0022-538X/80/09-0741/07$02.00/0
Black
Beetle Virus: Propagation in Drosophila Line 1
Cells
and an
Infection-Resistant Subline Carrying Endogenous
Black
Beetle
Virus-Related Particles
PAUL FRIESEN,' PAUL SCOTTI,2 JOHN LONGWORTH,2ANDROLAND RUECKERTI*
BiophysicsLaboratoryofthe Graduate School and Biochemistry Department of the College of Agricultural andLifeSciences, University ofWisconsin-Madison, Madison, Wisconsin53706,'andDepartment of
Scientific and Industrial Research, Entomology Division, Auckland, New
Zealand2
Black beetle virus (BBV), oneofarecently discovered class of viruses with a
bipartite genome, multiplied readily in Schneider's line 1 of Drosophila cells.
Virusyields, onthe orderof100mg perliterofculture,wereunusually high and
represented some20% of the total cell protein within3 days after infection. A
derivative subline of these Drosophilacells wasfoundto beresistant to infection
by BBV. Theseresistant cells werealsofound to carrysmallamounts of
BBV-relatedparticles, possiblyamaturation-defectiveformof BBV.
Blackbeetle virus (BBV), originally isolated
(7) from the New Zealand black beetle
(Heter-onychus arator),ischaracterizedbyanisometric
137S virion (30nmindiameter) whichcontains
a
single major
coatprotein (molecular
weight
[mol
wt],40,000)andtwoRNA molecules ofmolwt 1.0x 106 (RNA 1) and0.5x106(RNA 2) (8).
These
properties
establish BBVas amemberofa
recently
discovered group of smalldivided-genome riboviruses whose prototype is
Noda-muravirus (10).
Theunusuallysmallgenomeof viruses of this
group,onlyhalfaslargeasthat of the
picorna-virusesorretroviruses, isespecially favorable for
molecular analysis. With only two RNAs, this
family
represents thesimplest
class ofviruses withasegmented
genome, and itsmembersaretherefore
potentially
useful models forstudying
geneinteraction.
Mostofour
knowledge
of themolecularbiol-ogyof thisgroupofviruseshas beengained from
studiesonNodamura virus. Translationin
cell-free extracts has shown that both RNAs from
Nodamura virus are active messengers (11);
RNA1codesfora
105,000-mol-wt protein
(p105)
which may mediate
synthesis
of viral RNA,whereas RNA2codesforacoat-related
protein
of mol wt 43,000 (p43) which is
probably
aprecursorofmature coatprotein (vp40).
Progress in understanding the molecular
bi-ology of members of this interesting group of
virusesrequirescellculturesystemssuitablefor
propagatingand
manipulating
virusundercon-trolled conditions. We describe here a system
involvingBBVandDrosophila line1cellswhich
allows suchstudies to be made. Thissystem has
led directly to the recognition of a subline of
Drosophila line 1 which carries endogenous
BBV-related particlesand is resistant to
infec-tionwithBBV.
MATERIALS AND METHODS
Cells. Two strains ofDrosophilaline 1 cells (15) wereused. The WR strain, kindlyprovided by Imo-gene Schneider (Department ofEntomology, Walter Reed Army InstituteofResearch,Washington,D.C.),
waspropagatedinSchneider's complete growth me-dium (14)containing5mgofbacteriologicalpeptone (Difco Laboratories, Detroit, Mich.) per ml and sup-plemented with 15% fetal bovineserum.The NZstrain was a subline of Schneider's WR strain, obtained through T. C. Grace (Canberra, Australia)and main-tainedfor several yearsatAuckland,using the above growth medium but withalowerserumconcentration (10%).Cellswereroutinely grownat26°C in stationary tissue culture flasks (Falcon Plastics, Oxnard, Calif.;
3024) using 15 ml of medium per flask. To passage
cells,confluentmonolayers(5x 107cells)weregently
dislodged by flushingwith a pipetteand diluted 20-fold intofresh medium.
Propagationofvirusin waxmoth larvae. Wax moths (Galleria mellonella) wereraisedat 30°C as described (1). Fourth- and fifth-instar larvae(12to15 mmlong)wereinjectedwith 10,l ofafilter-sterilized BBV suspensioncontaining about 106waxmoth 50% lethal doses(LDro)/ml(seebelow). An ISCO model M
microinjectorwasused forthispurpose. After10days at
300C,
dead larvae (typically 90 to 100% of thepopulation)werecollectedand storedat-20°C. Stock virus wasprepared by homogenizing BBV-infected larvaeinice-cold0.05Msodiumphosphate, pH 7.2, usinga Sorvall Omnimixer. The 10% larval extract was clarifiedby centrifugationand sterilized witha0.45-,um membranefilter(Millipore Corp., Bed-ford, Mass.).Anequal volumeofglycerolwasadded, and viruswasstoredat-20°C. Infectivitywasassayed in waxmothlarvae asdescribed (1);yields averaged 109to 10"' LDr)operlarva.
Propagation ofvirus in Drosophila cell
cul-ture.Confluentcellmonolayerswereresuspendedand washedinSchneider's medium containing0.5%bovine serumalbumin (BSA). Cell viability (usually greater 741
on November 10, 2019 by guest
http://jvi.asm.org/
than 95%) was determined by staining with 0.05% Trypanblue,and thecellconcentrationwasadjusted to4 x107cells/ml.Cells(108)wereseededinto75-cm2 flasks and infectedwithamultiplicityof1waxmoth
LD)r,/cell,using thevirus stocks describedabove.The infectedcultureswereagitated gentlyfor 30min and then dilutedto107cells/mlwithSchneider's medium containing 10% fetal bovineserum.Cultureswere in-cubatedat26°Cand frozen 4days laterat-20°C.
Purification ofvirusfromDro8ophilacell cul-ture. Viruswasreleased from infectedcellswiththree freeze-thaw cycles. Cell debris wasremovedby cen-trifugation, and virus was pelleted through a2.0-ml cushion of 30%sucrose in buffer A (0.05 M sodium phosphate, pH 7.2, and 0.1% ,B-mercaptoethanol [18-ME]) containing 0.5% BSA. Centrifugation was at 25,000rpminaSpincoSW25.1rotor(60,000xg)for 4hat6°C.Viruspelletswereresuspendedin bufferA. Two-millilitersamplesweremade 1% withrespectto sodium dodecyl sulfate (SDS) and centrifuged at 25,000rpmintheSW25.1rotor(60,000xg)for4.5 h at11°Con28-mi 15to45%(wt/wt) sucrosegradients inbuffer A. Fractions(0.6 ml)werecollected fromthe topof eachgradient, usinganISCOdensity gradient fractionator equipped with aflow cell (0.2-cm path length) andamodel UA-5 absorbancemonitor. Frac-tionswere assayed for virus by spectrophotometric (absorbancyat260nm [As2])andradioactivity mea-surements. The virus-containing fractions were pooled,diluted with bufferA,andpelleted througha
30%sucrosecushioncontainingthesamebuffer.The
viruspelletswereresuspendedinbuffer Aandstored at-70°C.Virus concentrationswerecalculated from optical density measurements at 260 nm, assuming that 1mg/mlhasanabsorbanceof 4.15percmoflight path (8).
Preparation of radiolabeled virus.'Virus
prop-agatedinDrosophilacellculturewasradiolabeledby adding 1.0 mCiofL-[4,5-3H]leucine (AmershamCorp., ArlingtonHeights, Ill., TRK.170)or0.1mCi of L-[U-'4C]leucine (Amersham, CFB.67) to each 10 ml of culture 24 h afterinfection. Incubation with radiolabel wascontinued for 3days. Recovery of input radiolabel inpurifiedvirusrangedfrom0.7 to1.2%.
Viruspropagated inwaxmoth larvae was radiola-beledbyinjectinginfected larvae with80,uCi (40,uCi/ larvaondays3 and4postinfection) of L-[4,5-3H]leu-cine. Virus washarvested from dead larvae 10 days after infection. About 1% ofthe input radioactivity wasrecovered inpurifiedvirus.
Electrophoretic analysis.Methods for SDS-poly-acrylamide gel electrophoresishavebeendescribedby Medappa etal. (9). Gelscontaining9.8%acrylamide, 0.3% (vol/vol) ethylene diacrylate, 0.1% N,N,N',N'-tetramethylethylenediamine,0.1% SDS(Pierce Chem-icalCo.,Rockford, Ill., lot4102-5),and 1.0 Mureain 0.1 M sodiumphosphate, pH7.2, werecastin glass tubes(0.6by20cm).Polymerizationwascatalyzed by the additionofammoniumpersulfate toafinal
con-centration of 0.05%. The electrode buffer, 0.1 M
so-dium phosphate, pH 7.2, containing 0.1% SDS and 0.05 Mneutralized3-mercaptopropionic acid,was
cir-culated betweenupperand lower buffer vessels. Virussampleswerepreparedforelectrophoresis by
adjustingeachto1%SDS,0.5Murea,0.1%,8-ME,and
10 mMsodium phosphate, pH 7.2. Samples were made 20% insucrose and 0.01% in bromophenol blue and thenheated for5minat100°C before electrophoresis.
Electrophoresiswasconducted at4V/cm until the
bromophenol blue marker migrated to the bottom of the gel (about15hfora20-cmgel). Gels were frac-tionated andanalyzedaspreviously described (3).
Preparation of antiserum. Virus used to raise antiserumwaspurifiedfrom BBV-infected wax moth larvae. To purify virus, frozen larvae were homoge-nized in ice-cold 0.05M sodium phosphate, pH 7.2, and carbontetrachloride (2:1 by volume). The heavier organic phasewasseparated by centrifugation at 2,600 xg,and thevirus-containing aqueous phase was clar-ifiedbycentrifugationat12,000xgfor 30 min. Virus was pelleted and sedimented on 15 to 45% sucrose densitygradientsasdescribed above. Afterrepelleting,
viruswassuspendedinbuffer A and storedat-70°C. Anti-BBVserum waspreparedbyimmunizing rab-bits withasingleinjectionof 0.5 mg ofpurifiedBBV
emulsifiedin Freundcomplete adjuvant.Serum was prepared5weeks later.
Immunoprecipitationassays.Serological assays wereconducted by thestaphylococcalprotein A-anti-body adsorbent method describedby Kessler (5). Ra-diolabeled virus inphosphate-bufferedsaline (2) con-taining 0.1% BSA(PBSA)wasincubated with appro-priate dilutions of rabbit anti-BBVseruminPBSA for 2.5hat roomtemperature. Staphylococcal
immuno-globulinG(IgG)adsorbent(IgGsorb;Enzyme Center, Inc.,Boston, Mass.) in NET buffer (150 mM NaCl, 5 mM EDTA, 50mM Tris, pH 7.4, and 0.02% sodium azide) containing 0.5% Nonidet P-40 and 0.1% BSA wasthenaddedto a final concentration of 1% (vol/
vol). After20minatroomtemperature,theIgG ad-sorbentwaspelletedwithanEppendorf microcentri-fuge model5412(12,000xg) and washed with NET, 0.5%NonidetP-40,and 0.1%BSA. The adsorbent was
resuspendedin0.1Msodiumphosphate,pH 7.2,
con-taining 1% SDS and heated for 5 min at 100°C to release radiolabeled virus. The inert adsorbent was removedbymicrocentrifugation,and the supernatant wasassayedforradioactivity.
Radioimmunecompetitionassays.Competition
assays were conducted by incubating increasing amountsofunlabeledantigenwithadilution ofserum
capable ofprecipitating 80%ofa radiolabeled virus probe (0.1 ,ug) for2.5hat roomtemperature. A stand-ardamountofprobe(0.1 ,tg)wasthenadded,and each sample was incubated foran additional 2.5 h. Virus complexedwithanti-BBVIgGwasprecipitated bythe addition ofstaphylococcal IgGadsorbent. The adsorb-ent waspelleted,washed, andassayedforradioactivity asdescribedabove.
Tryptic peptide analysis. Tryptic digestion of viralproteins and thechromatographyof theresulting peptideshas beendescribed(6).Differentiallylabeled virusparticlesweremixed andheatedinthepresence of 0.01% SDS for 5 min at 100°C. Samples were
adjusted to 0.1 M Tris-hydrochloride, pH 8.0, then reduced with 3.5 mg of dithiothreitol per ml, and alkylatedwith 9mg of iodoacetamideperml.Proteins were precipitated with 50% trichloroacetic acid and
suspendedin 0.1 M ammoniumbicarbonate,pH 8.0. After digestion with 0.05 mg of
on November 10, 2019 by guest
http://jvi.asm.org/
CULTURE OF BBV IN DROSOPHILA CELLS 743
nylchloromethylketone-treatedtrypsin (Worthington Biochemicals Corp.,Freehold, N.J.) perml, samples
were lyophilized and suspended in 0.2 M pyridine acetate,pH3.1.Digestswerefiltered to remove insol-ublematerial, and samples were chromatographed on a Chromabead type Pcation-exchange column. Frac-tions(1.5ml)werecollectedandassayed for radioac-tivity.
RESULTS
Preliminary studies on the propagation
of
BBV in cell culture. Studiesonthemulti-.plication
of the smalldivided-genome RNAvi-ruses have beenhinderedby the lack of a system
inwhich to propagate and adequately radiolabel
virus incell culture.
Although
Nodamuravirusgrows in hamster (BHK-21) and mosquito
(Aedes albopictus) cell culture (1),
multiplica-tion
apparently
occurs at such low levels thatvirus-specific
protein synthesis has not yet beendetected
(11). Since
theability
to radiolabelvirus and virus-specific proteins in culture is
crucial to studies on the molecular
biology
ofany
virus,
the search for such a cell culturesystem hascontinued.
Because no
plaque
assay is yet available forBBV, and because the
infectivity
assay based onability
to killwax moth larvae iscumbersome,the searchfora
susceptible
line of hostcellswascarriedout
by measuring
incorporation
ofradio-labeled uridine into virus-like particles. A
num-ber of insect and mammalian cell lines were
examined. Tothis
end,
virus-inoculatedcultureswere incubated for 5 days in the presence of
[3H]uridine
(50,ICi/ml).
Virus, released fromcells
by
three freeze-thawcycles,
waspelleted
and sedimented on sucrose
density gradients
essentially
asdescribed in Materials andMeth-ods. Virus
multiplication
wasthen monitoredbyexamining
thesegradients
forthe presence ofa137S
peak
ofradioactivity.
No evidence of
multiplication
was found intwomammaliancell lines
(baby
hamsterkidney
ormouseL-cells) norinseveral insectcell
lines,
including
those of themosquito (A. albopictus
andA.
aegypti),
thecabbage
looper
(Trichoplu-sia
ni),
the fall armyworm(Spodoptera
frugi-perda),
orline GM1of thefruitfly (Drosophila
melanogaster).
The virusdid, however,
multiply
well in Schneider's
Drosophila
line 1;thus,
10days afterinfection, 10-ml cultures seeded with
108 cells
and108
LD50
(about 10 ,ug) of virusyielded4to 8
A20
units,representing1 to 2mgofvirus.Despitethishigh yield of virus,roughly
25 to 50
times
thattypically
obtained frompi-cornavirus-infected
cultures, therewaslittlecy-topathic effect and virus remainedlargely
cell
associated.
Low yields of virus from a subline of
Drosophila line
1. Preliminary attempts inMadison togrow BBV inDrosophila line 1
(DL-1)cells obtained from New Zealand (NZ subline)
yielded only a few percent of the expected
amountsof virus. The lowyield was ultimately
tracedtotheidentity ofthecells used to
prop-agate the virus. Thus, low yields were obtained
with the NZsubline, whereas high yields of BBV
were produced by DL-1 cells obtained directly
fromImogene Schneider's
laboratory
(WRline).This is illustrated by an experiment in which
equal
numbersof cells from the WR line and theNZsubline of WR cells were infected in parallel,
incubated at
26°C,
andradiolabeled
by exposingthe culturesto
[3H]leucine
(100,uCi/ml),
begin-ning 1 day after infection. On the 4th day after
infection, virus was released by freezing and
thawing
andanalyzed
on sucrosedensity
gra-dientsasdescribed above.
The parental WR cells yielded a prominent
peak of
radioactivity
which coincided with the137S peak of optical density (Fig. 1A). As
ex-pected,
nosuch 137Speak
wasobserved in thegradient from mock-infected WR cellscarried in
parallel
(Fig.
1C). Incontrast, the BBV-infected
4,-DROSOPHILA (DL-1) CELLS Parental (WR) T Subilne (NZ)
II 20 30 40 10 20 30 40
FRACTION NUMBER
*0.2 o m
r-o0.4 32
*0.2
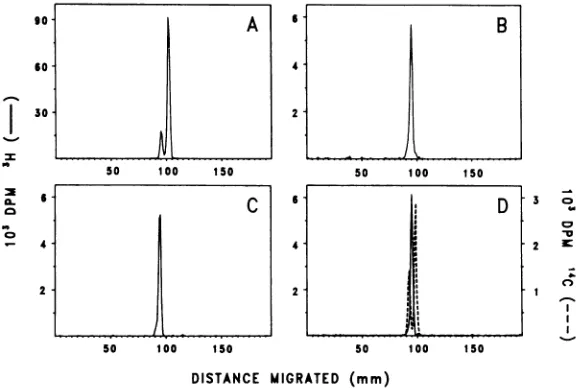
FIG. 1. Sucrosedensity gradient profiles showing
yieldsofBBV-likeparticlesfrom parental
Drosoph-ila line1cells and its NZ subline. Parental WR cells were (A) infected with BBV and(C) mock infected. NZ subline cellswere(B)infectedwith BBVand(D) mock infected. Cellmonolayerscontaining 108 cells wereexposedto0.025mlof5%extractsfrom BBV-infected (4 x 109LDr,o/ml) oruninfected waxmoth larvaeand incubatedfor4daysat26°C.
[3H]leucine
wasadded1dayafterinfection. Viruswasisolated,
purified, and centrifuged on sucrose density gra-dientsasdescribed inMaterialsand Methods. Sedi-mentationisfrom lefttoright.
VOL. 35, 1980
I
.F
m
a.
O
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.510.260.451.327.549.2]NZsubline yielded only3 to 10% as many137S
particles asthe WRline (compare Fig. 1B and
1A). From optical density measurements, the
totalyield of virus particles from theNZsubline
culturewasestimatedtobe about50,ug,whereas
the yield from the WR parental culture was
approximately 550
,tg.
Therecovery ofradioac-tivity inpurified viruswas106 dpm from theNZ
culture, compared with 30 x
106
dpm from theWRculture.
Unexpectedly, virus-like particles (137S)were
also found in themock-infected NZ cell cultures;
moreover,theywerepresentinamountssimilar
tothose obtainedfrom BBV-inoculated NZ
cul-tures(compareFig. lB and 1D). The possibility
that these particles arose from an undetected
viralagent,inadvertently introduced by the
ex-tractsof
healthy
larvaeused in mockinfection,
was ruled out in other experiments which
showed thesame 137S particles in NZcellsnot
previously exposedtolarvalextracts.Thus, the
NZsubline ofDrosophila cells carries
endoge-nous particles (DL-1 particles) whichsediment
likeBBVbut whichare notfound in the parental
WRcell line.
Electrophoretic differences in the coat
protein of BBV and DL-1 particles. The
sim-ilarityinsedimentation velocitiessuggested that
DL-1 particles were related to BBV. This was
confirmed byelectrophoretic analysis on
SDS-polyacrylamide gels. Each of the 137S peaks
from thesucrosegradients shown in Fig. 1
dis-playeda single major bandinthe region of the
40,000-dalton
coatprotein ofBBV(Fig. 2). ThatI
0
C5
90
60
30
6
4
2
A
50 100 1S0
.5 10.1.
so 1oo 1 so
DL-1 particles are not, however, identical to
BBVwasshownby differences in their electro-phoretic properties.
BBV wasfoundtocontaintwocoatproteins,
amajoronewith anapparentmolwtof40,000
(vp4O),andaminoroneof molwt44,000(vp44)
(Fig. 2); this agrees with the report of
Long-worth andCarey (8).vp44 may represent a
pre-cursor form of mature coat protein (vp4O) as
previously proposed for Nodamura virus (11);
indeed, the tryptic
profiles
of these two BBVproteinsare virtually identical (P. Friesen,
un-published data). We have observed that the
relative proportion ofvp44varies fromonevirus
preparation to another; however, it is not yet
clear whether this variation reflects different
contentsofprecursorproteinvp44inindividual
particles or amixture oftwo different kinds of
particles.DL-1 particles, unlike BBV, exhibited
noevidenceof a minorprotein (Fig. 2C).
A second difference in the proteins ofDL-1
particles
and BBVwasdemonstratedby
coelec-trophoresis of differentially labeled particles
(Fig. 2D). Theelectrophoretic mobility of DL-1
particlecoatprotein (molwt42,000)wasslightly
lower than that of the major BBVcoatprotein
(vp4O)buthigher than that ofvp44.
Finally,
electrophoretic
studies on the intactparticles revealedadifference innetcharge
be-tween BBV andDL-1 particles. AtpH 8.5 (1%
agarose in35 mM Trisbuffer), BBV migrated
towardthe
cathode,
whereas DL-1particles
mi-grated in the opposite direction (data not
shown).
4.
2
6
-4.'
2
B
50 100 150
.1
DD
50 100 150
3 o
0a
2 K
*1
n
DISTANCE MIGRATED (mm)
FIG. 2. Electrophoreticprofiles ofcoatproteinof the radioleucine-labeled virus-likeparticlesshown in Fig. 1. (A) FromFig. IA, BBV-infectedWRcells; (B)from Fig.
lB,
BBV-infectedNZcells; and(C)fromFig.ID,mock-infectedNZcells. (D)[3H]leucine-labeledDL-1particlesfrom Fig. IDversus
['4C]leucine-labeled
BBV. Virussamples weredisrupted and subjectedtoelectrophoresis onSDS-polyacrylamide tubegelsas described in Materialsand Methods.on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.510.119.407.428.622.2]Resistance of NZ cells to productive in-fection with BBV. Comparison of Fig. lBand 1D shows that inoculation of the NZ subline with BBV didnotsignificantly increasethe yield
of 137S material. Thecoat protein of particles from BBV-inoculated NZcells wasthat of DL-1 particles, not BBV (Fig. 2B). The sharpnessof
the peak and thepresenceof onlyaslight shoul-der in the region ofvp4O from BBV indicates that therewaslittle ifanyBBV protein in the
137S peak fromBBV-inoculated NZ cells. The absence of any detectable BBV in even this
smallpeak of virus-like material implies that NZ cellsareeffectively resistanttoproductive infec-tion by BBV.
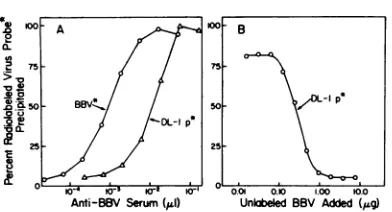
Serological relationship between BBV and DL-1particles. Both BBV (derived from the WRline) and DL-1 particles (derived from the NZsubline)wereprecipitated by antiserum raised against BBV (Fig. 3A). The capacity of this antiserumtoprecipitateaconstantnumber ofparticles was 12-foldgreater for BBV; thus, the precipitin titerwas90,000for BBV but only 7,000 for DL-1particles.
Further evidence for serological relatedness was provided by a radioimmune competition assay which measured the ability of unlabeled BBVtocompetewitharadiolabeled DL-1
par-ticle probe for a limited number of antibodies (Fig. 3B). Theassaydemonstrated that the
pre-cipitation of the DL-1 particle probe could be reduced to background levels when anti-BBV serum waspreincubated withexcessBBV. Thus, antibodies directed against BBV were also ca-pable of precipitating DL-1 particles.
Gel diffusion tests using antiserum raised against DL-1 particles independently confirmed that BBV and DL-1 particles have common surfaceantigens (datanotshown). The precipi-tation arcs of these tests indicated that both particleswereserologically related butnot
iden-tical. Thisnonidentityissupported bythe sub-stantialdifference inprecipitation titersof anti-BBVserumtowardeachparticle (Fig. 3A).
Similaritiesinthetrypticfingerprints of the coat proteins of BBV andDL-1 parti-cles. Comparison ofthe tryptic profiles of the coatprotein of BBV andDL-1 particles,
differ-entially labeledwithradioleucine,revealed sev-eral identical tryptic fragments along with a coupleof differences atfraction114(Fig. 4).The
smallnumber oftryptic peaks exhibited by both particlesmaybeduein parttothe insolubility ofafraction(20%) of the tryptic digest. Similar behavior has beenpreviously observed with No-damuravirus (11) andmaybe characteristicof
thecoatproteinof thisclass of viruses.Although
the degree of difference cannot be accurately
assessed because ofthesmallnumber of tryptic
0.U
!
0 S? .2!?
(
W.W* Kr" W^, 0.0 0.10 100
Anti-BBV Serum(ILI) Unbbeled BBV Added(/Lg)
FIG. 3. (A) Titration curves for BBV and DL-1 particles, usingantiserum directedagainstBBV. A fixed amount (0.1 pg) ofradioleucine-labeledprobe antigen (BBV, -5,000 dpm; DL-1 particles, -1,000
dpm) in PBSA was incubated with the indicated volume ofserum under standard assay conditions (2.5hatroom temperaturein atotal volumeof100
,ld). Radioactivityin the immune
complex
wasdeter-minedafterprecipitationwithstaphylcoccal IgG ad-sorbentasdescribed inMaterials and Methods. The precipitation titer, defined as thereciprocal ofthe volume(microliters) ofserumrequiredto half-precip-itate1 ngofvirus understandardassayconditions, was9x 104 forBBVand7x 103forDL-1 particles.
(B) Radioimmune competition assay ([3H]leucine
DL-1 particle probe versus BBV). The indicated amountsofunlabeled BBV in PBSA were preincu-batedfor2.5 hatroomtemperaturewithadilutionof
serumcapable ofprecipitating80%oftheradioactive
DL-1particle probe.A standard amount(0.1 pg) of DL-1particle probe(-1,000 dpm)in PBSAwasthen added, and incubationwascontinuedunder stand-ard conditionsforanadditional2.5 h.Radioactivity in thestaphylococcal IgGadsorbentprecipitatewas determinedasdescribed.
peaks observed, the similarities among those tryptic fragments displayed again suggest that
bothparticlesarerelated butnotidentical. DISCUSSION
We have shown that BBV multiplies well in
Schneider'sDrosophilaline1.However,the in-fection isnotverycytolytic and the infectedcells
remain intactforperiods of4or moredaysafter infection.Thus, arelatively long period of
syn-thesis probably accounts in part for the high yields of virus which rangefrom 1 to 2mg per
108cells(40mgequivalentwetweight) within3
days of infection. By the 3rd day postinfection, the cellmasshasincreasedtoabout 70mg(wet
weight). Assuming that protein constitutes 10%
of the cellular wetweight, 1.5 mg of virus cor-respondstoabout20%of thetotalcell-associated protein,a yield 100-foldgreaterthan that typi-callyobtained frompicornavirus-infected cells.
Although BBV multiplies well in one strain (WR line) of Schneider's line 1, it multiplies poorly, ifat all, in aderiVative strain (NZ sub-line). The observation that the resistant NZ
subline carries BBV-relatedparticles (DL-1 par-0-A
i° BBV* /
'5
._4.-,^f ,,}2 ,^f
lOO B
75
so
\,/)L~~~-I p*
25
.
VOL. 35, 1980
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.510.260.456.76.182.2]50 100 150
FRACTION NUMBER
500
'
300
-100 :
200 250
FIG. 4. Comparison of differentially radioleucine-labeled proteinsofBBVand DL-1particles by tryptic fingerprinting. A mixture of[14C]leucine-labeled BBV and [3Hlleucine-labeled DL-1 particles was heat denatured, digested with trypsin, and chromatographed on a cation-exchange column as described in Materials and Methods.
ticles)whereas thesusceptible WR line doesnot
suggeststhat resistance toinfectionby BBV is mediated by the DL-1 particle. However, further evidence is required to rule out the possibility that such resistance is an independent trait of cellscarrying DL-1 particles.
Recent studies (P. Scotti, unpublished data) show that DL-1particlescanmultiply in cells of Drosophilaline 2 and GM1. Thisinfectivity of
DL-1particlesnotonly establishes theagentas a virus (DLV), but may also open the way to clarifying the role of DLV in BBV resistance by determining its abilitytoalter thesusceptibility of DLV-infected cellstosuperinfection by BBV. Theproperties of DLV indicate that itbelongs
tothesamefamilyasBBV andNodamuravirus.
Each virus sedimentsat135to137S andcontains one majorcoatprotein in the molecularweight
range of40,000. Moreover, recent studies
indi-cate thatDLV, like BBV and Nodamura, con-tains two RNAs sedimenting at 15 and 22S, respectively.
Thediscovery of DLV raisesaquestionabout itsorigin. In light ofreportsof virus-likeparticles in the nucleus and cytoplasm of cultured Dro-sophilacells,including Schneider's line 1 (4, 12, 16), it is possible that DLVwas acontaminant of theoriginal tissue explant of the cell line. It could then have been eitherlost from the WR line ormaintained ina suppressedstate. Alter-natively, the virus could have arisen in the NZ
subline because of derepression at some later
stage ofsubcultivation.None of these explana-tions, however, accountsfortheremarkable co-incidence that the immuneDrosophilacellline carriesanagentclosely relatedtoBBV, avirus
originallyisolated fromblackbeetles.
A more likely way ofexplaining this coinci-dence is that DLV arose from an inadvertent infection of line 1 with BBV itself. Such an infection might easily have been overlooked since BBV does notcause extensive cytopathic effects inDrosophila cells. Continued subculti-vation of the infected cells may then have
se-lected formutantsmorecompatible with contin-ued cell growth, thus leadingto the
establish-mentofapersistent infection.
The rather small differences in the tryptic profiles of thecoatproteins (Fig. 4)are
consist-entwith thehypothesis that DLV isamutantof BBV. These tryptic similarities and the minor shift inelectrophoretic mobilityoftheDLV coat
proteinsuggestthatrelatively fewchanges have occurred. The apparent increase in molecular
weight of the DLV protein might be accounted forbya changeassmallas asingle amino acid residue since it has been demonstrated that re-placement of such a residue can significantly alter SDSbindingand thus the relativemobility of certainpolypeptidesonSDS-gels (see discus-sion in reference6).
On the other hand, our electrophoretic and serological studies suggest that BBV and DLV differsubstantiallymorethan wouldbeexpected
for asimpleselection ofviralmutants. For
ex-ample, DLV appears to lack the minor coat
found in BBV. Moreover, the particles have opposite chargesat pH 8.5 and have a 12-fold difference in immune reactivity, both implying substantial differences in the surfaces of BBV
and DLV.
Apossible resolutiontotheseapparently con-tradictory findings is suggested by an analogy with poliovirus. The surface antigenicity of poliovirus is radicallyalteredbyalarge confor-mational change which occursduringthe
mat-urationstepin whichthe virionacquires infec-tivity (reviewedinreference13);moreover,
mat-urationisaccompanied by cleavageofeachpro-.
tein subunit in theprecursorparticle (provirion). AnanalogousprocessinBBV,wherebythe
pro-tein subunits(vp44) of the provirionarecleaved
toformthemajorcoatprotein(vp4O)of mature
virions, could also be responsible formajor an-tigenic differences between the BBV provirion and the mature virus particle. This raises the
possibility, then, that DL-1 particles represent uncleaved provirions ofa maturation-defective
a-,
.0
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.510.88.411.64.181.2]747
mutant of BBV and that the single peak of
protein in DL-1 particles (Fig. 2C) represents
the precursorprotein, not the mature coat
pro-tein, ofthe defectiveforn of BBV. It should be
possible to test this hypothesis by comparing
DL-1 particle coat protein with the cell-free
translation product directed by its mRNA. ACKNOWLEDGMENTS
Thisresearch was supported in part by grantMV-33I from theAmerican Cancer Society and by Public Health Service grant CA-08662 from theNational Cancer Institute. P.F. was supported by Public Health Service training grant 5-T32-GM07215 from the National Institute of General Medical Sciences.
Wethank Harry Coppel, Department of Entomology, Uni-versity ofWisconsin-Madison, for the gift of wax moths, for instruction on propagationand injection of larvae, and for useful discussions. We also thank DanOmilianowskifor chro-matography of tryptic digests.
LITERATURE CITED
1. Bailey, L,J.F.E.Newman, andJ. S.Porterfield. 1975.Themultiplicationof Nodamura virus in insect andmammaliancellcultures.J. Gen.Virol.26:15-20. 2. Dulbecco,R.,andM.Vogt.1954.Plaqueformation and
isolationofpure lines withpoliomyelitisviruses. J.Exp. Med. 99:167-182.
3. Gilson,W.,R.Gilson,and R. R.Rueckert 1972. An automatic highprecision acrylamide gel fractionator. Anal. Biochem. 47:321-328.
4.Hirumi,H. 1976.Viral, microbial,and extrinsic cell
con-tamination of insect cellcultures, p. 233-268. In K. Maramorosch (ed.), Invertebrate tissue culture: re-search applications. AcademicPress, Inc.,New York andLondon.
5. Kessler, S. W. 1976. Cell membrane antigen isolation with the Staphylococcal protein A-antibody. J. Immu-nol. 117:1482-1490.
6.Kew, 0. M., M. A.Pallansch, D.R.Omilianowski, and R.R.Rueckert. 1980. Changes in three of four coat proteinsof oral poliovaccine derived from type 1 poliovirus. J. Virol. 33:256-263.
7. Longworth, J.F., and R. D. Archibald. 1975. A virus ofblack beetle, Heteronychus arator (F) (Coleoptera: Scarabaeidae).N.Z. J. Zool. 2:233-236.
8. Longworth,J.F.,andG. P.Carey.1976.Asmall RNA virus with adivided genome from Heteronychus arator (F)[Coleoptera:Scarabaeidae].J.Gen. Virol. 33:3140. 9.Medappa, K. C.,C. McLean, and R. R. Rueckert. 1971. Onthe structureofrhinovirus 1A. Virology 44:259-270. 10. Newman, J. F.E.,and F.Brown. 1973. Evidence for a divided genome in Nodamuravirus, anarthropod-borne picornavirus. J. Gen. Virol. 21:371-384.
11.Newman,J. F.E.,T.Matthews,D. R.Omilianowski, T.Salerno,P.Kaesberg, and R. Rueckert. 1978. In vitrotranslation of the two RNAs of Nodamura virus, anovel mammalian virus with a divided genome. J. Virol.25:78-85.
12.Plus, N. 1978.Endogenous viruses ofDrosophila mela-nogastercelllines: theirfrequency, identification,and origin.In Vitro 14:1015-1021.
13.Rueckert,R.R. 1976. Onthestructureand morphogen-esis ofpicornaviruses,p. 131-213. In H.Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology. PlenumPublishing Corp.,New York.
14.Schneider,L.1964.Differentiation of larvalDrosophila eye-antennal discs in vitro. J.Exp.Zool. 156:91-104. 15. Schneider, L.1972.Celllines derived fromlateembryonic
stages ofDrosophilamelanogaster. J.Embryol. Exp. Morphol.27:353-365.
16. Williamson,D.L,andR. P.Kernaghan.1972. Virus-like particles in Schneider'sDrosophila cell lines.Dros. Infect. Serv. 48:58-59.
VOL. 35, 1980

![FIG. 4.Materialsfingerprinting.denatured, Comparison of differentially radioleucine-labeled proteins of BBV and DL-1 particles by tryptic A mixture of [14C]leucine-labeled BBV and [3Hlleucine-labeled DL-1 particles was heat digested with trypsin, and chromatographed on a cation-exchange column as described in and Methods.](https://thumb-us.123doks.com/thumbv2/123dok_us/1492686.101940/6.510.88.411.64.181/materialsfingerprinting-denatured-comparison-differentially-radioleucine-hlleucine-chromatographed-described.webp)