0022-538X/96/$04.0010
Copyrightq1996, American Society for Microbiology
CREB and CREB-Binding Proteins Play an Important Role in
the IE2 86-Kilodalton Protein-Mediated Transactivation of the
Human Cytomegalovirus 2.2-Kilobase RNA Promoter
RUTH SCHWARTZ, BRIAN HELMICH,ANDDEBORAH H. SPECTOR*
Department of Biology, University of California, San Diego, La Jolla, California 92093-0357 USA
Received 18 December 1995/Accepted 19 June 1996
The human cytomegalovirus (HCMV) immediate-early region 2 86-kDa protein (IE2 86) is the major transactivator of the promoter for the 2.2-kb class of early RNAs (open reading frame UL 112–113). Previously, we reported that a DNA segment on this promoter between nucleotides (nt)2113 and 259 was critical for activation by IE2 86 in vivo and could be bound by IE2 86 in vitro (R. Schwartz, M. H. Sommer, A. Scully, and D. H. Spector, J. Virol. 68:5613–5622, 1994). With a set of site-specific mutations within nt284 to261, we have localized the essentialcis-acting sequences to nt272 to261, which contain an ATF/CREB-binding site. The IE2 86-binding site between nt2113 and285 is not essential for activation of the promoter by IE2 86 in transient-expression assays, but its presence can enhance the level of activation mediated through the se-quences located between nt284 and259. Electrophoretic mobility shift assays with a segment containing nt
284 to259 and nuclear extracts from human cells permissive for the HCMV infection revealed a complex band pattern. However, by supershift analysis with specific antibodies, we were able to identify CREB as the major ATF/CREB family member in the protein-DNA complexes. Further evidence that CREB is a target for IE2 86-mediated induction, is provided by the finding that IE2 86 activates the somatostatin promoter to high levels. Although the binding of IE2 86 to nonphosphorylated full-length CREB orDCREB is minimal, IE2 86 does form complexes with p300 and the CREB-binding protein (CBP), which in turn bind to CREB and can serve as adaptor proteins for CREB function. In addition, the in vivo functional relevance of the interaction between IE2 86 and CBP is indicated by the ability of IE2 86 to enhance transcriptional activation mediated by a GAL4-CBP fusion protein brought to a promoter by GAL4-binding sites.
Human cytomegalovirus (HCMV) is an opportunistic agent in immunocompromised individuals and is the major viral cause of birth defects in newborns (1, 41). Similar to other herpesviruses, HCMV gene expression is temporally regulated (10, 39, 59, 63, 64). The immediate-early (IE) gene products, which rely primarily on host factors for their expression, are synthesized initially after viral infection. Of this group of genes, the two genetic units in the major IE region designated IE1 and IE2 have been studied in greatest detail. At least three mRNAs are encoded by this region and are translated into proteins of 72 kDa (IE1 72; ppUL123), 86 kDa (IE2 86; ppUL122a) and 55 kDa (IE2 55; ppUL122b) (10, 18, 21, 57, 58, 60, 63, 65).
IE2 86 plays a major role in the activation of HCMV early promoters, as well as of heterologous cellular and viral pro-moters (9, 13, 14, 16, 28, 35, 38, 45, 62). IE2 86 is also capable of down-regulating its own expression through direct DNA
binding to acisrepression signal (CRS) near the cap site of its
promoter (5, 6, 17, 22, 32, 34, 37, 43–45, 56, 68). In addition, IE2 86 has the ability to interact with several cellular proteins, including the TATA box-binding protein (TBP), TFIIB, Sp1, Tef-1, c-Jun, JunB, p53, and Rb (4, 12, 15, 16, 23, 35, 48, 50–53).
Following the expression of the IE genes and before the onset of viral DNA replication, there is induction of the early class of RNAs. It appears that transcription of the early genes requires the prior synthesis of one or more IE proteins, but the
mechanism of this activation and the importantcis-acting
ele-ments andtrans-acting factors have yet to be fully defined. For
several years, our laboratory has focused on this question and has used as a model the HCMV early promoter for the 2.2-kb class of RNAs (open reading frame UL 112–113), which en-codes four nuclear phosphoproteins (28, 54, 55, 66, 67). Al-though the function of these proteins in the infection is un-known, they appear to be required for HCMV DNA replication in a transient-complementation assay (42) and can cooperate with the HCMV proteins IRS/TRS, UL 36–38, and IE1/IE2 to stimulate expression from the promoters for six HCMV replication proteins (20, 25).
In our initial transient-expression studies, we found that activation of the 2.2-kb RNA promoter in HCMV-infected cells was mediated through a major regulatory domain located
between nucleotides (nt)2113 and259 (54). We also noted
that this region contained a consensus ATF/CREB-binding site
between nt 271 and 266 and proposed that ATF/CREB or
some related factor might be involved in regulating this gene. Subsequently, we showed that IE2 86 plays a major role in the induction of this promoter and that the previously identified
region between nt2113 and259 is essential for this specific
activation (28, 47). Recently, we and others reported that IE2 86 is able to bind with high affinity to three domains on this
promoter bounded by nt2286 to2257, nt2248 to2218, and
nt2148 to2120 (3, 47). In addition, the results of our
exper-iments revealed that IE2 86 is capable of binding with lower
affinity to the sequences located between nt2113 and285,
which are within the domain that appeared in our studies to be the most biologically relevant (47). The recent work of Arlt et al. (3), however, suggests that the IE2 86-binding sites on this promoter may be playing only an accessory role and that the
* Corresponding author. Mailing address: Department of Biology, 0357, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0357. Phone: (619) 534-9737. Fax: (619) 534-6083. Electronic mail address: dspector@ucsd.edu.
6955
on November 9, 2019 by guest
http://jvi.asm.org/
major positive element for IE2 86-mediated transactivation is
located between nt284 and232. Moreover, their data
indi-cate that the strong IE2 86-binding sites cannot increase tran-scription when just a TATA box is present but can enhance the overall level of activation by IE2 86 when placed upstream of
nt2117 on this promoter.
In this study, we have further characterized the cis-acting
sequences and trans-acting factors required for activation of
the HCMV early 2.2-kb RNA promoter, focusing on the rela-tive importance of the ATF/CREB and the IE2 86 DNA-binding sites. By mutational analysis, we have shown that the
region between nt 272 and 261, which contains the ATF/
CREB-binding site, is essential for activation by IE2 86 and that either a strong or weak IE2 86 DNA-binding site
posi-tioned between nt 2113 and 285 can enhance the level of
activation through this downstream site. We demonstrate that a main candidate for transactivation of the 2.2-kb RNA pro-moter by IE2 86 in permissive cells is the transcription factor CREB, but a role for other factors which bind to this promoter cannot be excluded. In addition, we present evidence that IE2 86 has the ability to activate through a prototypical CREB site. Although IE2 86 is not capable of binding full-length CREB directly (50) and it has very little affinity for
nonphosphory-latedDCREB (31), we provide evidence that IE2 86 can
inter-act with the proteins p300 and CREB-binding protein (CBP), which bind to CREB and function as transcriptional coactiva-tors (2, 7, 30, 36). Furthermore, IE2 86 can enhance the acti-vation mediated by full-length CBP fused to GAL4 of a target promoter containing GAL4-binding sites. These results sug-gest that there is a functional interaction between IE2 86 and CBP.
MATERIALS AND METHODS
Virus and cells.Human U373-MG astrocytoma-glioblastoma cells were a gift from R. LaFemina (Merck Sharpe & Dohme) and were maintained in Dulbec-co’s modified Eagle’s medium (Gibco BRL) supplemented with high glucose and containing 5% fetal bovine serum and Mito Plus serum extender (Collaborative Research). HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco BRL) supplemented with high glucose and containing 10% fetal bovine serum.
Molecular cloning.Restriction enzymes were obtained from Bethesda Re-search Laboratories, Inc., or Boehringer Mannheim Biochemicals and used as recommended by the manufacturers. CompetentEscherichia coliDH5a (Be-thesda Research Laboratories) were transformed with recombinant plasmids as recommended by the suppliers.
Construction of plasmids p148CAT and p93CAT, which contain various lengths of the early promoter for the 2.2-kb class of RNAs fused to the chlor-amphenicol acetyltransferase (CAT) gene, has been described previously (54). Plasmid Del lacks sequences located between nt284 and259 of the promoter but contains sequences up to nt2113. Plasmids A, B, and C have sequences up to nt2113 in the promoter and contain substitutions of the weak IE2 86-binding site located between nt2108 and295 for either the CRS consensus element (32), a nonconsensus sequence, or the strong binding sequence on the 2.2-kb RNA promoter located between nt2143 and2130, respectively. Plasmid D is a 59deletion mutant with promoter sequences up to nt284. Mutant plasmids
284CAT,278CAT,272CAT, and266CAT have sequences up to nt2113 in the promoter and contain site-specific mutations formed by substituting 6 nt with theEcoRI restriction site downstream from nt284,278,272, and266, respec-tively. Plasmid Comb contains the three mutations present in plasmids278CAT,
272CAT, and 266CAT. Plasmids Del, A, B, C, D, 284CAT, 278CAT,
272CAT,266CAT, and Comb were constructed by PCR amplification and subcloning of the desired fragment into the p148CAT vector previously digested withXbaI andSstI.
The oligonucleotides used for PCR amplification of the mutated DNA frag-ments in plasmids A, B, C, and D were, respectively, 59GCTCTAGAGTCCC AGTCGTTTAGTGAACCGTACTGTTTAACCACGTTG 39, 59GCTCTAGA GTCCCAGTTAAGGTAATATAATTACTGTTTAACCACGTTGCG 39, 59G CTCTAGAGTCCCAGTCGATTTGCAGTCCGTACTGTTTAACCACGTTG 39, and 59GCTCTAGAGTCCCACGTTGCGTCGTGAC 39. Each oligonucle-otide was used in conjunction with oligonucleoligonucle-otide 59TTTAGCTTCCTTAGC TCCTG 39. The PCR products of the above mutants were digested withXbaI and
SstI, loaded on a 3% NuSieve (FMC BioProducts) agarose gel, and purified with the Mermaid kit (Bio101). The desired fragments were then ligated into the p148CAT vector previously digested withXbaI andSstI.
The oligonucleotides used for PCR amplification of the mutated DNA frag-ment in mutant Del were 59GCTCTAGAGTCCCAGTTACTTTAATAAACG TACTGTTTAAGGGTGTTGCTAGGCGGG 39and 59TTTAGCTTCCTTAG CTCCTG 39. The cloning procedure for plasmid Del was the same as for plasmids A, B, C, and D.
The mutant DNAs cloned in plasmids278CAT,272CAT, and266CAT were constructed by two PCR amplification steps. In the first PCR for each mutant, oligonucleotide 59 CTGCAGGTCGACTCTAGA 39 was used in conjunction with oligonucleotide 59CACGAATTCACGTGGTTAAACAGTACGTT 39, 59
CAAGAATTCGACGCAACGTGGTTAAACA 39 or 59CACGAATTCCGTC ACGACGCAACGTG 39, respectively, for mutants278CAT,272CAT, and
266CAT. In the second PCR for each mutant, oligonucleotide 59TTTAGCTT CCTTAGCTCCTG 39was used in conjunction with oligonucleotide 59GCTCT AGAGTCCCACGTGAATTCGTGACGTTGTTTGTGGG 39, 59GCTCTAGA GTCCCACGTTGCGTCGAATTCTTGTTTGTGGGTGTTGCTAG 39, or 59
GCTCTAGAGTCCCACGTTGCGTCGTGACGGAATTCGTGGGTGTTGC TAGGCG 39, respectively. The first PCR product for each mutant was digested withXbaI andEcoRI, and the second PCR product was digested for each mutant withEcoRI andSstI. Triple ligations were performed for each mutant containing both digested PCR products into the p148CAT vector previously digested with
XbaI andSstI.
The mutation in plasmid Comb was obtained by two rounds of PCR amplifi-cation. For the first round of reactions, two separate PCRs were performed. The oligonucleotides used for the first PCR were 59CTGCAGGTCGACTCTAGA 39 and 59 GAATTCGAATTCGAATTCACGTGGTTAAACAGTACGTT 39, and for the second PCR, the oligonucleotides used were 59TTTAGCTTCCTT AGCTCCTG 39and 59CGTGAATTCGAATTCGAATTCGTGGGTGTTGCT AGGCG 39. The products of both PCRs were subjected to a second round of PCR amplification with the external oligonucleotides 59CTGCAGGTCGACT CTAGA 39and 59TTTAGCTTCCTTAGCTCCTG 39. The PCR product thus obtained was digested withXbaI andSstI, loaded on a 3% NuSieve (FMC BioProducts) agarose gel, and purified with the Mermaid kit (Bio101). The mutated PCR fragment was then ligated into the p148CAT vector previously digested withXbaI andSstI. Plasmid284CAT was constructed in the same way as plasmid Comb. The specific oligonucleotides used to amplify the desired mutation in plasmid284CAT were 59GAATTCTTAAACAGTACGTTTATT AAAGT 39and 59GAATTCTGCGTCGTGACGTTGTTTG 39.
DNA sequence analysis.The presence of the desired mutations in all the plasmids constructed was determined by the dideoxy-chain termination method of DNA sequencing (46), using the Sequenase version 2.0 DNA-sequencing kit (Amersham). [a-35
S]dATP (1000Ci/mmol) was purchased from Amersham. Transient-expression assays in U373-MG cells.Human U373-MG astrocyto-ma-glioblastoma cell monolayers were transfected with plasmid DNAs by the DEAE-dextran technique previously described by Staprans et al. (54). In all experiments, at least two flasks per construct were transfected. All of the con-structs were assayed in at least three independent experiments. PlasmidD(271), which contains the somatostatin promoter sequences attached to a CAT reporter gene, was a kind gift of M. Montminy, Salk Institute (40).
Transient-expression assays in HeLa cells.HeLa cells were transfected by the calcium phosphate coprecipitation method with the GibcoBRL calcium phos-phate transfection kit. In all experiments, at least two flasks per construct were transfected. All of the constructs were assayed in at least two independent experiments. The cells were transfected with 5mg of the pGAL4-CAT reporter pGAL4/E1b TATA containing five GAL4-binding sites (33), 5mg of the coding plasmid for effector protein GAL4-CBP (full length) or GAL4-CBP (1678-2441) (7, 30), 5mg of a protein kinase A coding plasmid, and different quantities of pSGIE86 (28). The amount of DNA was kept constant in all the transfections by adding plasmid pSG5. The cells were harvested 48 h posttransfection and assayed for CAT activity as described previously (54). The GAL4-CBP constructs were kindly provided by R. Kwok and R. Goodman, Oregon Health Sciences Univer-sity.
Nuclear extract preparation.Nuclear extracts were prepared from U373-MG cell monolayers by modifications to the methods used by Dignam et al. (11) and Shapiro et al. (49) as previously described (28).
In vitro translation of proteins.In vitro transcription-translation reactions were carried out with the TNT coupled reticulocyte lysate system (Promega) as specified by the manufacturer. A vector containing the coding region for ATF-2 was generously provided by M. Green, Massachusetts Medical Center, Worces-ter, Mass. T7-CREB, T7DCREB and SP6CBP were kind gifts from M. Mont-miny. T3-p300 was generously provided by R. Eckner, Dana Farber Cancer Institute.
Gel shift analysis.In vitro-translated proteins or U373-MG nuclear extracts were incubated for 30 min at 228C with various 59 32P-end-labeled double-stranded DNA sequences in the presence of 2 to 10mg of dA-dT in a binding buffer containing 25 mMN-2-hydroxyethylpiperazine-N9-2-ethanesulfonic acid (HEPES; pH 7.9), 100 mM KCl, 20% glycerol, 0.1% Nonidet P-40, 10mM ZnSO4, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM sodium metabisulfite, 1 mM benzamidine, and 1 mM dithiothreitol. The samples were then loaded on a 5% nondenaturing TBE polyacrylamide gel, and the gel was dried and subjected to autoradiography. When the formed DNA-protein com-plexes were supershifted with antibodies, the nuclear extracts were first
on November 9, 2019 by guest
http://jvi.asm.org/
bated with 0.4mg of antibody for 20 min at 228C and then further incubated for 30 min at 228C in the presence of labeled DNA.
The DNA sequences used for gel shift analysis were nt284 to259 of the 2.2-kb RNA promoter (w) (54); a consensus activating transcription factor (ATF)-binding sequence (40), i.e., 59AGAGATTGCCTGACGTCAGAGAGC TAG 39and 59CTAGCTCTCTGACGTCAGGCAATCTCT 39(a); and a mu-tant ATF-binding sequence, i.e., 59 AGAGATTGCCTGTGGTCAGAGAGC TAG 39and 59CTAGCTCTCTGACCACAGGCAATCTCT 39(n). The mutant ATF-binding sequence was used as nonspecific DNA.
The oligonucleotides used for gel shift analysis of DNA withEcoRI mutations within nt284 to259, which were labeled mutants I through IV and Comb, were 59GAATTCTGCGTCGTGACGTTGTTTGT 39and 59ACAAACAACGTCA CGACGCAGAATTC 39(I), 59CCACGTGAATTCGTGACGTTGTTTGT 39 and 59ACAAACAACGTCACGAATTCACGTGG 39(II), 59CCACGTTGCG TCGAATTCTTGTTTGT 39 and 59 ACAAACAAGAATTCGACGCAACG TGG 39(III), 59CCACGTTGCGTCGTGACGGAATTCGT 39and 59ACGA ATTCCGTCACGACGCAACGTGG 39(IV), and 59CCACGTGAATTCGAA TTCGAATTCGT 39 and 59 ACGAATTCGAATTCGAATTCACGTGG 39 (Comb).
Anti-CREB, anti-ATF-2 and anti-ATF-4 antibodies were obtained from Santa Cruz Biotechnology.
Protein-binding assays.Protein-protein binding assays of glutathione-S -trans-ferase (GST) fusion products were performed as previously described (28, 50).
RESULTS
Localization of the sequences in the region between nt2113 and259 required for activation of the 2.2-kb RNA promoter.
We recently demonstrated that specific stimulation of the HCMV 2.2-kb RNA promoter by the IE2 86 protein in tran-sient-expression assays and superinfection experiments
re-quires sequences located between nt2113 and259 relative to
the transcription start site (47). Activation of this promoter is dependent on the presence of IE2 86, and no basal activity is observed in U373-MG cells transfected with the 2.2-kb RNA promoter alone. A promoter construct containing 113 nt of upstream sequence is maximally activated by IE2 86, although
during HCMV infection the sequences bounded by nt 2224
and2113 do contribute to a small extent to full activation. We
also showed that IE2 86 binds with different affinities to several distinct regions upstream of the transcription start site of the 2.2-kb RNA promoter. There are high-affinity binding regions
bounded by nt2286 to2257, nt2248 to2218, and nt2148
to2120, and a lower-affinity binding site bounded by nt2113
to285, which is located within the region that is most
biolog-ically relevant based on transfection and superinfection exper-iments.
To further delineate the sequences between nt 2113 and
259 required for activation by IE2 86, we prepared two
pro-moter deletion mutants and attached them to the CAT gene.
Plasmid D contains a 59deletion to nt284, and plasmid Del
has wild-type promoter sequences up to nt2113 with an
in-ternal deletion of nt284 to259. These constructs, as well as
the promoter construct containing wild-type sequences up to nt
2113 (p148CAT) and the construct containing a 59deletion to
nt258 (p93CAT), were independently cotransfected with the
IE2 86 expression vector pSGIE86 into U373-MG astrocyto-ma-glioblastoma cells (which are fully permissive for HCMV infection), and CAT activity was measured 48 h later. CAT activity was also assayed in extracts prepared from cells trans-fected with the promoter CAT constructs in the absence of IE2 86. In accord with our previous results, the basal activity of all constructs in the absence of IE2 86 was at background levels (data not shown). As shown in Fig. 1, deletion of sequences
located 59 to nt284 reduced activation by IE2 86 twofold.
However, when nt284 to259 were deleted, activation by IE2
86 was reduced 20-fold even when nt 2113 to285 were
re-tained. This result indicates that the factor(s) that interacts
through nt 284 to 259 is crucial for the IE2 86-mediated
transactivation of the promoter.
The above experiment indicated that the weak IE2 86-bind-ing site, although not essential for IE2 86-mediated activation, did contribute to full stimulation of the promoter. To further assess the relative importance of the weak IE2 86-binding site
(nt2113 to285), we constructed a series of additional
pro-moter mutations in this region. Plasmids containing the CAT reporter gene linked to the various mutated promoters were cotransfected with the IE2 86 expression vector pSGIE86 into U373-MG astrocytoma-glioblastoma cells, and CAT activity was assessed 48 h later. When the weak binding site was con-verted into a strong binding region corresponding to either the consensus CRS motif or the IE2 86-binding site bounded by nt
2143 and2130 of the 2.2-kb RNA promoter (plasmids A and
C, respectively), activation by IE2 86 remained at wild-type levels (Fig. 1). On the other hand, when the weak binding site was mutated to a nonconsensus sequence (plasmid B), activa-tion of the promoter by IE2 86 was reduced to the same level as observed when the sequence was deleted (approximately twofold). No activity above background levels was detected with any of these constructs in the absence of IE2 86 (results not shown). These results indicate that transactivation by IE2 86 is not affected by the strength of the binding site bounded by
nt 2113 and285 on the promoter. Moreover, although the
presence of an IE2 86-binding site does modestly increase the level of activation, another factor(s), which acts through nt
284 to259, probably plays the major role in IE2 86-mediated
regulation of this promoter.
IE2 86 activates the promoter in vivo through the ATF/ CREB-binding site.To define precisely which regions within nt
284 to259 of the 2.2-kb RNA promoter were important for
IE2 86 transactivation, we constructed four plasmids with base
substitutions such that singleEcoRI sites were created within
nt284 to279,278 to273,272 to267, and266 to261 in
the wild-type background of p148CAT. These mutants are
labeled284CAT,278CAT,272CAT, and266CAT,
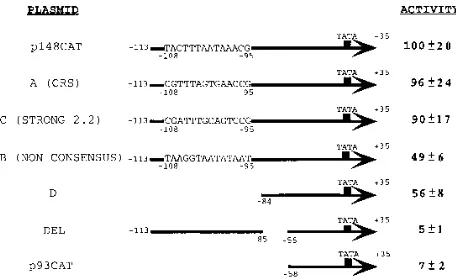
[image:3.612.321.549.70.210.2]respec-tively. Each of these promoter-CAT constructs was then tested for CAT activity following cotransfection with and without pSGIE86. No activity above background levels was detected
FIG. 1. Schematic representation of the early 2.2-kb RNA promoter muta-tion plasmids and their observed CAT activities following cotransfecmuta-tion with pSGIE86. Duplicate flasks of permissive U373-MG cells were cotransfected by the DEAE-dextran method, and the cells were harvested 48 h posttransfection and assayed for CAT activity as described in Materials and Methods. CAT activity relative to p148CAT, the wild-type construct, is shown. Nucleotide num-bers relative to the transcription start site are indicated. Plasmid Del lacks nt284 to259 of the promoter but contains sequences up to2113. Plasmids A, B, and C contain substitutions of the weak IE2 86-binding site located between nt2108 and295 for either the consensus CRS element (32), a nonconsensus sequence, or the strong binding sequence on the 2.2-kb RNA promoter located between nt
2143 and2130, respectively. Plasmid D contains wild-type sequences up to nt
284.
on November 9, 2019 by guest
http://jvi.asm.org/
with any of these reporter constructs in the absence of IE2 86 (results not shown). As shown in Fig. 2, the activation of
284CAT and278CAT by IE2 86 is reduced less than twofold
relative to that of the wild type. However, the mutations in
plasmids 272CAT and 266CAT cause a sevenfold drop in
CAT activity compared with the wild-type plasmid, indicating
that nt272 to261 are critical for IE2 86-mediated
transacti-vation of the 2.2-kb RNA promoter. In the report by Staprans et al. (54), we had initially noted that a sequence similar to the
ATF/CREB-binding site was located between nt271 and266
and had proposed that HCMV infection might activate the promoter through this region. The results of the above muta-tional analysis support this hypothesis and suggest that IE2 86 may effect activation of the promoter by interacting with the ATF/CREB family of transcription factors.
Identification of site-specific nuclear factors in cells permis-sive for HCMV infection. We used gel retardation assays to determine whether factors in the U373-MG nuclear extracts
could bind to the sequences located between nt284 and259
of the 2.2-kb RNA promoter. As shown in Fig. 3A, lane 2, we
observed at least four bands when using a wild-type32P-labeled
probe containing the nt284 to259 sequences. Binding to the
labeled probe is specifically inhibited by an excess of unlabeled
wild-type284 to259 oligonucleotide, whereas the complexes
are unaffected by nonspecific DNA (lanes 3 and 4, respective-ly).
Since the gel shift analysis with U373-MG nuclear extracts and the wild-type probe of the promoter gave several bands, we
proceeded to define further the domains of the protein-DNA
interactions with mutant probes containing theEcoRI site
sub-stitutions described above. The fragments (nt 284 to259)
containing theEcoRI sites within nt284 to279,278 to273,
272 to267, and 266 to 261 are labeled I, II, III and IV,
respectively, in Fig. 4. Figure 4A, lane 3, shows that mutant I
(containing an EcoRI site within nt 284 to 279) binds the
proteins with less intensity than does the wild-type probe but the general pattern of binding does not change. This is further supported by the data in Fig. 4B, lane 5, which show that excess unlabeled mutant I DNA efficiently competes for the binding of labeled wild-type DNA to the nuclear extract. The observa-tion that the pattern of bands generated with mutant I is the same as the one obtained with the wild-type probe, although the intensity is lower in some experiments, suggests that these sequences might contribute to the overall strength of the
bind-ing. With mutant II (containing anEcoRI site within nt278 to
273), there is a complete loss of band 1 and the intensity of
band 3 seems to be decreased (Fig. 4A, lane 4 and Fig. 4B, lane
6). Mutants III and IV (containingEcoRI sites within nt272
to267 and266 to261, respectively) do not yield band 2 and
show a significant reduction in the intensity of band 3; the intensity of band 1 is also reduced in these mutants (Fig. 4A, lanes 5 and 6, and Fig. 4B, lanes 7 and 8). When using a DNA construct that harbors mutations II, III, and IV, none of the bands are observed (Fig. 4A, lane 1; Fig. 4B, lane 9). Although there is an apparent loss of band 4 with mutants II, III, and IV, this result is not reproducibly seen (see Fig. 6B), and we there-fore believe that it may be nonspecific. These results indicate
that nt278 to273 are involved in forming the complex
[image:4.612.78.285.74.351.2]cor-responding to band 1 and that nt272 to261 are necessary for
FIG. 2. Schematic representation of the early 2.2-kb RNA promoter mutant plasmids and their observed CAT activities following cotransfection with pSGIE86. (A) Triplicate flasks of permissive U373-MG cells were cotransfected by the DEAE-dextran method as in Fig. 1. CAT activity relative to p148CAT is shown. Nucleotide numbers relative to the transcription start site are indicated. X indicates the position of eachEcoRI substitution. (B) Sequences within nt284 to261 in the wild-type (WT),284CAT,278CAT,272CAT,266CAT, and Comb plasmids. TheEcoRI substitutions are underlined.
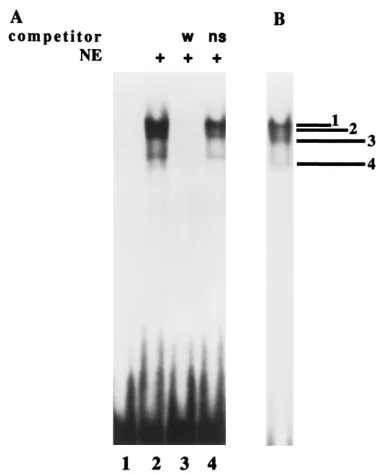
FIG. 3. Gel shift analysis of U373-MG nuclear extracts binding to nt284 to
259 of the 2.2-kb RNA promoter. (A) DNA binding was performed in the presence of nuclear extract (NE) as described in Materials and Methods, and the complexes were subjected to electrophoresis through a 5% nondenaturing poly-acrylamide gel. The labeled DNA fragment contained wild-type sequences from nt284 to259. Unlabeled competitor wild-type (w) DNA or non-specific (ns) DNA (100 ng) was used in lanes 3 and 4, respectively. Lane 1 contains labeled DNA only. Lanes 2, 3, and 4 contain nuclear extract. (B) A sample identical to that in lane 2 but from another experiment is included for a better representation of the band pattern formed.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:4.612.340.533.406.643.2]bands 2 and 3. It also appears that there may be cooperative interactions between the factors binding to these sites and that adjacent sequences contribute to the strength of the binding.
ATF family members bind to the 2.2-kb RNA promoter.The above results showed that several proteins bind to sequences
between nt284 and259 of the 2.2-kb RNA promoter.
More-over, since the sequence with similarity to the ATF/CREB site was altered in mutants III and IV, it seemed likely that one or more ATF/CREB family members might be present in bands 2 and 3. However, because the sequence in the 2.2-kb RNA promoter does diverge from the consensus site, we proceeded to test whether this domain on the promoter could bind to in vitro-translated ATF/CREB proteins. As shown in Fig. 5A, lanes 2 and 3 and lanes 6 and 7, respectively, both ATF-2 and CREB are capable of binding to the 2.2-kb RNA promoter.
As a complement to the above experiment, we also tested whether the U373-MG nuclear extracts contain a protein ca-pable of binding to the consensus ATF DNA sequence. Figure 5B shows a gel shift analysis with nuclear extracts and either labeled consensus ATF probe or the wild-type 2.2-kb RNA promoter probe. The ATF consensus probe yields a single band that migrates at approximately the same position as band 2 generated by incubating nuclear extract proteins with the wild-type 2.2-kb RNA promoter (lanes 1 and 4, respectively). As expected, the wild-type promoter sequence efficiently com-petes for binding of the nuclear extract to either the ATF DNA or the wild-type 2.2-kb RNA promoter sequences (lanes 2 and 5, respectively). In contrast, the ATF DNA competes well for the binding of the nuclear extract to the labeled consensus ATF probe (lane 3) but is less effective as a competitor for complexes formed between the nuclear extracts and the
wild-type 2.2-kb RNA promoter sequences (lane 6). These results suggest that U373-MG cells contain several proteins capable of binding to the 2.2-kb RNA promoter and that at least one of these corresponds to a member of the ATF/CREB family of transcription factors.
Gel shift analysis with specific antibodies reveal that CREB is the major ATF family member binding to the promoter.To identify which of the ATF/CREB family members in the U373-MG nuclear extracts bind to the wild-type promoter or the ATF DNA, we performed gel supershift analysis with spe-cific antibodies. As shown in Fig. 6A, lanes 3 and 8, we ob-tained a supershift when we used an anti-CREB antibody. This antibody does not cross-react with other members of the ATF family of transcription factors or with the naked DNA (results not shown). Moreover, antibodies specific for ATF-2 (lanes 4 and 9) and ATF-4 (lanes 5 and 10) had no effect on the gel-shift pattern.
[image:5.612.103.510.71.316.2]With the consensus ATF probe, it appears that CREB is the major ATF/CREB family protein in the U373-MG cells inter-acting with this sequence, since most of the complex is super-shifted by the anti-CREB antibody. In contrast, with the wild-type promoter, CREB appears to be only one of several proteins forming the complexes and band 2 seems to be most strongly affected by the presence of the anti-CREB antibody. Since CREB is heat stable (19, 61), the identity of the binding factor as CREB was further assessed by heat denaturing the nuclear extract and supershifting with the CREB anti-body. The results are shown in Fig. 6B, lanes 1 and 2, and provide additional support for the conclusion that band 2 of the untreated nuclear extract contains CREB or a very similar protein. Further evidence that the CREB-binding region is
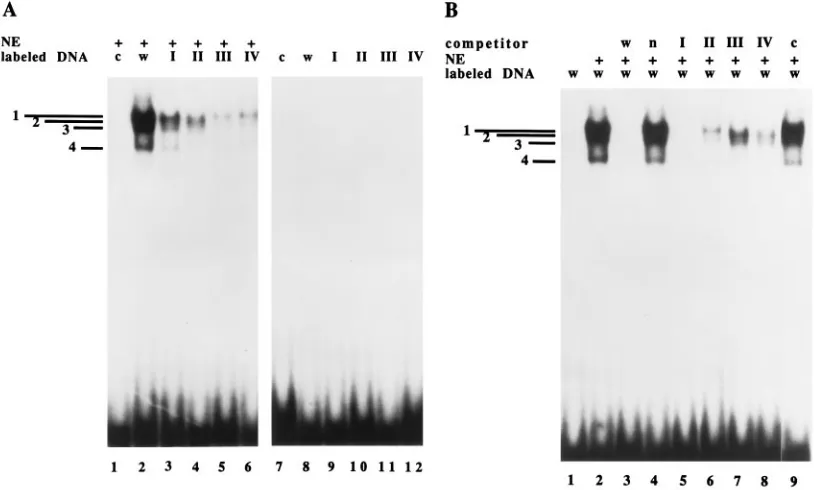
FIG. 4. Gel shift analysis of U373-MG nuclear extracts binding to wild-type or mutant sequences encompassing nt284 to259 of the 2.2-kb RNA promoter. The gel shift assay was performed as described in Materials and Methods. (A) Lanes 1 to 6 contain nuclear extract (NE); lanes 7 to 12 contain DNA only. Lanes 1 and 7 contain labeled Comb DNA (c) (which containsEcoRI substitutions within nt278 to273,272 to267, and266 to261). Lanes 2 and 8 contain wild-type sequences (w). Lanes 3 and 9 contain anEcoRI site within nt284 and279 of the promoter (mutation in site I). Lanes 4 and 10 contain anEcoRI site within nt278 to273 of the promoter (mutation in site II). Lanes 5 and 11 contain anEcoRI site within nt272 to267 of the promoter (mutation in site III). Lanes 6 and 12 contain anEcoRI site within nt266 to261 of the promoter (mutation in site IV). (B) Unlabeled DNA competition with the differentEcoRI substitution mutants of the binding obtained with the wild-type sequences. All the lanes contain labeled wild-type sequences284 to259 of the 2.2-kb RNA promoter (w). Lanes 2 to 9 contain nuclear extract (NE). Lanes 3 to 9 contain 100 ng of various unlabeled DNAs used as competitors: wild-type (w), nonspecific (n), mutant I, mutant II, mutant III, mutant IV, and Comb DNA (c), respectively.
on November 9, 2019 by guest
http://jvi.asm.org/
missing in mutant III (containing anEcoRI site within nt272
to267) and very weak in mutant IV (containing anEcoRI site
within nt 266 to 261) is provided by the observation that
anti-CREB antibody did not supershift any bands when using mutant III DNA and supershifted a minimal amount of protein when using mutant IV DNA (Fig. 6B, lanes 10 and 12). In Fig. 6B, band 4 is present in all of the mutants and is eliminated in all cases whenever antibody is added. Although this complex
may contain a CREB-related protein which binds with low affinity to multiple sites in this region, we believe that it is most probably nonspecific. Taking into consideration all of these results, we conclude that the transcription factor CREB or a
closely related protein binds within nt272 to261 of the 2.2-kb
RNA promoter and is part of the complex contained in band 2.
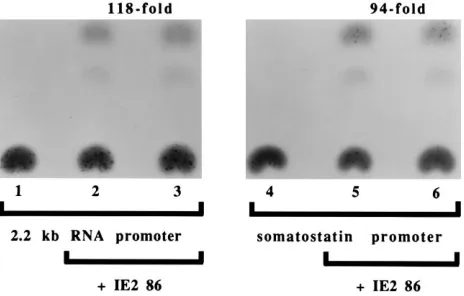
IE2 86 can transactivate through a classic CREB site.To further assess the functional interaction between IE2 86 and CREB, we cotransfected U373-MG cells with a construct con-taining the somatostatin promoter, which has the prototype CREB site, attached to a CAT gene, and an IE2 86 coding plasmid. As shown in Fig. 7, IE2 86 is capable of activating the somatostatin promoter 100-fold relative to the basal level ob-served with the reporter construct alone (compare lanes 5 and 6 with lane 4). Interestingly, the level of activation of the somatostatin promoter by IE2 86 is the same as for the 2.2-kb RNA promoter (compare lanes 2 and 3 with lane 1). These results indicate that IE2 86 has the ability to transactivate through a prototypical CREB site.
IE2 86 binds p300 and CBP.The above finding that CREB plays an important role in IE2 86 transactivation of the pro-moter was surprising since we had previously demonstrated that IE2 86 does not directly bind to full-length CREB (50); however, Lang et al. have recently shown that CREB-A
(DCREB), which contains a deletion of amino acids 88 to 101,
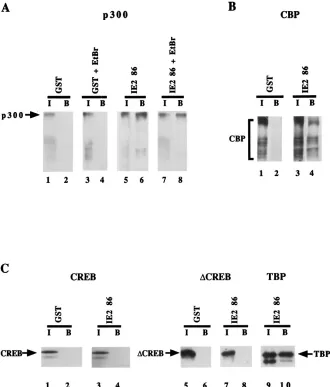
binds to IE2 86 in vitro after being phosphorylated by protein kinase A (31). We reasoned that full-length CREB and IE2 86 could be interacting through an adaptor protein such as p300 or CBP, both of which bind CREB and function as coactivators (2, 7, 30, 36); therefore, we tested whether IE2 86 could inter-act in vitro with these proteins. The GST-IE2 86 fusion protein was immobilized on glutathione-agarose beads and incubated
with [35S]methionine-labeled in vitro-synthesized p300 or CBP.
Following incubation, the protein complexes were washed ex-tensively and the bound material was analyzed by sodium do-decyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In vitro translation of CBP results in several protein bands. Nevertheless, we report our binding assays with the full array of bands obtained. As shown in Fig. 8A and B, GST-IE2 86 but not the control GST bound to both p300 and CBP. The binding affinity of IE2 86 to p300 is approximately five times weaker than the strong binding interaction with TBP (results not shown), and the in vitro translation CBP products bind to GST-IE2 86 with a lower affinity than p300 does. For compar-ative purposes, we also show that IE2 86 does not bind to full-length CREB, as previously published by Sommer et al. (50) (Fig. 8C), and that the binding to nonphosphorylated
DCREB is very weak, as reported by Lang et al. (31). These
experiments were run at the same time as a positive control-binding experiment involving GST-IE2 86 plus labeled TBP (Fig. 8C, lanes 9 and 10). To eliminate the possibility that contaminating DNA from the bacterial lysates was mediating the interaction between p300 or CBP and IE2 86, we repeated the experiments in the presence of ethidium bromide. Ethidium bromide had no effect on the level of binding of IE2 86 to p300 or CBP, and thus it appears that the interaction is bacterial DNA independent (results not shown for CBP). We can therefore conclude that IE2 86 binds to both p300 and CBP in vitro.
[image:6.612.79.269.69.483.2]IE2 86 can activate CBP in vivo. To determine whether there was a functional interaction between IE2 86 and CBP/ p300 in vivo, we assayed gene expression from a CAT reporter construct driven by a promoter containing five GAL4-binding sites when it was cotransfected into HeLa cells with plasmids encoding IE2 86 and a GAL4-CBP (full-length) fusion protein. As shown in Fig. 9, IE2 86 was capable of enhancing the
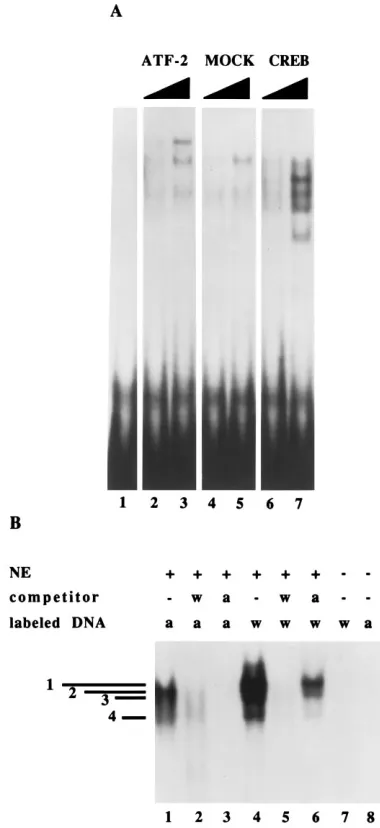
FIG. 5. Gel shift analysis of in vitro-translated proteins binding to the wild-type promoter and of permissive cell nuclear extracts binding to either the consensus ATF site or to the wild-type promoter. (A) Gel shift analysis of complexes formed between in vitro-translated ATF-2 or CREB and the wild-type nt284 to259 of the 2.2-kb promoter. Lane 1 contains DNA only. Lanes 2 and 3 contain increasing amounts of in vitro-translated ATF-2. Lanes 4 and 5 contain increasing amounts of in vitro mock translation mix. Lanes 6 and 7 contain increasing amounts of in vitro-translated CREB. (B) Gel shift analysis of U373-MG nuclear extracts binding to the consensus ATF sequence or to nt284 to259 of the 2.2-kb RNA promoter. The DNA was incubated with the nuclear extract (NE) and subjected to electrophoresis through a 5% nondenaturing polyacrylamide gel. The labeled DNA fragments contained either wild-type (w) sequences from nt284 to259 (lanes 4 to 7) or consensus ATF (a) sequences (lanes 1 to 3 and 8). Lanes 1 to 6 contain U373-MG nuclear extracts (NE). Unlabeled competitor wild-type DNA (w) or ATF consensus DNA sequences (a) (100 ng) were used in lanes 2 and 5 and lanes 3 and 6 respectively. Lanes 7 and 8 contain only labeled wild-type or consensus ATF DNA, respectively.
on November 9, 2019 by guest
http://jvi.asm.org/
activity of GAL4-CBP up to 7.5-fold in a dose-dependent man-ner. As a control, IE2 86 and the reporter plasmid were co-transfected in the absence of GAL4-CBP. Under these condi-tions, IE2 86 had no effect on the promoter (results not shown). Further specificity of the interaction was provided by the finding that activation of the reporter construct by a fusion protein of GAL4 linked to only the carboxy terminus of CBP (amino acids 1678 to 2441) was not responsive to IE2 86. These results indicate that IE2 86 and CBP can interact in vivo and lead to further CBP activation.
DISCUSSION
IE2 86 plays a key role in the activation of various HCMV early promoters. It is also capable of transactivating numerous viral and cellular promoters in addition to down-regulating its own expression (5, 6, 17, 26–28, 32, 34, 37, 38, 43–45, 54). The mechanism by which IE2 86 can regulate the activity of so many different promoters is not fully understood. Neverthe-less, the emerging picture for the way this protein functions seems to include both DNA-protein and protein-protein inter-actions.
We have used the promoter for the 2.2-kb class of HCMV early RNAs, which depends upon IE2 86 for its activation (28, 54, 55), as a model system for investigating the mechanisms underlying IE2 86 function. Recently, we and others reported that the IE2 86 protein can bind to various sites located on the
2.2-kb RNA promoter (3, 47). It binds with high affinity to the
regions bounded by nt2286 to2257, nt2248 to2218, and nt
2148 to2120, and with lower affinity to the sequences located
between nt2113 and285. Since transfection and
superinfec-tion experiments indicated that nt2113 to259 (which lack a
strong IE2 86-binding site) were essential for induction of the promoter (47), we first assessed the relative importance of the
region between nt 284 and 259 and of the weak IE2
86-binding site between nt2113 and285. Our data show that in
transient-transfection assays, high-level activation of this pro-moter by IE2 86 exhibits an absolute requirement for the
sequences between nt 284 and 259 and can occur without
direct binding of IE2 86 to the DNA. However, the IE2 86 DNA-binding sites do appear to serve an accessory function as evidenced by the fact that when either a strong or a weak
binding site is positioned between nt2113 and285, the
over-all level of activation is increased twofold. These results are similar to those recently published by Arlt et al. (3), who
localized the essential cis-acting sequences to the region
be-tween nt284 and232 and found that the presence of a strong
IE2 86-binding site placed upstream of nt2117 enhanced the
induction of this promoter by IE2 86.
Since the most relevant region for mediating IE2 86 trans-activation on the 2.2-kb RNA promoter seemed to be bounded
by nt284 to259, we focused on further characterizing the
cis-acting sequences and trans-acting factors interacting with
those sequences. Site-specific mutational analysis showed that
FIG. 6. Supershift analysis of factors bound to the 2.2-kb RNA promoter. (A) Supershift analysis with ATF/CREB antibodies and U373-MG nuclear extracts bound to wild-type sequences284 to259 of the 2.2-kb RNA promoter or to the consensus ATF sequence. The gel shift procedure was performed as described in Materials and Methods. Lanes 1 to 5 contain labeled wild-type sequences284 to259 of the 2.2-kb RNA promoter (w). Lanes 6 to 10 contain labeled consensus ATF DNA (a). Lanes 1 and 6 contain DNA only. Lanes 2 to 5 and 7 to 10 contain U373-MG nuclear extract (NE). Lanes 3 and 8 contain anti-CREB antibody. Lanes 4 and 9 contain anti-ATF-2 antibody. Lanes 5 and 10 contain anti-ATF-4 antibody. (B) Gel shift analysis of heat-treated or untreated nuclear extracts (NE) incubated with wild-type or mutant sequences284 to259 of the 2.2-kb RNA promoter. Heat-treated nuclear extracts (D) were heated at 658C for 10 min (lanes 1 and 2). Lanes 3 to 12 contain untreated U373-MG nuclear extracts. Lanes 2, 4, 6, 8, 10, and 12 contain anti-CREB antibody. Lanes 1 to 4 contain labeled wild-type sequences284 to259 of the promoter. Lanes 5 and 6 contain labeled DNA mutated in site I (EcoRI site within nt284 to279). Lanes 7 and 8 contain labeled DNA mutated in site II (EcoRI site within nt278 to273). Lanes 9 and 10 contain labeled DNA mutated in site III (EcoRI site within nt272 to267). Lanes 11 and 12 contain labeled DNA mutated in site IV (EcoRI site within nt266 and261). To facilitate visualization of the bands, lanes 1 and 2 and lanes 7 to 12 are longer exposures of the same gel.
on November 9, 2019 by guest
http://jvi.asm.org/
the sequences between nt272 and261 were critical for trans-activation by IE2 86. These results, coupled with our previous observation that a consensus ATF/CREB-binding site was
lo-cated between nt271 and266, further supported our earlier
hypothesis that activation of this promoter might involve the ATF/CREB family of transcription factors (54). Additional support for this comes from the recent report by Lukac and colleagues, which shows that IE2 86 can activate a minimal
promoter containing thehsp70TATA box and an ATF/CREB
site in CV-1 cells (35). Although the ATF/CREB site contri-bution to the transactivation of this promoter seems to be
dominant, mutational analysis of nt284 to259 also suggests
that there may be other cooperative interactions between the various subregions of the promoter.
To identify the cellular factors that might interact with this major regulatory region on the promoter, we utilized a variety of gel retardation assays that included wild-type promoter, mutant promoter, and ATF consensus DNA probes (alone or in combination for competition assays), proteins from nuclear extracts or synthesized through in vitro translation reactions, and antibodies specific for members of the ATF/CREB family of transcription factors. Gel retardation analysis with a probe
containing the wild-type nt 284 to259 promoter sequences
and U373-MG nuclear extracts generated four bands. Three of the bands represent complexes with specific sequences, al-though cooperative interactions may influence the strength of the binding. Band 1 is a DNA-protein complex involving the
sequence between nt278 and273, and bands 2 and 3 involve
nt272 to261. Because this latter region contains a site similar
to the ATF/CREB consensus site, it seemed likely that these bands would contain one or more of the ATF/CREB transcrip-tion factors. Additranscrip-tional evidence that the wild-type promoter could interact with ATF/CREB factors was provided by the experiments showing that both in vitro-translated ATF-2 and CREB formed complexes with the DNA.
To simplify interpretation of the complex pattern of bands observed in the mobility shift assays with the U373-MG nu-clear extracts and wild-type promoter sequences, we also used the consensus ATF DNA sequence as a probe. This probe generated a band that comigrated with the above-described band 2 formed from the interaction of nuclear extract proteins
with the wild-type promoter sequences. In competition assays, we found that although the presence of excess unlabeled wild-type promoter sequences competed well with the formation of complexes with either probe, the ATF consensus sequence competed much more effectively for the binding of the nuclear proteins to the ATF consensus probe than to the wild-type promoter probe. These results suggest that at least one of the several proteins in U373-MG cells binding to the 2.2-kb RNA promoter belongs to the ATF/CREB family of transcription factors.
By supershift analysis with an anti-CREB antibody that does not cross-react with other members of the ATF family of tran-scription factors, we identified one of the binding factors in the U373-MG cells as CREB or a very closely related protein. CREB seems to be the major nuclear extract protein that interacts with the ATF consensus probe, as evidenced by the large fraction of the complex that is supershifted when the anti-CREB antibody is present. In contrast, with the wild-type promoter probe, it appeared that a smaller fraction of the complexes were supershifted by the anti-CREB antibody, with band 2 being most strongly affected. Preliminary results of supershift analysis with a highly specific antibody to ATF-1 suggest that ATF-1 present in the U373-MG nuclear extracts also binds to the promoter but to a smaller extent than CREB. Although the identities of the other proteins in the complexes are unknown, it is unlikely that any correspond to ATF-2 or ATF-4, since antibodies specific for these factors did not change the pattern of bands generated with the nuclear extract. The identification of the binding factor as CREB was further supported by the demonstration that the supershifted complex contained a factor resistant to heat denaturation, as is CREB. In addition, anti-CREB antibody did not supershift any of the bands when the probe contained mutations in the sequence
between nt272 and267 and supershifted only a very small
amount of complex when the probe contained mutations in the
sequence between nt266 and261. We conclude from these
experiments that band 2 reflects the interaction of the
tran-scription factor CREB with the sequence between nt272 and
261 and that this region on the promoter is critical for IE2
86-mediated transactivation. However, we cannot exclude the possibility that other factors that bind to this domain, partic-ularly those that form the complex corresponding to band 3, also contribute to activation of this promoter by IE2 86.
Further evidence that IE2 86 can indeed activate through CREB arose from experiments in which we tested the ability of IE2 86 to transactivate the somatostatin promoter, which con-tains the prototypical CREB-binding site. IE2 86 was capable of stimulating the somatostatin promoter by 100-fold. The fact that the fold activation of the somatostatin promoter by IE2 86 is identical to that obtained for the 2.2-kb RNA promoter strongly suggests that IE2 86 acts through the CREB site on the 2.2-kb RNA promoter. Experiments indicating that IE2 86
can also activate through DCREB have been published
re-cently by Lang et al. (31).
[image:8.612.62.294.71.219.2]A major question raised by our studies involves how CREB transactivates the 2.2-kb RNA promoter in conjunction with IE2 86. Although IE2 86 is able to form complexes with mul-tiple cellular transcription factors and regulatory proteins, we have been unable to detect any binding of in vitro-translated full-length CREB to IE2 86 under the same experimental con-ditions that allow efficient complex formation between IE2 86 and TBP, TFIIB, Rb, c-Jun, and JunB (48, 50, 51). In view of these results, we considered the possibility that full-length CREB and IE2 86 do not bind to each other directly to trans-activate the 2.2-kb RNA promoter. There are at least two cellular factors, p300 and CBP, that bind to CREB after it has
FIG. 7. Activation of the somatostatin promoter by IE2 86. Duplicate flasks of permissive U373-MG cells were cotransfected by the DEAE-dextran method, and cells were harvested 48 h posttransfection and assayed for CAT activity as described in Materials and Methods. The cells were cotransfected with 2mg of either theD(271) somatostatin promoter construct or 148CAT (the 2.2-kb RNA promoter construct) in the presence or absence of 1mg of pSGIE86. Lanes 1 to 3 were transfected with 148CAT. Lanes 4 to 6 were transfected withD(271). Lanes 2, 3, 5, and 6 contain pSGIE86. The fold activation by IE2 86 of the two promoters is shown at the top.
on November 9, 2019 by guest
http://jvi.asm.org/
been phosphorylated at serine 133 by protein kinase A (7, 30, 36). Since both p300 and CBP function as coactivators for CREB (2, 36), it seemed possible that IE2 86 was interacting with CREB through either of these proteins. The results of our experiments showing that IE2 86 is capable of binding to p300 and to CBP in vitro suggest that activation of the 2.2-kb RNA promoter might involve a multiprotein complex including CREB, p300 or CBP, and IE2 86. At this point, however, we cannot exclude the possibility that the binding of IE2 86 to p300 or CBP serves to modulate the transcriptional adaptor properties of the p300/CBP proteins. There is precedent for such a mechanism, since the adenovirus E1A protein, through its binding to CBP or p300, interferes with the ability of these proteins to serve as coactivators for CREB (2, 36). Alterna-tively, IE2 86-mediated activation through CREB may be in-direct and involve the regulation of protein kinase A and hence
the phosphorylation state of CREB. Further in vivo experi-ments will be required to resolve these possibilities, and such studies are in progress.
Just prior to submission of this communication, Lang et al. (31) shared with us a preprint of their studies on the activation of the same HCMV early promoter. There are a number of important differences in both the experimental approaches and results, but taken together, the data are complementary and lead to similar conclusions. In their studies, Lang et al. showed
that prokaryotically expressedDCREB protects a region on the
2.2-kb RNA promoter between nt 278 and 256. However,
their mutational analysis did not establish a clear role for the ATF/CREB-binding site in the context of the wild-type pro-moter. When they exchanged two nucleotides within this site
that abolished binding ofDCREB in DNase I protection
[image:9.612.142.472.70.457.2]ex-periments, activation of the promoter by IE2 86 was reduced
FIG. 8. Protein-protein interaction between IE2 86 and p300, CBP, CREB,DCREB, and TBP. p300, CBP, CREB,DCREB, and TBP were independently translated in vitro in the presence of [35
S]methionine. Each radiolabeled protein was incubated with GST or a GST–IE2 86 fusion protein immobilized on glutathione-agarose beads. Following incubation, an aliquot was removed (input) and the remaining beads were washed extensively. The proteins in the input (I) and bound (B) fractions were resolved by SDS-PAGE. (A) IE2 86 binding to p300. The input sample represents 1% of the total reaction. (B) IE2 86 binding to CBP. The input sample represents 20% of the total reaction. A shorter exposure of the input lane with respect to the bound lane is shown. (C) IE2 86 binding to CREB,DCREB, and TBP. The input samples represent 10% of the total reactions. In the binding reactions performed in the presence of ethidium bromide, the proteins were bound to glutathione-agarose beads and the mix was incubated in the presence of 100mg of ethidium bromide on ice for 30 min. Radiolabeled p300 or CBP was added, and the protocol was continued as described above, except that all the washes were performed in the presence of ethidium bromide. All the binding-reaction mixtures reported in this paper were washed with (20 mM Tris-HCl [pH 8], 1 mM EDTA, 0.5% Nonidet P40) NETN containing 100 mM NaCl except for those used to test CBP binding, which were washed with NETN containing 200 mM NaCl.
on November 9, 2019 by guest
http://jvi.asm.org/
less than twofold relative to the wild type. In contrast, the mutations introduced into the ATF/CREB site in our experi-ments reduced the level of activation sevenfold and signifi-cantly affected the binding of the U373-MG nuclear factors to the promoter. Since Lang et al. did not examine the binding of proteins in the permissive cell extracts that bound to either the wild-type or mutant 2.2-kb RNA promoter, it is possible that they inadvertantly created another transcription factor-binding site which, in the context of the wild-type promoter, could mediate activation by IE2 86. They were able to show, how-ever, with engineered constructs that contained oligonucleo-tides corresponding to either genuine or mutated ATF/CREB sites cloned as single copies or multimers just upstream of the TATA box of this promoter, that IE2 86 could mediate acti-vation through this site. They also observed in transient-ex-pression assays a strong stimulation of transcription from a reporter construct containing five GAL4-binding sites up-stream of the B-globin TATA box when it was cotransfected
with expression plasmids for both a GAL4-DCREB fusion
pro-tein and IE2 86 (31). Experiments were also performed to
determine whether IE2 86 can bind toDCREB, a protein that
contains a deletion of amino acids 88 to 101 (69). Their data indicated that there was a weak interaction between GST–IE2
86 and in vitro-translated DCREB. In our experiments,
al-though some binding can be observed, it is minimally above
background levels. However, it appears that DCREB
phos-phorylated by protein kinase A in vitro does bind more strongly to IE2 86 (31). Since p300 or CBP binds to protein kinase A-phosphorylated CREB (7, 30, 36), our results, coupled with those of Lang et al., suggest that strong activation of this HCMV early promoter through its CREB site may result from the ability of IE2 86 to interact with both proteins in the phosphorylated CREB-p300 or CREB-CBP complex.
In addition to showing a physical interaction between IE2 86 and CBP or p300, we have assessed the functional interaction in vivo. Our results show that IE2 86, in a dose-dependent fashion, can significantly increase the activation of a target
promoter containing GAL4-binding sites by a GAL4-CBP (full-length) fusion protein. This activation is specific and does not occur if the fusion protein contains only the carboxy ter-minus (amino acids 1678 to 2441) of CBP linked to GAL4. Interestingly, by itself the fusion protein with full-length CBP is a weaker transactivator than the one containing only the carboxy-terminal region (30). Since preliminary experiments in our laboratory suggest that IE2 86 can physically interact with the carboxy-terminal domain, it is tempting to speculate that as a result of its interaction with IE2 86, the full-length CBP undergoes a conformational change to the more active form. There is increasing evidence that in addition to CREB, CBP may function through a number of other transcriptional acti-vators (8, 24, 29), and thus some of the apparent promiscuity of IE2 86 as an activator may be due to its interaction with CBP or p300.
In conclusion, we have shown that activation of the 2.2-kb RNA promoter by IE2 86 requires sequences located between
nt272 and261. Within this region, there is a binding site for
the ATF/CREB family of transcription factors, and in permis-sive cells, CREB is the major family member which binds to
this cis-acting element. The precise mechanisms underlying
activation by IE2 86 through this site in vivo remain to be elucidated, but the data presented here, coupled with the data reported by Lang et al. (31), suggest that proteprotein
in-teractions between IE2 86 and either phosphorylatedDCREB
or the CREB coactivators CBP and p300 may play a crucial role. In transient-expression assays, direct binding of IE2 86 to the DNA appears to be less important for stimulation of this promoter, although the presence of the IE2 86 DNA-binding
site positioned between nt2113 and285 does contribute to
full activation through the essential downstream element.
ACKNOWLEDGMENTS
We thank Chuck Clark for excellent technical assistance and Bryan Salvant for helping with the construction of some of the mutant pro-moter plasmids. We also thank Isaac Engel, Lee Cranmer, Elizabeth Fortunato, Juan Carlos Gonzalez-Armas, Thomas Moreno, Christo-pher Morello, Franziska Ruchti, Steven Rodems, Marvin Sommer, and Maziar Younessian for stimulating discussions and critical reviews of the manuscript.
This investigation was supported by NIH grant CA-34729.
REFERENCES
1.Alford, C. A., and W. J. Britt.1990. Cytomegalovirus, p. 1981–2010.InB. N. Fields and D. M. Knipe (ed.), Virology, 2nd ed. Raven Press, New York. 2.Arany, Z., D. Newsome, E. Oldread, D. M. Livingston, and R. Eckner.1995.
A family of transcriptional adaptor proteins targeted by the E1A oncopro-tein. Nature (London)374:81–84.
3.Arlt, H., D. Lang, S. Gebert, and T. Stamminger.1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol.68:4117–4125. 4.Caswell, R., C. Hagemeier, C.-J. Chiou, G. Hayward, T. Kouzarides, and J.
Sinclair.1993. The human cytomegalovirus 86K immediate early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J. Gen. Virol.74:2691–2698. 5.Cherrington, J. M., E. L. Khoury, and E. S. Mocarski.1991. Human
cyto-megalovirus IE2 negatively regulatesagene expression via a short target sequence near the transcription start site. J. Virol.65:887–896.
6.Chiou, C.-J., J. Zong, I. Waheed, and G. S. Hayward.1993. Identification and mapping of dimerization and DNA-binding domains in the C terminus of the IE2 regulatory protein of human cytomegalovirus. J. Virol.67:6201–6214. 7. Chrivia, J. C., R. P. S. Kwok, N. Lamb, M. Hagiwara, M. R. Montminy, and
R. H. Goodman.1993. Phosphorylated CREB binds specifically to the nu-clear protein CBP. Nature (London)365:855–859.
8. Dai, P., H. Akimaru, Y. Tanaka, D. X. Hou, T. Yasukawa, C. Kanei-Ishii, T. Takahashi, and S. Ishii.1996. CBP as a transcriptional coactivator of c-Myb. Genes Dev.10:528–540.
9. Davis, M. G., S. C. Kenney, J. Kamine, J. S. Pagano, and E.-S. Huang.1987. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 84:8642–8646.
FIG. 9. In vivo interaction between IE2 86 and CBP. HeLa cells were trans-fected by the calcium phosphate coprecipitation procedure with 5mg of the pGAL4-CAT reporter pGAL4/E1b TATA containing five GAL4-binding sites (33), 5mg of the coding plasmids for the effector proteins GAL4-CBP (full length) or GAL4-CBP (amino acids 1678 to 2441) (C terminal) (7, 30), or 5mg of a protein kinase A coding plasmid and different quantities of pSGIE86 (28), and harvested 48 h posttransfection as discussed in Materials and Methods. Activation of the GAL4-CAT reporter construct by GAL4-CBP (full length) or GAL4-CBP (amino acids 1678 to 2441) in the absence of IE2 86 was set to 1.
on November 9, 2019 by guest
http://jvi.asm.org/
10. DeMarchi, J. M.1981. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology114:23–28.
11. Dignam, J. D., R. M. Lebovitz, and R. G. Roeder.1983. Accurate transcrip-tion initiatranscrip-tion by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res.11:1475–1489.
12. Furnari, B. A., E. Poma, T. F. Kowalik, S.-M. Huong, and E.-S. Huang.1993. Human cytomegalovirus immediate-early gene 2 protein interacts with itself and with several novel cellular proteins. J. Virol.67:4981–4991.
13. Geist, L. J., M. M. Monick, M. F. Stinski, and G. W. Hunninghake.1994. The immediate early genes of human cytomegalovirus upregulate tumor necrosis factor-alpha gene expression. J. Clin. Invest.93:474–478. 14. Ghazal, P., J. Young, E. Giuletti, C. DeMattei, J. Garcia, R. Gaynor, R. M.
Stenberg, and J. A. Nelson.1991. A discretecis element in the human immunodeficiency virus long terminal repeat mediates synergistictrans ac-tivation by cytomegalovirus immediate-early proteins. J. Virol. 65:6735– 6742.
15. Hagemeier, C., R. Caswell, G. Hayhurst, J. Sinclair, and T. Kouzarides. 1994. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J.13:2897–2903.
16. Hagemeier, C., S. Walker, R. Caswell, T. Kouzarides, and J. Sinclair.1992. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton imme-diate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J. Virol.66:4452– 4456.
17. Hermiston, T. W., C. L. Malone, and M. F. Stinski.1990. Human cytomeg-alovirus immediate-early two-protein region involved in negative regulation of the major immediate-early promoter. J. Virol.64:3532–3536.
18. Hermiston, T. W., C. L. Malone, P. R. Witte, and M. F. Stinski.1987. Identification and characterization of the human cytomegalovirus immedi-ate-early region 2 gene that stimulates gene expression from an inducible promoter. J. Virol.61:3214–3221.
19. Hurst, H. C., N. Masson, N. C. Jones, and K. A. W. Lee.1990. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol. Cell. Biol.10:6192–6203.
20. Iskenderian A. C., L. Huang, A. Reilly, R. M. Stenberg, and D. G. Anders. 1996. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of repli-cation genes. J. Virol.70:383–92.
21. Jahn, G., E. Knust, H. Schmolla, T. Sarre, J. A. Nelson, J. K. McDougall, and B. Fleckenstein.1984. Predominant immediate-early transcripts of hu-man cytomegalovirus AD169. J. Virol.49:363–370.
22. Jupp, R., S. Hoffmann, A. Depto, R. M. Stenberg, P. Ghazal, and J. A. Nelson.1993. Direct interaction of the human cytomegalovirus IE86 protein with thecisrepression signal does not preclude TBP from binding to the TATA box. J. Virol.67:5595–5604.
23. Jupp, R., S. Hoffmann, R. M. Stenberg, J. A. Nelson, and P. Ghazal.1993. Human cytomegalovirus IE86 protein interacts with promoter-bound TATA-binding protein via a specific region distinct from the autorepression domain. J. Virol.67:7539–7546.
24. Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S.-C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfield.1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell85:403–414.
25. Kerry, J. A., M. A. Priddy, T. Y. Jervey, C. P. Kohler, T. L. Staley, C. D. Vanson, T. R. Jones, A. C. Iskenderian, D. G. Anders, and R. M. Stenberg. 1996. Multiple regulatory events influence human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J. Virol.70:373–382. 26. Kline, J. N., L. J. Geist, M. M. Monick, M. F. Stinski, and G. W.
Hun-nighake.1994. Regulation of expression of the IL-1 receptor antagonist (IL-1ra) gene by products of the human cytomegalovirus immediate early genes. J. Immunol.152:2351–2357.
27. Klucher, K. M., D. K. Rabert, and D. H. Spector.1989. Sequences in the human cytomegalovirus 2.7-kilobase RNA promoter which mediate its reg-ulation as an early gene. J. Virol.63:5334–5343.
28. Klucher, K. M., M. Sommer, J. T. Kadonaga, and D. H. Spector.1993. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol. Cell. Biol.13:1238– 1250.
29. Kwok, R. P. S., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H.-M. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman.1996. Control of CAMP-regulated enhancers by the viral transactivator tax through CREB and the co-activator CBP. Nature (London)380:642–646.
30. Kwok, R. P. S., J. R. Lundblad, J. C. Chrivia, J. P. Richards, H. P. Bach-inger, R. G. Brennan, S. G. E. Roberts, M. R. Green, and R. H. Goodman. 1994. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature (London)370:223–226.
31. Lang, D., S. Gebert, H. Arlt, and T. Stamminger.1995. Functional interac-tion between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J. Virol.69:6030–6037.
32. Lang, D., and T. Stamminger.1993. The 86-kilodalton IE-2 protein of
human cytomegalovirus is a sequence-specific DNA-binding protein that interacts directly with the negative autoregulatory response element located near the cap site of the IE-1/2 enhancer-promoter. J. Virol.67:323–331. 33. Lillie, J. W., and M. R. Green.1989. Transcription activation by the
adeno-virus E1a protein. Nature (London)338:39–44.
34. Liu, B., T. W. Hermiston, and M. F. Stinski.1991. Acis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol.65:897–903.
35. Lukac, D. M., J. R. Manuppello, and J. C. Alwine.1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: require-ments for simple promoter structures and interactions with multiple com-ponents of the transcription complex. J. Virol.68:5184–5193.
36. Lundblad, J. R., R. P. S. Kwok, M. E. Laurance, M. L. Harter, and R. H. Goodman.1995. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature (London)374: 85–88.
37. Macias, M. P., and M. F. Stinski.1993. Anin vitro system for human cytomegalovirus immediate early 2 protein (IE-2)-mediated site-dependent repression of transcription and direct binding of IE2 to the major immediate-early promoter. Proc. Natl. Acad. Sci. USA90:707–711.
38. Malone, C. L., D. H. Vesole, and M. F. Stinski.1990. Transactivation of human cytomegalovirus early promoter by gene products from the immedi-ate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J. Virol.64:1498–1506.
39. McDonough, S. H., and D. H. Spector.1983. Transcription in human fibro-blasts permissively infected by human cytomegalovirus strain AD169. Virol-ogy125:31–46.
40. Montminy, M. R., K. A. Sevarino, J. A. Wagner, G. Mandel, and R. H. Goodman.1986. Identification of a cyclic-AMP responsive element within the rat somatostatin gene. Proc. Natl. Acad. Sci. USA83:6682–6686. 41. Nankervis, G. A., and M. L. Kumar.1978. Diseases produced by
cytomega-loviruses. Med. Clin. North Am.62:1021–1035.
42. Pari, G. S., and D. G. Anders.1993. Eleven loci encodingtrans-acting factors are required for transient complementation of human cytomegalovirus ori-Lyt-dependent DNA replication. J. Virol.67:6979–6988.
43. Pizzorno, M. C., and G. S. Hayward.1990. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major im-mediate-early promoter through a target sequence located near the cap site. J. Virol.64:6154–6165.
44. Pizzorno, M. C., M.-A. Mullen, Y.-N. Chang, and G. S. Hayward.1991. The functionally active IE2 immediate-early regulatory protein of human cyto-megalovirus is an 80-kilodalton polypeptide that contains two distinct acti-vator domains and a duplicated nuclear localization signal. J. Virol.65:3839– 3852.
45. Pizzorno, M. C., P. O’Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988.trans-activation and autoregulation of gene expression by the immedi-ate-early region 2 gene products of human cytomegalovirus. J. Virol.62: 1167–1179.
46. Sanger, F., S. Nicklen, and A. R. Coulson.1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA74:5463–5467. 47. Schwartz, R., M. H. Sommer, A. Scully, and D. H. Spector.1994. Site-specific
binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J. Virol.68:5613–5622.
48. Scully, A. L., M. H. Sommer, R. Schwartz, and D. H. Spector.1995. The human cytomegalovirus IE2 86 kDa protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol.69:6533–6540.
49. Shapiro, D. J., P. A. Sharp, W. W. Wahli, and M. J. Keller.1988. A high-efficiency HeLa cell nuclear transcription extract. DNA7:47–55. 50. Sommer, M. H., A. L. Scully, and D. H. Spector.1994. Trans-activation by
the human cytomegalovirus IE2 86-kDa protein requires a domain that binds to both TBP and RB. J. Virol.68:6223–6231.
51. Sommer, M. H., and D. H. Spector.Unpublished data.
52. Spector, D. J., and M. J. Tevethia.1994. Protein-protein interactions be-tween human cytomegalovirus IE2-580aa and pUL84 in lytically infected cells. J. Virol.68:7549–7553.
53. Speir, E., R. Modali, E.-S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein.1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science265:391–394.
54. Staprans, S. I., D. K. Rabert, and D. H. Spector.1988. Identification of sequence requirements andtrans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J. Virol.62:3463–3473. 55. Staprans, S. I., and D. H. Spector.1986. 2.2-Kilobase class of early tran-scripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J. Virol.57:591–602.
56. Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal.1990. Promoter-specifictransactivation and repression by human cytomegalovirus immediate-early proteins involves common and unique pro-tein domains. J. Virol.64:1556–1565.
57. Stenberg, R. M., D. R. Thomsen, and M. F. Stinski.1984. Structural analysis of the major immediate early gene of human cytomegalovirus. J. Virol. 49:190–199.