0022-538X/11/$12.00 doi:10.1128/JVI.05691-11
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
Molecular Determinants of Arenavirus Z Protein
Homo-Oligomerization and L Polymerase Binding
䌤
Maria Eugenia Loureiro,
1Maximiliano Wilda,
1Jesica M. Levingston Macleod,
1†
Alejandra D’Antuono,
1Sabrina Foscaldi,
1Cristina Marino Buslje,
2and Nora Lopez
1*
Centro de Virología Animal (CEVAN), Instituto de Ciencia y Tecnología Dr. Cesar Milstein, Consejo Nacional de Ciencia y Tecnología (CONICET), Saladillo 2468, Buenos Aires C1440FFX, Argentina,1and Fundacio´n Instituto Leloir,
Avenida Patricias Argentinas 435, Buenos Aires 1405, Argentina2
Received 14 July 2011/Accepted 14 September 2011
The arenavirus Z is a zinc-binding RING protein that has been implicated in multiple functions during the viral life cycle. These roles of Z involve interactions with viral and cellular proteins that remain incompletely understood. In this regard, Z inhibits viral RNA transcription and replication through direct interaction with the viral L polymerase. Here, we defined the L-binding domain of Tacaribe virus (TCRV) Z protein and the structural requirements mediating Z homo-oligomerization. By using site-directed mutagenesis, coimmuno-precipitation, and functional assays, we showed that residues R37, N39, W44, L50, and Y57, located around the zinc coordination site I, play a critical role in the Z-L interaction. We also found that Z protein from either TCRV or the pathogenic Junin virus (JUNV) self-associates into oligomeric forms in mammalian cells. Importantly, mutation of the myristoylation site, the strictly conserved residue G at position 2, severely impaired the ability of both TCRV Z and JUNV Z to self-interact as well as their capacity to accumulate at the plasma membrane, strongly suggesting that Z homo-oligomerization is associated with its myristoylation and cell membrane targeting. In contrast, disruption of the RING structure or substitution of W44 or N39, which are critical for L protein recognition, did not affect Z self-binding. Overall, the data presented here indicate that homo-oligomerization is not a requirement for Z-L interaction or Z-mediated polymerase activity inhibition.
Tacaribe virus (TCRV), a nonpathogenic member of the
Arenaviridae family, is closely related to the known South American pathogens that cause severe hemorrhagic disease in humans. In particular, TCRV displays a close genetic and antigenic relationship with Junin virus (JUNV), the etiological agent of Argentine hemorrhagic fever, a recognized major public health problem in certain agricultural areas of Argen-tina (9, 13, 33).
Like all arenaviruses, TCRV is an enveloped virus whose genome consists of two single-stranded, negative-sense RNA segments named S and L. The S RNA encodes the nucleopro-tein (N; ca. 64 kDa) and the glycopronucleopro-tein precursor (GPC; ca. 70 kDa). The L segment encodes the viral RNA-dependent RNA polymerase (L protein; ca. 220 kDa) and a small protein called Z (ca. 11 kDa) (4, 15). Both L and N proteins are the minimaltrans-acting factors required to drive full-cycle repli-cation and transcription of the viral genome (20, 26, 30). Are-naviruses replicate in the cytoplasm, and budding of new par-ticles takes place at the plasma membrane of infected cells (4, 10).
The Z protein of arenaviruses contains a conserved
37-amino-acid RING domain that coordinates two zinc ions and that is surrounded by less conserved N- and C-terminal regions (22, 51). Several lines of experimental evidence indicate that the Z protein has multiple functions during the viral life cycle. A possible regulatory role on cell growth has been proposed since Z has been shown to interact with several cellular proteins, including ribosomal protein P0 (3), promyelocytic leukemia protein (PML) (2), eukaryotic translation initiation factor 4E (eIF4E) (5, 24), and the proline-rich homeodomain protein (12, 48).
Z is a structural component of the virions, forming a layer beneath the viral envelope (34, 41). Moreover, the Z protein plays an essential role in virion assembly and budding, sharing several characteristics with other negative-strand RNA virus matrix proteins. Expression of Z protein in mammalian cells, in the absence of other viral proteins, leads to the assembly and budding of virus-like particles (VLPs) (8, 36, 45, 46, 50). Fur-thermore, the Z protein C-terminal region contains proline-rich late domains (PTAP and/or PPPY), which are important for the recruitment of host proteins that assist in the late stages of budding (36, 49). In addition, Z has been demonstrated to associate with cellular membranes (8, 36, 46). N-terminal myristoylation is required for both Z plasma membrane tar-geting and particle budding (37, 47).
As in the case of the matrix proteins of other enveloped RNA viruses such as rhabdovirus M and filovirus VP40 (17, 19), bacterially expressed Z proteins from the Old World arenaviruses lymphocytic choriomeningitis virus (LCMV) and Lassa fever virus (LASV) were reported to exhibit
self-assem-* Corresponding author. Mailing address: CEVAN, Instituto de Ciencia y Tecnología Dr. Cesar Milstein, CONICET, Saladillo 2468, Buenos Aires C1440FFX, Argentina. Phone and fax: 54 11 4687 6735. E-mail: nlopezcevan@centromilstein.org.ar.
† Present address: Department of Microbiology, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, NY 10029.
䌤Published ahead of print on 28 September 2011.
12304
on November 7, 2019 by guest
http://jvi.asm.org/
bly propertiesin vitro(25). Self-interaction of matrix proteins is believed to be critical for their function as viral budding-driving factors. However, the relevance of arenavirus Z protein self-association under conditions present in mammalian cells has not been determined.
The Z protein also interacts with other viral proteins, in-cluding the nucleoprotein and the L polymerase (8, 23, 42). Indeed, we previously demonstrated that the interaction of TCRV Z protein with two noncontiguous regions in the L polymerase inhibits viral RNA replication and transcription (23, 52). Nevertheless, the precise molecular determinants in Z mediating its interaction with L have remained elusive.
In this study, we delimited the L-binding domain of Z pro-tein and identified residues in Z that are essential for the interaction with the L polymerase. Moreover, we present the first evidence of Z protein oligomerization within mammalian cells and define the requirements for Z self-association. Find-ings reported here indicate that homo-oligomerization is not required for the interaction between Z and L proteins.
MATERIALS AND METHODS
Cells, virus, and sera.BSR (a clone of BHK-21 cells) and CV1 cells were
grown in a 5% CO2atmosphere at 37°C by using Glasgow minimum essential
medium (Invitrogen) and Dulbecco’s modified Eagle’s medium (Invitrogen), respectively. Growth medium was supplemented with 10% fetal bovine serum
(FBS; Invitrogen) and penicillin (100 U/ml)-streptomycin (100g/ml)
(Invitro-gen). Recombinant vaccinia virus vTF7-3, which expresses the T7 RNA poly-merase (16), was kindly provided by Bernard Moss (National Institutes of Health, Bethesda, MD). Rabbit monospecific serum recognizing the recombi-nant TCRV L was obtained as described before (40). Primary antibodies used
were serum against glutathioneS-transferase (GST) (GE Healthcare), rabbit
antihemagglutinin (anti-HA) polyclonal antibody (Santa Cruz Biotechnology), and mouse anti-Flag M2 monoclonal antibody (MAb; Sigma-Aldrich).
Plasmids.Plasmid pWT expresses a TCRV minigenome RNA, consisting of
the S genome 5⬘untranslated region (UTR) sequence, followed by a
171-nucle-otide spacer sequence, the complete S intergenic sequence, and then the chlor-amphenicol acetyltransferase (CAT) open reading frame (ORF) in an antisense
orientation and the complete S genome 3⬘UTR sequence (29). pGEM
vector-based plasmids pN and pL (30) as well as pTM1 vector-vector-based plasmids pTacV N and pTacV L (8) encode the TCRV N and L proteins, respectively. Plasmids expressing the GST protein (pgst) and the GST-tagged TCRV Z protein (pgstZ) were described previously (23). Plasmid pgstZ(36-95) encodes a peptide com-prising residues 36 to 95 of TCRV Z fused at its N terminus to GST (23).
To generate plasmid pgstZ(36-85), a DNA fragment was obtained by PCR
using plasmid pgstZ as template. The forward primer contained (5⬘to 3⬘) a
sequence corresponding to nucleotides (nt) 107 to 128 of the TCRV Z ORF,
including a MfeI restriction site. The reverse primer contained (5⬘ to 3⬘) an
EcoRI site followed by the sequence complementary to two stop codons and to positions 255 to 237 of the TCRV Z ORF. The amplified fragment was digested with MfeI and EcoRI and inserted into pgstZ(36-95) previously digested with the same enzymes.
For plasmid pgstZ(40-95) construction, a DNA fragment encoding amino acids (aa) 40 to 95 of TCRV Z ORF was obtained by PCR, using plasmid pgstZ
as template. Forward primer contained (5⬘to 3⬘) a BamHI site followed by a start
codon, and the sequence comprised between nt 118 and 137 of the TCRV Z ORF. Reverse primer RevpGEM4 was described before (23). The PCR product was digested with BamHI and EcoRI, and the excised DNA fragment was inserted between the BamHI and EcoRI sites of plasmid pgst.
The pTM1 vector-based plasmids pTCRV Z-ha and pJUNV Z-ha, formerly designated pTacV Z-HA and pJunV Z-HA, respectively, express the HA-tagged version of TCRV Z protein and JUNV Z protein, respectively (8). Plasmids pTCRV Z-Flag and pJUNV Z-Flag express the Z protein from either TCRV or JUNV joined at their C termini with the Flag epitope (DYKDDDDK). For their construction, either pTCRV Z or pJUNV Z (8) was used as template to obtain the corresponding Flag-tagged ORF by PCR. Each PCR product was then cloned between the NcoI and SacI sites of the pTM1 vector (kindly provided by B. Moss).
Amino acid substitutions in the TCRV Z or JUNV Z ORFs were introduced
by using a QuikChange mutagenesis kit (Stratagene) with primers containing the mutated sequences and plasmid pgstZ, pgstZ(36-85), pTCRV Z-ha, or pJUNV Z-ha as template, as indicated. This strategy was also employed to introduce a stop codon in pgstZ(36-85) at positions corresponding to residue T82 or L80 of the TCRV ORF, generating plasmids pgstZ(36-81) and pgstZ(36-79), respec-tively. All constructs were checked by dideoxynucleotide double-stranded DNA sequencing (Macrogen Inc.). All primer sequences and vector maps are available upon request.
Plasmid pCMV-T7pol expresses the bacteriophage T7 RNA polymerase under the control of the cytomegalovirus promoter (39) and was kindly provided by Martin A. Billeter (University of Zurich, Irchel, Switzerland).
DNA transfections.Subconfluent monolayers of CV1 cells grown in 12-well dishes were infected with 3 to 5 PFU of vTF7-3 per cell. The inoculum was removed, and the cells were washed and then transfected with plasmid DNA, as follows. For Z-L interaction analysis (Fig. 1 and 2), 100 ng of pL and 200 ng of the plasmid expressing the GST-tagged Z protein (gstZ), GST, or each of the indicated mutant gstZ proteins were used per well. The amounts of plasmid DNA used for the evaluation of Z-Z interactions were (unless otherwise indi-cated) 500 ng of either pTCRV Z-Flag or pJUNV Z-Flag and 250 ng of the plasmids expressing wild-type or mutant HA-tagged versions of Z protein from either TCRV or JUNV. For cross-linking experiments, 500 ng of the indicated wild-type or mutant Z-expressing plasmid was added to the transfection mix.
For VLP generation, BSR cells grown in 12-well plates were transfected with
1g of pCMV-T7pol and 2g of plasmid pTCRV Z-ha per well. The
transfec-tion conditransfec-tions used for determinatransfec-tion of CAT activity are indicated in the legends to Fig. 1 and 5. All transfections were performed using Lipofectamine 2000 reagent (Invitrogen), according to the supplier’s instructions. When re-quired, the total amount of transfected DNA was kept constant by the addition of vector pTM1 DNA. All plasmids were purified by Qiagen tip-100 (Qiagen Inc.).
Analysis of protein interactions by coimmunoprecipitation.Radiolabeling of
transfected CV1 cells with [35
S]methionine-cysteine mix (150Ci/ml; NEG 772;
NEN PerkinElmer) and preparation of cell lysates were performed as previously
described (23). Aliquots corresponding to about 1⫻105to 2⫻105cells were
immunoprecipitated with either anti-L or anti-GST serum, and the immunopre-cipitated proteins were resolved by two-step SDS-PAGE in gels containing 8%
and 12% polyacrylamide, along with14
C-labeled markers, and then visualized by exposure to X-ray films (Kodak), as described previously (23). When indicated, quantification of the protein bands was performed by densitometry using ImageJ software (1). Z-L binding (Z-L%) corresponds to the amount of L coimmuno-precipitated with gstZ or gstZ mutants using anti-GST serum and normalized for L protein expression, as determined by immunoprecipitation with anti-L serum. Binding of gstZ or gstZ(36-85) to L was taken as 100%, as indicated.
For Z-Z interaction analysis, transfected CV1 cell monolayers were washed twice with phosphate-buffered saline (PBS) at 18 h posttransfection and then lysed by adding buffer RIPA (50 mM HCl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton X-100) containing protease inhibitors (2
g/ml aprotinin, 20g/ml phenylmethylsulfonyl fluoride, 50g/mlN-␣-tosyl-L
-lysine chloromethyl ketone [Sigma-Aldrich]). Cell lysates were clarified from
nuclei and cellular debris by centrifugation at 16,000⫻gfor 20 min at 4°C, and
aliquots of cytoplasmic extracts corresponding to about 2⫻105cells were
immunoprecipitated with either anti-HA antibody or anti-Flag M2 MAb, follow-ing the procedure previously described (8, 23). Immunoprecipitated proteins were resuspended in Novex Tricine sample buffer (Invitrogen), resolved by SDS-PAGE, and visualized by Western blotting.
Chemical cross-linking.Subconfluent monolayers of CV1 cells, transfected as indicated above, were washed twice with PBS at 18 h posttransfection and then incubated with 0.5 ml of PBS containing the indicated concentration of dithio-bis(succinimidyl propionate) (DSP; Thermo Scientific) for 2 h at 4°C. The cross-linking reaction was stopped by replacing the DSP solution with buffer Tris-HCl (20 mM, pH 7.4), followed by incubation of cell monolayers for 15 min at 4°C. Cells were harvested by scraping into buffer TEN (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA) and recovered by low-speed centrifugation. Cells were resuspended in Novex Tris-glycine sample buffer (Invitrogen) and subjected to three cycles of freezing and thawing. Proteins were resolved by denaturing SDS-PAGE after being incubated for 5 min at 100°C with or without addition of
-mercaptoethanol to a final concentration of 10%, as indicated, and then were
visualized by Western blotting.
Western blotting.Purified VLPs or cellular lysates were resolved by SDS-PAGE in gels containing 12% polyacrylamide and then transferred to a nitro-cellulose membrane. Immunoblotting was carried out following the procedure previously described (27). Detection was performed with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific), followed by exposure to X-ray
on November 7, 2019 by guest
http://jvi.asm.org/
films (Kodak). Horseradish peroxidase-conjugated antimouse or antirabbit sec-ondary antibodies were provided by Jackson ImmunoResearch.
CAT assay.CAT activity was assayed in extracts from transfected CV1 or BSR cells, as described previously (8, 30). CAT activity was calculated by determining the percentage of radioactivity associated with monoacetylated chloramphenicol species relative to total radioactivity, as described before (30).
Indirect immunofluorescence and confocal microscopy.BSR cells grown on
glass coverslips within 12-well dishes were transfected with 1g of pCMV-T7pol
and 0.5g of the indicated wild-type or mutant Z-expressing plasmid per well. At
24 h after transfection, cells were fixed, permeabilized, and then probed with rabbit anti-HA antibody, followed by incubation with Alexa Fluor 568 goat anti-rabbit immunoglobulin G (Invitrogen) to detect the HA-tagged Z proteins, as described previously (8, 27). Confocal images were collected using an LSM 510 Meta confocal microscope (Zeiss) with an X63 Plan-Apochromat oil immer-sion objective (numerical aperture, 1.4; Zeiss), as indicated before (27).
Modeling the L-binding domain of TCRV Z protein.A model of TCRV Z protein residues 36 to 81 was built with the Modeller program (version 9v8) (14), using LASV Z protein (Protein Data Bank [PDB] accession no. 2KO5) from residues 27 to 72 (51) as a template. The sequence identity is 50% in the modeled region. The cycle of realignment, modeling, and structure evaluation was re-peated until no further improvements on the structure were observed. Evalua-tion of the model with the Verify3D program (32) produced all positive scores, and assessment of the Ramachandran plot (31) showed that only 1 residue is in a disallowed region of the plot. The model was also evaluated by superposition with its own template structure using the combinatorial extension method (44), showing a root mean square deviation (RMSD) of 0.4 Å in a total of 46 out of 46 residues modeled. The above-described appraisal indicates the correctness of the model. All graphics were done with Chimera software (38).
RESULTS
Amino acids around the zinc coordination site I of TCRV Z protein are critical for binding L protein.In order to precisely map the L-binding domain within the TCRV Z protein, we initially introduced a series of deletions into a sequence en-coding residues 36 to 95 of TCRV Z fused to GST [gstZ(36-95); see Materials and Methods]. This fusion protein binds to L and mediates levels of viral RNA synthesis inhibition com-parable to those of either wild-type Z protein or its GST-tagged version (gstZ) (23). The binding of the truncated Z mutants to the L protein was evaluated by coimmunoprecipi-tation using antibodies against L or Z protein, as previously described (23). Control immunoprecipitations were performed using extracts from cells coexpressing L along with either GST or gstZ. The analysis of proteins precipitated with serum anti-L showed that similar levels of L were expressed under all the transfection conditions (Fig. 1, lower panels). Likewise, gstZ and each of the Z mutants were readily detected upon immu-noprecipitation of the lysates with the anti-GST antibody (Fig. 1, upper panels). As expected, L coimmunoprecipitated with gstZ (lane 1) but not with GST (lanes 5 and 9), which further confirmed the findings from previous studies indicating that
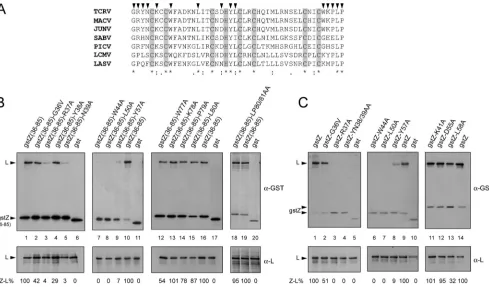
FIG. 1. Effect of deletions in TCRV Z on L binding and Z-mediated reporter gene expression inhibition. CV1 cell monolayers were transfected to express TCRV L protein (⫹), along with gstZ (lane 1) or each of the Z mutant proteins fused to GST, as indicated on top (lanes 2 to 4 and 6 to 8). As a control, L was coexpressed with GST (lanes 5 and 9). Cells were radiolabeled and lysed as indicated in Materials and Methods. Aliquots of the cell extracts were immunoprecipitated using serum against GST (␣-GST panels) or L (␣-L panels). The immunoprecipitated proteins were resolved by SDS-PAGE. Z-L binding values (Z-L%), calculated as indicated in Materials and Methods, correspond to the mean of three independent experiments, with relative intrasample variability ranging from 1% (lane 2) to 12% (lane 3). For determination of CAT activity (CAT%), vTF7-3-infected CV1 cell monolayers were transfected with plasmids expressing a TCRV minigenome RNA and TCRV N and L proteins (pWT, pN, and pL, respectively), along with plasmids expressing either GST, gstZ, or each of the indicated Z mutants, as previously described (23). CAT activities in transfected-cell lysates were quantified; mean values of three independent experiments are shown, with relative intrasample variability ranging from 2% (lane 2) to 14% (lane 4). Molecular masses of markers are indicated on the right of each panel.␣, anti.
on November 7, 2019 by guest
http://jvi.asm.org/
the interaction with L is mediated through the Z portion of the fusion protein (23). In agreement with previously published results, L coprecipitated with mutant gstZ(36-95) at levels sim-ilar to those detected for gstZ (compare lanes 1 and 2) (23). The results also revealed that residues 36 to 39 were required for Z to retain its L-binding capacity, as mutant gstZ(40-95) failed to coimmunoprecipitate with L (lane 4). Deletion of 10 residues from the C terminus of gstZ(36-95) led the resultant mutant, gstZ(36-85), to display wild-type levels of L binding (compare lane 3 to lanes 1 and 2), indicating that residues 86 to 95 were dispensable for this interaction. However, mutant gstZ(36-79), which carries a deletion comprising residues 80 to 95, was unable to bind L (lane 8), whereas mutant gstZ(36-81) coimmunoprecipitated with L at levels comparable to those of gstZ(36-85) (compare lanes 6 and 7), suggesting that the se-quence encompassing amino acids 36 to 81 is sufficient to sustain the binding of Z to L.
To further support these results, the ability of mutant Z proteins to inhibit viral RNA synthesis was measured in a TCRV minireplicon system, which reconstitutes viral transcrip-tion and replicatranscrip-tion from plasmid-supplied TCRV N and L proteins and a TCRV S RNA analog encoding the reporter
gene chloramphenicol acetyltransferase (CAT) (30). Quantifi-cation of the results (shown at the bottom of each panel in Fig. 1) evidenced that, as expected, mutant gstZ(36-95) displayed levels of reporter gene expression inhibition similar to those shown by wild-type gstZ (compare lanes 1 and 2 to the control without Z in lane 5). As predicted by the coimmunoprecipita-tion experiments (upper panels), wild-type levels of CAT ex-pression inhibition were also promoted by mutant gstZ(36-85) (lane 3). Moreover, mutant gstZ(36-81) displayed levels of CAT activity inhibition comparable to those of mutant gstZ(36-85) (lanes 6 and 7). In contrast, almost no inhibitory effect on CAT activity was observed in cells coexpressing mu-tant gstZ(40-95) or gstZ(36-79) along with the rest of the system components (lanes 4 and 8). These results, which were consistent with those presented above, further supported the suggestion that the minimal essential determinants for binding the L polymerase, as well as for viral RNA synthesis inhibition, are contained within the region of Z (here referred to as the L-binding domain) comprising residues 36 to 81.
Next, we sought to identify individual residues important for Z-L interaction within the L-binding domain. As it was previ-ously shown that the integrity of the RING structure is
neces-FIG. 2. Mapping of L-binding sites on the TCRV Z region comprising residues 36 to 81. (A) Multiple-sequence alignment of arenavirus Z protein sequences (Clustal method). The region shown corresponds to residues 36 to 81 of TCRV Z protein. GenBank accession numbers of the Z protein sequences used are as follows: NP_694847.1 (TCRV), AAT40445.1 (JUNV), NP_899214.1 (Machupo virus [MACV]), YP_089659.1 (Sabia virus [SABV]), YP_138535.1 (Pichinde virus [PICV]), AAX49343.1 (LCMV), and AAO59510.1 (LASV). Strictly conserved amino acids are marked with asterisks. The positions within the TCRV Z sequence that were mutated are indicated on top with arrowheads. Amino acids of the RING motif are shadowed. (B and C) CV1 cell monolayers were transfected with pL along with either pgstZ(36-85) (B, lanes 1, 10, 16, and 19), pgstZ (C, lanes 1, 9, and 14), the plasmids expressing each of the gstZ(36-85) mutants (B, lanes 2 to 5, 7 to 9, 12 to 15, and 18), or those expressing gstZ mutants (C, lanes 2 to 4, 6 to 8, and 11 to 13), as indicated. In lanes 6, 11, 17, and 20 (B) and lanes 5 and 10 (C), pL was cotransfected with the GST-expressing plasmid. Aliquots of the cell lysates were immunoprecipitated using monospecific serum against either GST (␣-GST, top panels) or L (␣-L, bottom panels). The immunoprecipitated proteins were resolved by SDS-PAGE, and Z-L binding (Z-L%) was calculated as described in Materials and Methods. Mean values of three independent experiments are shown; standard deviations ranged from 4 to 10%.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.47.536.68.355.2]sary for Z-mediated inhibition of viral RNA synthesis and Z-L interactions (11, 23), the conserved cysteine and histidine res-idues that define the RING motif of TCRV Z were excluded from this analysis. To examine the relevance of the sequences flanking the RING domain for L binding, amino acids 36 to 39 and 77 to 80 were individually changed in gstZ(36-85), gener-ating mutants 85)-G36V, 85)-R37A, 85)-Y38A, 85)-N39A, 85)-W77A, gstZ(36-85)-K78A, gstZ(36-85)-P79A, and gstZ(36-85)-L80A (Fig. 2A). Also, a double alanine substitution was introduced at positions 80 and 81, producing mutant gstZ(36-85)-LP80/ 81AA. To identify strictly conserved residues within the RING domain that could contribute to the Z-L interaction, a group of representative arenavirus Z protein sequences were aligned and a number of amino acids were selected as targets for site-directed mutagenesis (Fig. 2A). Thus, residues W44, L50, and Y57, which are strictly conserved in all arenavirus Z se-quences, were individually changed to alanine in gstZ(36-85). Then, the ability of Z mutants to interact with L was analyzed in coimmunoprecipitation experiments (Fig. 2B; the estimated L-binding capacities are indicated at the bottom). Mutants gstZ(36-85)-K78A, gstZ(36-85)-P79A, gstZ(36-85)-L80A, and gstZ(36-85)-LP80/81AA exhibited levels of interaction with L protein similar to those observed for gstZ(36-85) (compare lanes 13 to 15 to lane 16 and lane 18 to lane 19). A second set of mutants, including gstZ(36-85)-G36V, gstZ(36-85)-Y38A, and gstZ(36-85)-W77A mutants, showed capacities to bind L that, albeit reduced, were still measurable (lanes 2, 4, and 12). In contrast, substitution of alanine for residue R37, N39, W44, L50, or Y57 completely abolished or drastically impaired the ability of the resulting Z mutant proteins to associate with L (lanes 3, 5, and 7 to 9). These results indicated that C-terminal residues K78, P79, L80, and P81 appeared to be unessential for L binding and revealed that while residues G36, Y38, and W77 would contribute partially, the conserved residues W44, L50, and Y57 within the RING domain, as well as N-terminal amino acids R37 and N39, were crucial for Z-L interaction.
To rule out the possibility that the observed defects in Z-L interaction were due to structural constraints resulting from the close proximity of the GST tag to the N-terminal region of Z(36-85), single or double substitutions were generated at crit-ical positions in full-length gstZ, and the ability of the resulting mutant proteins to interact with the L polymerase was evalu-ated as before (Fig. 2C). Consistent with the results presented in Fig. 2B, binding of mutant gstZ-G36V to L was 50% that of wild-type gstZ (Fig. 2C, lane 2). Moreover, little or no binding between L and mutant R37A, YN38/39AA, gstZ-W44A, gstZ-L50A, or gstZ-Y57A was detected (lanes 3, 4, and 6 to 8). These results further supported the conclusion that Z residues R37, N39, W44, L50, and Y57 are critical for binding L protein.
We then examined the contribution of other well-conserved residues located near critical amino acids within the RING domain to the interaction with L. Residues K41, D55, and L58 (Fig. 2A) were individually replaced with alanine in gstZ, and the resulting mutants were analyzed for their capacity to bind L by coimmunoprecipitation. The results showed that mutants gstZ-K41A and gstZ-D55A displayed almost unaffected abili-ties to coimmunoprecipitate with L (Fig. 2C, lanes 11 and 12),
while levels of L binding about 30% those of gstZ were exhib-ited by mutant gstZ-L58A (Fig. 2C, lane 13).
Altogether, these results demonstrated that whereas amino acids G36, Y38, L58, and W77 would contribute at some extent to the L-binding properties of Z, residues R37, N39, W44, L50, and Y57 play an essential role in Z-L interaction.
The G residue at position 2, the N-terminal acceptor for protein myristoylation, is required for Z homo-oligomeriza-tion.Several lines of evidence indicate that the Z protein plays multiple roles in the arenavirus life cycle. Nevertheless, no data indicating that Z can form oligomers in mammalian cells with-out the participation of other viral proteins were available. Furthermore, whether monomeric or oligomeric Z was able to interact with L was a matter of speculation. Accordingly, we first examined whether Z protein self-associates both in cells and in Z-containing VLPs. We used plasmid pTCRV Z-ha (see Materials and Methods), which encodes an HA-tagged version of TCRV Z, as it was previously demonstrated that short C-terminal tags do not interfere with Z protein self-budding ability (8, 27, 36). BSR cells were transfected with pTCRV Z-ha, cell lysates were prepared, and culture supernatants were collected and ultracentrifuged on sucrose gradients to purify VLPs. Samples were divided into aliquots that were either heated at 100°C or incubated at room temperature and then resolved by SDS-PAGE and analyzed by Western blotting us-ing anti-HA antibody (Fig. 3A). Consistent with previous find-ings (23), monomeric Z protein migrated as a major band with an apparent molecular mass of nearly 14 kDa both in cells and in VLPs (Fig. 3A). In addition to Z monomeric form, two bands migrating near the 29-kDa and the 38-kDa markers, respectively, were visualized both in cell lysates and in VLPs, upon incubation of samples at room temperature prior to SDS-PAGE (lanes 2 and 4). Heating at 100°C caused these two bands to be strongly reduced in VLPs and completely absent from cell lysates (lanes 1 and 3). Thus, these results suggested that Z could self-associate to form oligomers.
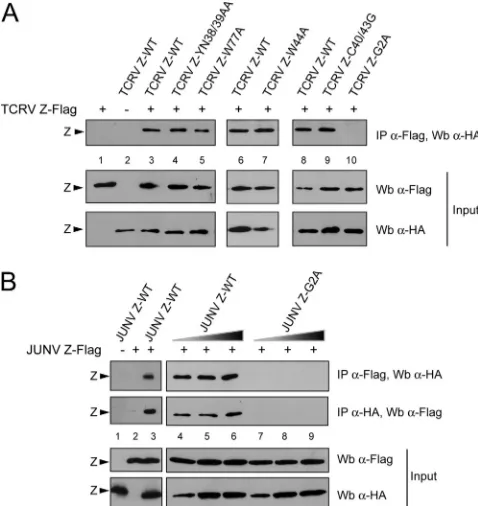
Next, Z-Z interactions were studied by coimmunoprecipita-tion (Fig. 3B). In order to avoid nonspecific oligomerizacoimmunoprecipita-tion that could be associated with the GST moiety, we did not use gst-fused Z protein in these experiments. Instead, lysates from cells expressing TCRV Z-ha along with a Flag-tagged version of TCRV Z (TCRV Z-Flag) were subjected to immunopre-cipitation with anti-HA or anti-Flag antibody. Control immu-noprecipitations were carried out using extracts from cells expressing either protein alone. Western blotting of the pre-cipitated proteins (IP panels) revealed that Z-Flag and Z-ha specifically coimmunoprecipitated (lane 3), since essentially no Z-Flag was precipitated with serum against HA and no Z-ha was pulled down with anti-Flag antibody from control lysates (lanes 2 and 1, respectively). These results confirmed that Z has the ability to self-interact.
We then investigated the requirements for TCRV Z protein self-binding. To this aim, we generated a panel of Z mutants by introducing single or double substitutions within TCRV Z-ha (hereinafter, TCRV Z wild type [TCRV Z-WT]). Thus, changes that totally disrupted (W44A and YN38/39AA) or partially affected (W77A) Z-L interactions (Fig. 2) were made to obtain mutants TCRV Z-W44A, TCRV Z-YN38/39AA, and TCRV Z-W77A. To examine the relevance of the RING struc-ture for Z-Z interaction in mammalian cells, mutant TCRV
on November 7, 2019 by guest
http://jvi.asm.org/
Z-C40/43G, which carries a double substitution at positions C40 and C43 (Fig. 2A) that disrupts the RING folding, was also constructed. In addition, we generated a mutant in which the strictly conserved G at position 2 was changed to alanine (TCRV Z-G2A). Because the 35 N-terminal residues of TCRV Z were found to be dispensable for its association to L (Fig. 1), mutant TCRV Z-G2A was predicted to fully retain the L-binding capacity of wild-type Z.
Then, Z mutants were assayed for their ability to self-inter-act by coimmunoprecipitation. Plasmids encoding TCRV Z-WT or each of the Z mutants were transfected along with the TCRV Z-Flag-expressing plasmid. Western blot analysis of the cell lysates using either anti-HA or anti-Flag antibody confirmed that all the mutants were expressed at levels com-parable to those of Z-WT and that Z-Flag was detected at similar levels under all the transfection conditions (Fig. 4A, Input). Additional aliquots of the cell extracts were incubated with anti-Flag antibody, and the precipitated proteins were analyzed by immunoblotting, using anti-HA antibody. As shown in Fig. 4A (IP panel), both mutants Z-YN38/39AA and Z-W44A as well as mutant Z-W77A coimmunoprecipitated with Z-Flag at levels comparable to those of wild type Z-ha (compare lanes 4 and 5 to lane 3 and lane 7 to lane 6),
indicating that residues Y38, N39, W44, and W77 were not required for Z-Z interactions. Similarly, mutant protein TCRV Z-C40/43G, which is unable to bind L (23), displayed wild-type levels of self-interaction (compare lane 9 to lane 8). In con-trast, mutation of the G residue at position 2 critically impaired the ability of Z to self-associate, as very low or undetectable levels of mutant TCRV Z-G2A were coprecipitated with TCRV Z-Flag (lanes 8 and 10). The results for controls con-firmed that Z-Flag and Z-ha were not cross-detected by anti-HA and anti-Flag antibodies, respectively (lanes 1 and 2). These results indicated that neither the integrity of the RING structure nor key residues required for Z-L contacts were involved in Z-Z interaction. Moreover, the data revealed that amino acid G2, which is the N-terminal acceptor residue for protein myristoylation and is dispensable for binding L protein, is required for Z self-association.
[image:6.585.300.539.70.323.2]In order to assess whether our observations could be ex-tended to Z protein from the pathogenic JUNV, similar coim-munoprecipitation experiments were carried out (Fig. 4B). The results showed that wild-type JUNV Z-ha (JUNV Z-WT) specifically coimmunoprecipitated with JUNV Z-Flag (lanes 1 to 3). Moreover, upon coexpression of JUNV Z-Flag with
FIG. 3. Z protein self-interacts. (A) BSR cells expressing TCRV Z-ha were lysed at 48 h posttransfection in nonreducing SDS-PAGE sample buffer (Invitrogen), and VLPs were purified from the culture supernatants, as described before (8). After addition of -mercapto-ethanol, aliquots corresponding to about 1⫻104to 5⫻104cells from
both cell extracts and VLPs were incubated at either 100°C or room temperature (RT) for 5 min and then subjected to SDS-PAGE. Re-solved proteins were analyzed by Western blotting using anti-HA an-tibody. The molecular masses of the markers are indicated on the right. (B) CV1 cells were transfected with plasmids pTCRV Z-ha and pTCRV Z-Flag, either separately (lanes 1 and 2) or together (lane 3), as indicated. Cell extracts, obtained at 24 h posttransfection, were analyzed by Western blotting (Wb) with the indicated antibody either before (Input) or after being immunoprecipitated with anti-Flag (IP ␣-Flag) or anti-HA (IP␣-HA) antibody.
FIG. 4. Effect of substitutions in Z on Z-self interaction. (A) CV1 cells were transfected with pTCRV Z-Flag (⫹) along with the plasmids expressing TCRV Z-ha (TCRV Z-WT) (lanes 3, 6, and 8) or each of the indicated TCRV Z-ha mutants (lanes 4, 5, 7, 9, and 10). As a control, pTCRV Z-Flag (lane 1) and pTCRV Z-ha (lane 2) were transfected alone. (B) CV1 cell monolayers were transfected with pJUNV Z-Flag (⫹) along with 120 ng (lanes 4 and 7), 250 ng (lanes 5 and 8), or 500 ng (lanes 6 and 9) of the plasmids encoding either wild-type JUNV Z-ha (JUNV Z-WT) or mutant JUNV Z-G2A, as shown. Control monolayers were transfected with pJUNV Z-Flag (lane 2) or the JUNV Z-WT-expressing plasmid (lane 1) alone. (A and B) Aliquots of the cell extracts were analyzed by Western blotting (Wb) with the indicated antibody, either before (Input) or after being immunoprecipitated with anti-Flag (IP␣-Flag) or anti-HA (IP␣-HA) antibody, as indicated.
on November 7, 2019 by guest
http://jvi.asm.org/
increasing amounts of mutant JUNV Z-G2A, no coimmuno-precipitation was detected, indicating that substitution of ala-nine for residue G2 completely abrogated JUNV Z self-bind-ing capacity (compare lanes 7 to 9 with lanes 4 to 6). It is interesting to note that previously generated mutant JUNV Z proteins with changes at positions C39/42 (which disrupt the RING structure), G35, L79, or P80, or within the PTAP motif (8), as well as JUNV Z mutant proteins carrying a single alanine substitution of residue K40, W43, L49, Y56, or L57, exhibited unmodified capacities to self-interact compared to JUNV Z-WT (data not shown). Altogether, these results dem-onstrated that, as shown for TCRV Z, the G residue at position 2 appeared to be essential for JUNV Z self-interaction, while other residues or the RING structure did not.
[image:7.585.79.502.69.409.2]We then investigated the possibility that mutant proteins TCRV Z-G2A and JUNV Z-G2A could form oligomers that, being unstable, would be undetectable by coimmunoprecipita-tion. In order to stabilize such hypothetical Z-Z interactions we used DSP, which is a thiol-cleavable and membrane-per-meant cross-linking reagent (spacer arm of 12 Å). Cells ex-pressing Z-WT or mutant Z-G2A from either TCRV or JUNV were incubated with the addition of DSP in the culture me-dium. Aliquots of total cell extracts were subsequently sepa-rated in nonreducing SDS-polyacrylamide gels, followed by immunoblotting with anti-HA antiserum (Fig. 5A). In addition to Z monomer, protein complexes that may correspond to dimeric (28 kDa) and trimeric (42 kDa) forms of Z were observed upon incubation of cells transiently expressing TCRV
FIG. 5. Mutation of residue G2 to alanine affects self-association and intracellular localization of Z protein. (A and B) Analysis of Z-Z interaction by chemical cross-linking. CV1 cells were transfected with plasmids encoding TCRV Z-ha (TCRV Z-WT; A, lanes 1 to 4; B, lanes 1 and 5), TCRV Z-G2A (A, lanes 5 to 8), JUNV Z-ha (JUNV Z-WT; A, lane 9), JUNV Z-G2A (A, lane 10), or each of the mutant TCRV Z-ha proteins indicated in panel B. Cells were incubated with DSP at the concentrations indicated below each panel, as described in Materials and Methods. Aliquots of the DSP-treated cell lysates were incubated for 5 min at 100°C with (B, lane 5) or without (A, lanes 1 to 10; B, lanes 1 to 4)-mercaptoethanol (M-OH) and then resolved on SDS-polyacrylamide gels and analyzed by Western blotting, using anti-HA antibody. Bands consistent with monomeric (I), dimeric (II), trimeric (III), or tetrameric (IV) forms of Z are indicated. The molecular masses of markers are indicated on the right of each panel. (C) Intracellular distribution of Z-WT and mutant Z-G2A proteins. BSR cells expressing TCRV Z-WT (frame A), TCRV Z-G2A (frame B), JUNV Z-WT (frame C), or JUNV Z-G2A (frame D) were fixed, and the HA-tagged Z proteins were stained by immunofluorescence and visualized by confocal microscopy as indicated in Materials and Methods. (D) Mutant Z-G2A mediates wild-type levels of polymerase activity inhibition. BSR cells grown in 12-well plates were transfected with 1g of pCMV-T7pol, 3g of pWT, 3g of pTacV N, and 1g of pTacV L. When indicated, 0.15g of either pTCRV Z-ha (Z-WT) or the plasmids encoding each of the mutant TCRV Z proteins was added to the transfection mix. Cell lysates, obtained at 48 h posttransfection, were used to assay CAT activity (CAT%), as indicated in Materials and Methods. Values correspond to the mean of two independent experiments; relative intrasample variability ranged from 1% to 4%. MAc, monoacetylated chloramphenicol; NAc, nonacetylated chloramphenicol.
on November 7, 2019 by guest
http://jvi.asm.org/
Z-WT with 0.1 mM DSP (lane 2). Treatment with increasing concentrations of DSP led to the detection of larger amounts of trimers as well as less resolved higher oligomeric forms of TCRV Z-WT (lanes 3 and 4). In contrast, mutant TCRV Z-G2A was predominantly detected as a monomer, and small amounts of dimeric and essentially no trimeric or higher oligomeric forms were observed in TCRV Z-G2A-expressing cells incubated with DSP at concentrations up to 0.5 mM (lanes 6 to 8). Cross-linking experiments also revealed that mutant JUNV Z-G2A exhibited a severely impaired ability to form homo-oligomers compared to JUNV Z-WT (compare lanes 9 and 10). Further cross-linking analysis (Fig. 5B) showed that, as observed for TCRV Z-WT (lane 1), mutant proteins TCRV Z-YN38/39AA, TCRV Z-W44A, and TCRV Z-W77A were detected as a ladder of protein bands displaying apparent molecular masses consistent with monomeric, dimeric, trim-eric, tetramtrim-eric, and higher oligomeric forms of Z (lanes 2 to 4). Treatment with -mercaptoethanol prior to SDS-PAGE caused the cross-linking bonds to be mostly reduced (shown for TCRV Z-WT in lane 5), confirming the involvement of disul-fide bonds in cross-linked Z protein complex formation. Re-markably, the putative dimer, which was observed to be the most prominent band among Z complexes (Fig. 5B, lanes 1 to 4; see also Fig. 5A), was still detectable after reduction (lane 5), suggesting that intermonomeric interactions within the dimer would be very stable. These results clearly showed that wild-type Z from TCRV or JUNV as well as mutants TCRV Z-YN38/39AA, TCRV Z-W44A, and TCRV Z-W77A were able to self-cross-link into oligomeric forms. Moreover, these data confirmed that the conserved residue G at position 2 in both JUNV Z and TCRV Z proteins is required for Z homo-oligomerization in mammalian cells.
Previous studies have shown that residue G2 is crucial for association of LASV Z to cellular membranes (7, 47). In order to examine the relevance of residue G2 for the plasma mem-brane localization of TCRV Z and JUNV Z proteins, BSR cells were transfected with plasmids encoding WT or G2A-mutated Z proteins, and their intracellular distribution was analyzed by indirect immunofluorescence. As illustrated by representative cells displayed in Fig. 5C, both TCRV Z-WT and JUNV Z-WT proteins accumulated at the plasma mem-brane (frames A and C). In contrast, mutants TCRV Z-G2A (frame B) and JUNV Z-G2A (frame D) showed no distinct plasma membrane localization but exhibited a diffuse cytoplas-mic distribution pattern. Thus, these results indicated that res-idue G2 is required for plasma membrane targeting of both TCRV Z and JUNV Z proteins.
Finally, control experiments were carried out in which Z mutants were assayed for their abilities to repress viral RNA synthesis by using a previously established TCRV minirep-licon system (8). As shown in Fig. 5D, mutants TCRV Z-YN38/39AA, TCRV Z-W44A, and TCRV Z-C40/43G failed to inhibit CAT expression, whereas mutant TCRV Z-G2A displayed levels of reporter gene expression inhibi-tion comparable to those of Z-WT.
Taken together, these results indicated that association of Z to cellular membranes and homo-oligomerization are not re-quired for Z to recognize and bind the L polymerase.
DISCUSSION
Arenavirus Z protein is involved in a number of protein-protein interactions that are important at different steps during the viral life cycle. To gain insight into Z protein functions, we have analyzed the molecular determinants involved in the rec-ognition of TCRV Z with the L polymerase, as well as those engaged in Z homo-oligomerization.
Residues within the region comprising residues 36 to 81 of TCRV Z protein are critical for L binding. Previous studies showed that the RING domain, although necessary, is not sufficient for Z-mediated inhibition of RNA synthesis by the L polymerase (11, 23). In the case of TCRV Z, the C-terminal sequence comprising residues 78 to 95 was suggested to harbor important determinants for Z-L interaction (23). Here we present new data demonstrating that the region spanning res-idues 36 to 81 of TCRV Z contains all the molecular determi-nants required for L polymerase binding and for viral RNA synthesis inhibition. This 46-residue region, defined here as the L-binding domain, comprises the entire RING domain plus 4 N-terminal (GRYN) and 5 C-terminal (WKPLP) residues (Fig. 1 and 2A).
Key residues within the L-binding domain were identified by analyzing a panel of mutant Z proteins for their L-binding capacity. Examination of the mutant carrying substitutions at amino acids located C terminal to the RING revealed that while mutant gstZ(36-85)-W77A showed a somewhat reduced ability to associate with the L polymerase compared to the wild-type protein, K78A, P79A, L80A, and LP80/81AA substi-tutions had little or no effect on Z-L binding (Fig. 2). These results suggest that residues K78, P79, L80, and P81 would be not directly engaged in binding L protein. However, as deletion of the conserved residues L80 and P81 abrogated the L-bind-ing ability of Z (Fig. 1), it seems likely that they may be required for maintaining the overall structure of the L-binding domain. An earlier report showed that residue P72 of LASV Z, which is homologous to P81 of TCRV Z, is required for LASV Z-mediated inhibition of reporter gene expression in an LCMV-minireplicon system (6). The apparent discrepancies with our results could be explained by consideration of the fact that these analyses were carried out in a heterologous context. Alternatively, the observed differences might be linked to the amino acid sequence divergence of the L and Z proteins from Old World and New World arenaviruses.
The integrity of the Z RING structure has been demon-strated to be required for many of the protein-protein inter-actions in which Z protein is involved, including its interaction with L (8, 23, 24, 48, 51). However, with the exception of amino acid W36 of LCMV Z, which was implicated in Z-mediated inhibition of LCMV polymerase activity (11), no other residues within the RING domain had been found to be engaged in L binding. In this work, we went further by demonstrating not only that residue W44 of TCRV Z (homologous to W36 of LCMV Z) is required but also that other conserved residues, namely, L50, Y57, and, to a lesser extent, L58, are important for direct Z-L association (Fig. 2).
TCRV Z residues Y57 and L58 are comprised in a sequence motif, YLCX (where X denotes any amino acid), which is conserved within the RING domain of all known arenavirus Z proteins. Recent studies showed that mutation of Y57 in
on November 7, 2019 by guest
http://jvi.asm.org/
TCRV Z almost completely blocked the incorporation of N protein into Z-directed VLPs, though it had no effect on Z budding activity (18, 50). Similar results were observed for Mopeia virus Z protein, whose YLCL sequence was also shown to be needed for its interaction with a cellular protein (AIP1) of the multivesicular body pathway (45). In light of our findings demonstrating that TCRV Z residues Y57 and L58 are important for L binding (Fig. 2), a more complex role could be envisaged for the YLCX motif of arenavirus Z protein.
Residues located around the zinc-binding site I (positions 30 to 39) of LASV Z have been shown to interact with eIF4E (51). Z-eIF4E interaction reduces the affinity of eIF4E for its ligand, the 7-methylguanosine cap (m7G cap) on the 5⬘ end of
mRNAs, resulting in selective translation repression (5, 24). Interestingly, the homologous region in TCRV Z (residues N39 to K48) includes amino acids N39 and W44 and is located next to residues G36, R37, and Y38, all of which play a critical role in L binding (Fig. 2), suggesting that Z-L and Z-eIF4E interactions may be mutually exclusive. It would be of great interest to investigate whether these interactions are modu-lated to maintain the balance between viral replication and cell growth control that may be required for arenavirus chronic infections.
In order to provide a hypothesis on the contribution of key residues to Z-L interaction, we built a model of the TCRV Z protein region comprising residues 36 to 81 (L-binding do-main), taken as a template LASV L protein from residues 27 to 72 (PDB accession no. 2KO5). Superimposition of the three-dimensional (3D) model with the template showed that not only the cysteine and histidine residues that coordinate the two zinc atoms but also the conserved residues analyzed in this work, i.e., G36, K41, W44, L50, Y57, and L58, display the same orientation as the corresponding residue in the LASV Z struc-ture (shown for W44, L50, and Y57 in Fig. 6). Interestingly, in both the template and the modeled structures, residues L50 and Y57 are located at a physical contact distance (⬍5 Å). Moreover, in the TCRV Z model, these amino acids are also
predicted to be in contact with residue Y38 (Fig. 6). On the basis of these observations, it is tempting to speculate that the conserved residues L50 and Y57 as well as residue Y38 might be engaged in hydrophobic interactions required for protein structure stabilization. Consistent with our results (Fig. 2), the 3D model also predicts that residues R37 and N39, as well as residue W44, are exposed and therefore available for interac-tion with L (Fig. 6).
Molecular determinants of Z homo-oligomerization.To gain insight into the molecular mechanism governing the protein-protein interactions in which Z is involved, we characterized the oligomeric state of TCRV Z protein in mammalian cells. Specifically, we employed SDS-PAGE under either nonboiling or denaturing conditions, coimmunoprecipitation assays, and chemical cross-linking to demonstrate that both TCRV Z and JUNV Z proteins self-associate into oligomeric forms consis-tent with Z dimers, trimers, tetramers, and higher-order com-plexes (Fig. 3 to 5). Interestingly, the major oligomeric form was represented by the putative dimer, which was resistant to reduction (Fig. 5), suggesting that it may be stabilized by strong intradimeric interactions. Overall, our findings suggest that Z homo-oligomerization may result from consecutive addition of monomeric building blocks.
Mutation of the strictly conserved residue G at position 2 drastically impaired the capacity of Z protein from either TCRV or JUNV to self-associate into oligomers, indicating that amino acid G2 plays a critical role in Z-Z interaction. In contrast, mutants TCRV Z-C40/43G, TCRV Z-YN38/39AA, TCRV Z-W44A, and TCRV Z-W77A showed wild-type abil-ities to self-interact (Fig. 4 and 5). These results indicate that neither the RING structure nor residues W44 and N39 (criti-cally involved in L binding) are important for Z homo-oli-gomerization. Thus, it may be concluded from our data that Z homo-oligomerization is not required for L recognition. This conclusion is further supported by the fact that change of residue G2 did not alter the ability of Z to inhibit TCRV minigenome replication (Fig. 5D), suggesting that Z monomer might represent the functional unit for Z-mediated polymerase activity regulation.
[image:9.585.45.280.69.238.2]Myristoylation of Z protein has been demonstrated to be essential for the role of Z on viral morphogenesis. Indeed, mutation of the strictly conserved G at position 2, which is the acceptor site for myristate, abolishes the association of Z to cell membrane, as well as Z-mediated budding (8, 37, 47, 50). In accordance with these data, we found that mutants TCRV Z-G2A and JUNV Z-G2A were not membrane associated, whereas wild-type TCRV Z and JUNV Z proteins exhibited a well-defined plasma membrane localization (Fig. 5C). These results, along with our finding that the amino acid G2 is es-sential for Z protein homo-oligomerization, strongly suggest that this process is dependent on protein myristoylation and cell membrane targeting. One possible explanation is that as-sociation with the plasma membrane may enhance the local concentration of Z, facilitating homomultimerization through yet-undefined protein-protein contacts. Alternatively, mem-brane targeting might induce conformational changes in Z, thus triggering its homo-oligomerization, which could then sta-bilize membrane binding, as suggested for Ebola virus VP40 and HIV-1 Gag proteins (21, 28, 35, 43). The fact that change of residue G2 to alanine results in a budding-incompetent
FIG. 6. Modeling the L-binding domain of TCRV Z. Ribbon rep-resentation of the 3D model of the region spanning residues 36 to 81 of TCRV Z protein (orange), superimposed on the LASV Z structure (gray; PDB accession no. 2KO5). Zinc atoms are shown as light blue spheres. Relevant residues are displayed in stick mode. In the 3D model, residues predicted to be exposed are shown in yellow.
on November 7, 2019 by guest
http://jvi.asm.org/
phenotype (8, 37, 47, 50), while mutation of other residues examined in this report, including Y38, N39, W44, and W77, does not (data not shown), implicates that Z homo-oligomer-ization may be an essential step for particle assembly and budding. Interestingly, substitution of alanine for residue G2 did not affect the interaction between Z and N, as demon-strated by confocal microscopy (data not shown), suggesting that homo-oligomerization and plasma membrane targeting of Z are not required for its association to N protein.
In conclusion, using biochemical and functional analyses, we demonstrate that the L-binding domain of TCRV Z encom-passes residues 36 to 81. Within this region, residues R37, N39, W44, L50, and Y57, located around the zinc coordination site I of the RING domain, are critical for Z-L interaction. Mo-lecular modeling of the L-binding domain of TCRV Z suggests that residues R37, N39, and W44 might be directly involved in this interaction. Our results also demonstrate that arenavirus Z protein undergoes homo-oligomerization in mammalian cells and support the notion that myristoylation and membrane association are required for this process. An implication of these findings is that Z homo-oligomerization of the other members of the Arenaviridae family may present similar re-quirements. Finally, from the reported data, it emerges that homo-oligomerization and targeting of Z protein to the plasma membrane are not essential for its interaction with the L poly-merase.
A better understanding of the mechanisms by which protein-protein interactions involving Z are modulated may be used for the rational design of novel antiviral therapies against the highly pathogenic arenaviruses.
ACKNOWLEDGMENTS
We especially thank Osvaldo Rey (University of California, Los Angeles, CA), Andrea Gamarnik (Fundacio´n Instituto Leloir, Buenos Aires, Argentina), and María Teresa Franze-Ferna´ndez (University of Buenos Aires, Buenos Aires, Argentina) for helpful discussions and critical readings of the manuscript. We also thank Guillermo A. Go-mez (University of Queensland, Australia) for imaging advice. We are grateful to Martin A. Billeter (University of Zurich, Irchel, Switzer-land) and Bernard Moss (National Institutes of Health, Bethesda, MD) for generously providing reagents. The technical assistance of J. Acevedo and S. Rojana is acknowledged.
This work was supported by the Agencia Nacional de Promocio´n Científica y Tecnolo´gica (ANPCyT) and CONICET. M.E.L. is a re-cipient of a fellowship from CONICET. M.W. and S.F. are rere-cipients of a fellowship from ANPCyT (grants 2378/2006 and 1931/2008).
A.D., C.M.B., and N.L. are research investigators of CONICET.
REFERENCES
1.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram.2004. Image processing
with Image J. Biophotonics Int.11:36–42.
2.Borden, K. L., E. J. Campbell Dwyer, and M. S. Salvato.1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol.
72:758–766.
3.Borden, K. L., E. J. Campbell Dwyer, G. W. Carlile, M. Djavani, and M. S. Salvato.1998. Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P
proteins. J. Virol.72:3819–3826.
4.Buchmeier, M. J., J. C. de La Torre, and C. J. Peters.2007.Arenaviridae: the
viruses and their replication, p. 1791–1828.InD. M. Knipe et al. (ed.), Fields
virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA. 5.Campbell Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L.
Borden.2000. The lymphocytic choriomeningitis virus RING protein Z as-sociates with eukaryotic initiation factor 4E and selectively represses
trans-lation in a RING-dependent manner. J. Virol.74:3293–3300.
6.Capul, A. A., J. C. de la Torre, and M. J. Buchmeier.2011. Conserved
residues in Lassa fever virus Z protein modulate viral infectivity at the level
of the ribonucleoprotein. J. Virol.85:3172–3178.
7.Capul, A. A., et al.2007. Arenavirus Z-glycoprotein association requires Z
myristoylation but not functional RING or late domains. J. Virol.81:9451–
9460.
8.Casabona, J. C., J. M. Levingston Macleod, M. E. Loureiro, G. A. Gomez, and N. Lopez.2009. The RING domain and the L79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of
gly-coproteins into infectious chimeric arenavirus-like particles. J. Virol. 83:
7029–7039.
9.Charrel, R. N., X. de Lamballerie, and S. Emonet.2008. Phylogeny of the
genus Arenavirus. Curr. Opin. Microbiol.11:362–368.
10.Compans, R. W.1993. Arenavirus ultrastructure and morphogenesis, p.
3–16.InM. S. Salvato (ed.), TheArenaviridae. Plenum Press, New York, NY.
11.Cornu, T. I., and J. C. de la Torre.2002. Characterization of the arenavirus RING finger Z protein regions required for Z-mediated inhibition of viral
RNA synthesis. J. Virol.76:6678–6688.
12.Djavani, M., et al.2005. The proline-rich homeodomain (PRH/HEX) pro-tein is down-regulated in liver during infection with lymphocytic
choriomen-ingitis virus. J. Virol.79:2461–2473.
13.Enria, D. A., A. M. Briggiler, and Z. Sanchez.2008. Treatment of Argentine
hemorrhagic fever. Antiviral Res.78:132–139.
14.Eswar, N., et al.2006. Comparative protein structure modeling using
Mod-eller. Curr. Protoc. Bioinformatics Chapter5:Unit 5.6.
15.Franze-Ferna´ndez, M. T., S. Iapalucci, N. Lo´pez, and C. Rossi.1993.
Sub-genomic RNAs of Tacaribe virus, p. 113–132.InM. S. Salvato (ed.), The
Arenaviridae. Plenum Press, New York, NY.
16.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss.1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that syn-thesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. U. S. A.
83:8122–8126.
17.Graham, S. C., et al.2008. Rhabdovirus matrix protein structures reveal a
novel mode of self-association. PLoS Pathog.4:e1000251.
18.Groseth, A., S. Wolff, T. Strecker, T. Hoenen, and S. Becker.2010. Efficient budding of the Tacaribe virus matrix protein Z requires the nucleoprotein.
J. Virol.84:3603–3611.
19.Hartlieb, B., and W. Weissenhorn.2006. Filovirus assembly and budding.
Virology344:64–70.
20.Hass, M., U. Golnitz, S. Muller, B. Becker-Ziaja, and S. Gunther.2004.
Replicon system for Lassa virus. J. Virol.78:13793–13803.
21.Hoenen, T., et al.2010. Oligomerization of Ebola virus VP40 is essential for
particle morphogenesis and regulation of viral transcription. J. Virol.84:
7053–7063.
22.Iapalucci, S., et al.1989. The 5⬘region of Tacaribe virus L RNA encodes a
protein with a potential metal binding domain. Virology173:357–361.
23.Ja´camo, R., N. Lopez, M. Wilda, and M. T. Franze-Fernandez.2003. Tac-aribe virus Z protein interacts with the L polymerase protein to inhibit viral
RNA synthesis. J. Virol.77:10383–10393.
24.Kentsis, A., et al.2001. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly
inhibiting translation initiation factor eIF4E. J. Mol. Biol.312:609–623.
25.Kentsis, A., R. E. Gordon, and K. L. Borden.2002. Self-assembly properties
of a model RING domain. Proc. Natl. Acad. Sci. U. S. A.99:667–672.
26.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de La Torre.
2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA
analogs. J. Virol.74:3470–3477.
27.Levingston Macleod, J. M., et al. 2010. Identification of two functional
domains within the arenavirus nucleoprotein. J. Virol.85:2012–2023.
28.Li, H., J. Dou, L. Ding, and P. Spearman.2007. Myristoylation is required for human immunodeficiency virus type 1 Gag-Gag multimerization in
mamma-lian cells. J. Virol.81:12899–12910.
29.Lopez, N., and M. T. Franze-Fernandez.2007. A single stem-loop structure in Tacaribe arenavirus intergenic region is essential for transcription termi-nation but is not required for a correct initiation of transcription and
repli-cation. Virus Res.124:237–244.
30.Lopez, N., R. Ja´camo, and M. T. Franze-Fernandez.2001. Transcription and RNA replication of Tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol.
75:12241–12251.
31.Lovell, S. C., et al.2002. Structure validation by Calpha geometry: phi,psi
and Cbeta deviation. Proteins50:437–450.
32.Luthy, R., J. U. Bowie, and D. Eisenberg. 1992. Assessment of protein
models with three-dimensional profiles. Nature356:83–85.
33.Martínez-Peralta, L. A., C. E. Coto, and M. C. Weissenbacher.1993. The Tacaribe complex: the close relationship between pathogenic (Junín) and a
nonpathogenic (Tacaribe) arenavirus, p. 281–296.InM. S. Salvato (ed.), The
Arenaviridae. Plenum Press, New York, NY.
34.Neuman, B. W., et al.2005. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and
image analysis. J. Virol.79:3822–3830.
35.Panchal, R. G., et al.2003. In vivo oligomerization and raft localization of
on November 7, 2019 by guest
http://jvi.asm.org/
Ebola virus protein VP40 during vesicular budding. Proc. Natl. Acad. Sci.
U. S. A.100:15936–15941.
36.Perez, M., R. C. Craven, and J. C. de la Torre.2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies.
Proc. Natl. Acad. Sci. U. S. A.100:12978–12983.
37.Perez, M., D. L. Greenwald, and J. C. de la Torre.2004. Myristoylation of the
RING finger Z protein is essential for arenavirus budding. J. Virol.78:
11443–11448.
38.Pettersen, E. F., et al.2004. UCSF Chimera—a visualization system for
exploratory research and analysis. J. Comput. Chem.25:1605–1612.
39.Radecke, F., et al. 1995. Rescue of measles viruses from cloned DNA.
EMBO J.14:5773–5784.
40.Rossi, C., O. Rey, P. Jenik, and M. T. Franze-Fernandez.1996.
Immuno-logical identification of Tacaribe virus proteins. Res. Virol.147:203–211.
41.Salvato, M. S., K. J. Schweighofer, J. Burns, and E. M. Shimomaye.1992. Biochemical and immunological evidence that the 11 kDa zinc-binding pro-tein of lymphocytic choriomeningitis virus is a structural component of the
virus. Virus Res.22:185–198.
42.Schlie, K., et al.2010. Viral protein determinants of Lassa virus entry and
release from polarized epithelial cells. J. Virol.84:3178–3188.
43.Scianimanico, S., et al.2000. Membrane association induces a
conforma-tional change in the Ebola virus matrix protein. EMBO J.19:6732–6741.
44.Shindyalov, I. N., and P. E. Bourne.1998. Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng.
11:739–747.
45.Shtanko, O., S. Watanabe, L. D. Jasenosky, T. Watanabe, and Y. Kawaoka.
2011. ALIX/AIP1 is required for NP incorporation into Mopeia virus
Z-in-duced virus-like particles. J. Virol.85:3631–3641.
46.Strecker, T., et al.2003. Lassa virus Z protein is a matrix protein and
sufficient for the release of virus-like particles [corrected]. J. Virol.77:10700–
10705.
47.Strecker, T., et al.2006. The role of myristoylation in the membrane
asso-ciation of the Lassa virus matrix protein Z. Virol. J.3:93.
48.Topcu, Z., D. L. Mack, R. A. Hromas, and K. L. Borden.1999. The promy-elocytic leukemia protein PML interacts with the proline-rich homeodomain protein PRH: a RING may link hematopoiesis and growth control.
Onco-gene18:7091–7100.
49.Urata, S., T. Noda, Y. Kawaoka, H. Yokosawa, and J. Yasuda.2006. Cellular
factors required for Lassa virus budding. J. Virol.80:4191–4195.
50.Urata, S., J. Yasuda, and J. C. de la Torre.2009. The Z protein of the New World arenavirus Tacaribe virus has bona fide budding activity that does not
depend on known late domain motifs. J. Virol.83:12651–12655.
51.Volpon, L., M. J. Osborne, A. A. Capul, J. C. de la Torre, and K. L. Borden.
2010. Structural characterization of the Z RING-eIF4E complex reveals a
distinct mode of control for eIF4E. Proc. Natl. Acad. Sci. U. S. A.107:5441–
5446.
52.Wilda, M., N. Lopez, J. C. Casabona, and M. T. Franze-Fernandez.2008. Mapping of the Tacaribe arenavirus Z-protein binding sites on the L protein identified both amino acids within the putative polymerase domain and a region at the N terminus of L that are critically involved in binding. J. Virol.
82:11454–11460.