and various concentrations of[3H]dTTP (530cpm/pmol),10 ,ugof activated calf thymus DNA, 10 to 20
"g
of bovine serumalbumin,5tol1o0 glycerol,and enzyme. Incu-bationwas at37°Cfor 30 min.Acid-insolubleradioac-tivitywascollectedon anitrocellulose filter(Gelman
orMillipore,0.45 ,um)washedseveral times with 5%
trichloroacetic acid containing 2 mM sodium
pyro-phosphate,washedoncewith70%oethanol,dried,and measured inaliquid scintillationcounter. DNA
poly-merase ,B activity was assayed under similar
condi-tions except thatapH 9.0Tris-hydrochloride buffer, 50 FM ofnonradioactive triphosphates and 40 mM
KCl were used. The reaction mixture for assaying HSV-1 DNA polymerase activity contained 50 mM
Tris-hydrochloride (pH 8.3), 2 mM dithiothreitol, 4
mMMgCI2, 10
FzM
eachofdATP,dCTP,anddGTP, andvarious concentrations of[3H]dTTP(4,300cpm/ pmol), 5 ,ug ofactivatedcalfthymus DNA, 50 mMammoniumsulfate,10 FLgof bovineserumalbumin, 5
to10%7glycerol, and enzyme. Other conditionswere
similartothosedescribed above formeasuringDNA polymeraseaactivity.EBV DNApolymeraseactivity
wasassayedundersimilarconditions for DNA
poly-meraseaexcept that 4mMMgCl2and 100 mMKCI
wereused. VOL.43, 1982
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.504.267.458.330.413.2]334 NOTES
,uM BVaraUTP) are almost similar, the Km/Ki
ratio forDNApolymeraseawassix timesmore than thatfor the HSV-1 DNApolymerase.This
implies that BVaraUTP is less selective than
BVdUTP. Since the submission of this
manu-script, Ruth and Cheng reported a Kivalue of
0.013 ,uM for BVaraUTP of HSV-1 DNA
poly-merase(11).
DNA polymerases
require
all four naturaldeoxyribonucleoside triphosphates
foroptimum
activity. However, a limited DNA
synthesis
occurs whenoneor two
triphosphate
substratesare omitted from the reaction mixture.
Using
thisphenomenon,wetested whether BVaraUTP
can substitute dTTP in aDNA
polymerization
reaction. BVaraUTP was added to the HSV-1
DNApolymerase reactionmixture
containing
alltriphosphates except
dTTP,
and the reactionwasfollowed for180min
(Fig. 2).
The additionofBVaraUTP instead of dTTP did notenhance the
polymerization
reaction. There was no in-crease in DNAsynthesis
even when excessdTTPwasaddedatthe endof 120 min.
Howev-er, theadditionofdTTPtothereaction mixture
containing onlythe three nucleotides at120min
increasedDNA
synthesis
asexpected.
Theaddi-tion ofnew enzyme and dTTP to the reaction mixture containing the analog did not enhance
DNAsynthesis;theaddition of excesstemplate
anddTTP increasedDNAsynthesis only
slight-ly. It appears that BVaraUTP was not an alter-nate substrate for DNA polymerase and that it did not getinternally incorporatedinto replicat-ing DNA (Fig. 2). A similarpatternwasobtained for DNA polymerase a. Preincubation of the enzyme in the presence of different concen-trations ofBVaraUTP for 18 h at 40C did not alter the enzyme reaction (data not shown). These results areconsistent with the view that BVaraUTP may be a chain terminator for the
polymerizationreaction.
To study theeffect of BVaraU on DNA syn-thesis in virus-infected cells, Vero cells were
infectedwithHSV-1 and grown in the presence
of BVaraU; the viral and cellular DNAs were
separatedby isopycnic CsCl centrifugation (2).
FIG. 1. DNA polymerases a and a were purified
from leukocytesofapatient withacutemyelogenous leukemia. EBV DNA polymerasewaspurified froma
virus-producing cell line, P3HR-1,originally derived fromapatientwith Burkitt'slymphoma. The isolation proceduresweredescribed previously(1).TheHSV-1 DNApolymerasewaspurifiedfromKBcellsinfected with HSV-1 strain HF.Growth conditions and
isola-tion procedures of HSV-1-infected KB cells were
described by Shipman et al. (13). (A) Effect of BVaraUTP on HSV-1 DNA polymerase reaction in thepresenceof different concentrationsof[3H]dTTP with activatedDNA template.Symbols: 0,no
inhibi-tor, 0,1 ,LM BVaraUTP;and0,4,uM BVaraUTP.
(B) Effect ofBVaraUTPonDNApolymerasea
reac-tion in the presence of different concentrations of [3H]dTTP withactivated DNAtemplate. Otherassay
conditions wereoptimal for the DNA polymerase a
activity. Symbols: *,noinhibitor; A,0.5,M
BVar-aUTP;0,1p,MBVaraUTP;and0,4,uMBVaraUTP.
(C) Effect ofBVaraUTPonDNApolymerase
reac-tion in the presence of different concentrations of
[3H]dTTP withactivatedDNA template. Otherassay
conditions wereoptimal for the DNA polymerase ,B activity. Symbols: 0, no inhibitor; 0, 4 p,M
BVar-aUTP; and0,20P,MBVaraUTP.
A
B
C
I/dTTP. .M'
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.504.70.229.64.549.2]NOTES 335
DNA (2). Recently, Pelling et al. (10) demon-f strated that another arabinose analog,
9-P-D-arabinofuranosyladenine (araA), is not an
abso-lute chain terminator and is internally incorporated into viral and cellular DNAs.
The results presented in this communication demonstrate that BVaraU is approximately 10 times less active than BVdUrd against HSV-1 replication in PRK cells. However, recently Machida et al., usingdifferent HSV-1 strains and humanembryonic lung fibroblast cells, observed nodifference between BVaraU and BVdUrd in their abilities to inhibit replication of HSV-1
(0.032 ,ug/ml) and HSV-2 (100 ,ug/ml) (9). The
180 reason for differences in values of the anti-herpesvirus activity between the two
labora-FIG. 2. Time course of BVaraUTP inhibition of
HSV-1 DNA polymerase reaction. Activated DNA
wasusedastemplateand theconditionswereoptimal
for HSV-1 DNA polymerase. Symbols: 0, all four
nucleotidespresent; 0,dATP,dCTP,and [3H]dGTP
present;*,dATP, dCTP,and [3H]dGTP,plus 40,uM BVaraUTP present. At 120 min, 40 MMdTTP was
addedto thereaction mixturecontaining eitherthree
nucleotides (U) or three nucleotides plus 40 FM BVaraUTP(O) andthe reactionwasstoppedat30and
60min afterthe addition.
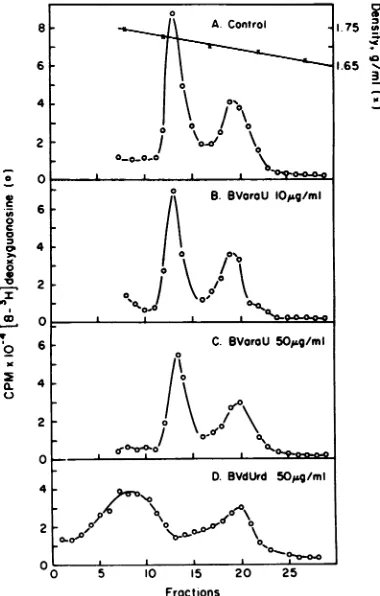
BVaraUinhibited thesynthesis ofbothviraland
cellular DNAs (Fig. 3). Forexample, the addi-tion of 50 ,ug of BVaraU per ml resulted in a
decrease in synthesis of both viral and cellular DNA by 37 and 28%, respectively. The inhibi-tionwasproportionaltotheconcentration of the nucleoside analogadded. The effect of the addi-tion of 50,ug ofBVdUrdperml isshown inFig.
3D. Wehave alsoexamined whether therewas anyqualitative change in the viral and cellular
DNAs synthesizedin the presenceof BVaraU.
Previously, it has been shown that growing virus-infected cells in the presence ofeither
5-iodo-2'-deoxyuridine (IdUrd)or
5'-amino-5-iodo-2'-deoxyuridine(AIdUrd)resultedin the
incorpo-ration of these analogs into DNA, which was
measured by an increase in densities of the
DNAs(7). Substitution of2%ormoreof
thymi-dinebyBVaraUinto DNA would have resulted
in an increase in densities ofviral and cellular
DNAs. Therewas no differencein densitiesof
either viral or cellular DNA isolated from the virus-infected cells grown in presence of
BVaraU (Fig. 3).This indicated that theanalog
waseithernotinternallyincorporatedor insuffi-ciently incorporated into replicating DNAs in virus-infected cells, which is consistent with
results shown inFig.2.However,virus-infected
cellsgrownin the presence of 50,ugof BVdUrd permlshowedanincreaseindensityofthe viral
DNA(from1.725to1.74g/ml)butnot in cellular
3
to
o . . , .
B.BVoraU I0jg/mI
C
ii 6
4
0~~~~~~~~~~ %
011 0
OI
09
OD 0
090--6 C. BVoroU 50#Lg/mI
a. 4 Ca
~~~~~~~~~~~~~00
2
o diOumi.0 i i
50Fg/mlo 144,u;and D. BVdUrd 50ag/mi
2 F.0
0 5 10 5 20 25
Fractions
FIG. 3. CsCl density gradient analysis ofcellular and viral DNA in HSV-1 (Cl101)-infectedVero cells
at a multiplicity of infection of four. After 1 h of contactat370C,residual viruswasremoved,and 4 ml ofgrowthmediumcontainingeithernonucleosideor
BVaraU was added. To each petri dish, 20 MCi of
[3H]deoxyguanosine (specific activity, 14.5 Ci/mmol)
wasadded andincubatedfor 24 h. (A) Cellular DNA
density,1.695g/ml;HSV-1 DNAdensity,1.725g/mlin
control;(B)BVaraU, 10iLg/mlor29 MLM;(C)BVaraU 50ILg/mlor144 MLM;and(D)BVdUrd50 pLg/mlor150
MLM.
00 0
20
CL
0
I 0
lo
0.
x
3CL
90 Time (min)
VOL.43,1982
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.504.64.252.56.236.2] [image:4.504.268.458.258.556.2]tories is not yetknown. HSV-1 DNA polymer-ase and cellular polymerase a are the most
sensitive to the BVaraUTPinhibitionamongthe
enzymestested. TheKivalues for BVaraUTPof
DNA polymerase specific to two strains of HSV-1, i.e., strain Cl 101 propagated in Vero cells and strain HF propagated in human KB
cells are similar (0.14 to 0.2 ,uM). The analog does not appearto get internally incorporated into replicating DNA. Our results show that
BVaraU wasapproximately100 times less
effec-tive ininhibiting the replication of HSV-2 than
HSV-1. Since HSV-2 markedly differs from
HSV-1 in its susceptibility to BVaraU, the dif-ferential sensitivity can be exploited in
distin-guishing the two types ofHSV.
This research wassupported byPublic Health Service grant CA28852-01 and AmericanCancerSocietygrant CH47-C.
LITERATURE CITED
1. Allaudeen, H. S., and J. R. Bertino. 1978. Isolation of herpesvirus-specific DNA polymerase from tissues of an American patient with Burkitt's lymphoma. Proc. Natl. Acad.Sci. U.S.A. 75:4504-4508.
2. Allaudeen, H. S., M. S. Chen, J. J.Lee, E. De Clercq, and W.H. Prusoff.1982. Incorporation of E-5-(2-halovinyl)-2'-deoxyuridines intodeoxyribonucleic acids of herpes sim-plex virus type-1 infected cells. J. Biol. Chem. 257:603-606.
3. Allaudeen,H. S., J. W. Kozarich, J. R. Bertino, and E. DeClercq. 1981.On the mechanism of selective inhibition ofherpesvirusreplication by E-5-(2-bromovinyl)-2' deox-yuridine. Proc.Nati.Acad. Sci.U.S.A. 78:2698-2702. 4. De Clercq, E., J. Descamps, G. F. Huang, and P. F.
Torrence. 1978. 5-Nitro-2'-deoxyuridine and 5-nitro-2'-deoxyuridine 5'-monophosphate: antiviral activity and
inhibition of thymidylate synthetase in vivo. Mol. Phar-macol. 14:422-430.
5. DeClercq, E., J.Descamps, P. De Somer, P. J. Barr, A. S. Jones, and R. T. Walker. 1979. (E)-5-(2-bromovinyl)-2'-deoxyuridine: a potent and selective antiherpes agent. Proc. Natl.Acad. Sci. U.S.A. 76:2947-2951.
6. De Clercq, E., J. Descamps, G.Verhelst, R. T. Walker, A.S. James, P. F. Torrence, and D.Shugar. 1980. Com-parative efficacy of antiherpes drugs against different strains of herpes simplex virus. J. Infect. Dis. 14:563-574. 7. Fisher, P. H., M. S.Chen, and W. H. Prusoff. 1980. The
incorporationof5-iodo-5'-amino-2',5'-dideoxyuridine and 5-iodo-2'-deoxyuridine into herpes simplex virus DNA. Biochim. Biophys. Acta 606:236-246.
8. Lineweaver, H., and 0. Burk. 1934. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56:658-666.
9. Machida, H., S. Sakata, A. Kuninaka, and H. Yoshino. 1981. Antiherpesviral and anticellular effects of 1-1-D-arabinofuranosyl-E-5-(2-halogenovinyl)uracils. Antimi-crob. AgentsChemother. 20:47-52.
10. PellIng, J. C., J. C. Drach, and C. Shipman, Jr. 1981. Internucleotide incorporation of arabinosyladenine into herpes simplex virus and mammalian cell DNA. Virology 109:323-335.
11. Ruth, J. L., and Y. C. Cheng. 1981.Nucleoside analogs with clinical potential in antivirus chemotherapy. The effect ofseveral thymidine and 2'-deoxycytidine analog 5'-triphosphatesonpurifiedhuman(a,>)and herpes sim-plexvirus (types 1, 2) DNA polymerases. Mol. Pharma-col. 20:415-422.
12. Sakata, S., S. Shibuya, H. Machida, H. Yoshino, K. Mirota, K. S. Senda, K. Ikeda, and Y. Mizuno. 1980. Synthesisand antiherpesviral activity of 5-C-substituted uracil nucleosides. Nucleic Acids Res. Symp. Ser. 8:539-542.
13. Shipman, C., Jr., S. M. Smith, R. M. Carlson, and J. C. Drach. 1976. Antiviral activity of arabinosyladenine and arabinosylhypoxanthinein herpessimplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Anti-microb.AgentsChemother. 9:120-127.
on November 10, 2019 by guest
http://jvi.asm.org/