Copyright© 1993, AmericanSocietyforMicrobiology
Mutational Analysis of the Cysteine Residues in the Hepatitis
B
Virus
Small
Envelope Protein
CONSTANCE M. T. MANGOLD* AND ROLF E. STREECK
InstitutfiirMedizinischeMikrobiologie, Johannes Gutenberg-Universitat Mainz,
Augustusplatz, D-55131 Mainz, Germany
Received8February1993/Accepted10May 1993
The smallenvelopeproteinofhepatitisBvirus is the
major
componentof the viralcoatand is alsosecreted from cells as a 20-nm subviral particle, even in the absence of other viral proteins. Suchempty
envelopeparticles are composed of
approximately
100 copies of this polypeptide and host-derived lipids and are stabilizedby extensive intermolecular disulfidecross-linking. To studythe contribution of disulfide bondstoassembly
andsecretion of the viralenvelope, singleand multiplemutantsinvolving all 14 cysteines inHepG2and COS-7cellswereanalyzed. Of the sixcysteineslocated outside theregion
carrying
the surfaceantigen, Cys-48,Cys-65,andCys-69wereeach foundtobe essential for secretion of 20-nmparticles, whereasCys-76, Cys-90,and Cys-221were dispensable. Byintroduction ofan additional cysteine substituting serine 58, the yield of secretedparticleswasincreased. Of fourmutantsinvolving the eight cysteines located in the antigenic region,only
the double mutant lacking Cys.121 and Cys-124 was secreted with wild-type efficiency. Secretion-competentenvelopeproteins wereintracellularly retained bysecretion-deficient cysteine mutants. According toalkylation
studies, both intracellularand secreted envelope proteins contained free sulfhydrylgroups.Disulfide-linked oligomerswerestudiedby gelelectrophoresisunder nonreducingconditions. The human hepatitis B virus (HBV) and the related
hepatotropic viruses discovered in woodchucks (WHV),
ground squirrels (GSHV), ducks (DHBV), and herons
(HHBV)areclassifiedasthefamilyofHepadnaviridae.The virion structures, genome sizes,and modes ofreplicationof
thesevirusesaresimilar. The prototype,HBV, has a diam-eterofapproximately42 nmwith a27-nmcore enclosing a 3.2-kb DNA genome. The lipid-containing envelope carries three closely related protein components. These
polypep-tides are synthesized by initiation of translation at three distinct in-frame AUG codons of the HBV S open reading
frame, givingrise tothe large, middle, and small envelope proteins, respectively. TheC-terminal 226 amino acids cor-respondingtothe small Sprotein containthemajor antigenic determinant,hepatitis Bsurfaceantigen (HBsAg),which is
exposedon the surface of viralparticles. (For reviews, see references 9and24.)
In addition to complete virions, noninfectious HBsAg
lipoprotein particlesin both filamentous andsphericalforms aresecreted from the livers ofpatientswith HBV infections. The spherical 20-nm particles, which are present in large
excess, arepredominantly composed ofapproximately 100
copiesof the small S protein and host-derivedlipids.
Cul-turedcellstransfectedwithvectorsencoding onlythe small
S
protein produce lipoprotein
particles virtually
identicalto the 20-nmparticles found in the blood of HBV carriers.All of the information needed for correct assembly of theenvelopeproteinswithlipids, particle formation, and secre-tionmusttherefore reside in thesmall Sprotein itself.
The Sproteinisinitially synthesized as atransmembrane
protein spanningthe membrane of the endoplasmic reticu-lum (ER) at least twice (6) and is partially glycosylated at
asparagine 146 (22). Secreted 20-nm particles contain both
unglycosylated P24 protein and its glycosylated derivate, GP27. Secreted GP27 carries a complex oligosaccharide
*
Corresponding
author.chain,whereasonlythehigh-mannose form, whichisabout
1 kDasmaller, isdetectedintracellularly (18).
The Sproteinsin the secretedviraland subviral particles areextensivelycross-linkedby disulfide bridges(10). It has been demonstrated that covalent cross-linking occurs
con-comitantlywith assembly andbudding oflipoprotein
parti-cles(11), but it is unclear whether theformation of disulfide bonds is pivotal for this process. In the present study, we have usedmutant envelope proteinsto approach this ques-tion.
The small, middle, and large HBV envelope proteins contain cysteine residues exclusivelyinthe 226-amino-acid
sequence common to all three polypeptides. Of the 14
cysteines, 8 arelocated in ahydrophilic regioncarrying the surface antigen. Only three of the remainingcysteine resi-duesareconserved among allhepadnaviruses(7, 15, 17, 25,
26).Wehavenowcarried out asystematic analysis of single andmultiplemutants toidentify thecysteinesindispensable
forassemblyand secretion of 20-nm emptyenvelope
parti-cles.
MATERUILSANDMETHODS
Recombinant plasmids and site-directed mutagenesis. For
expressionof the HBV S gene(ayw),plasmid pNI2
carrying
the human metallothionein IIA promoter was used (13). After removal of the uniqueAccI site ofpNI2, the 1.8-kb
XhoI-BglII
fragment of the HBV genome (nucleotides [nt]129 to 1986 [8]) was inserted into the polylinker of pNI2,
yielding plasmid pW. Plasmid pBAP contains amodified S genewitha36-bp insertionat ntposition303 andencodesa hybrid S protein, HBsPolioAg, carrying an 11-amino-acid insertioncorresponding toapoliovirus type 1 VP1 neutral-ization epitope (5). For epitope tagging, the 552-bp
XhoI-SpeI fragment ofpW (nt 129 to 681) was replaced by the
corresponding
588-bp fragment of pBAP; this construct isdesignatedpWP.
For site-directed mutagenesis, we used bacteriophage 4588
on November 9, 2019 by guest
http://jvi.asm.org/
CYSTEINES IN THE HBV ENVELOPE 4589 M13-derivedrecombinants. HBVmpl9 and HBVmp8 carry
the 2.3-kb BglII-BglII fragment (nt 2839 to 1986) and the EcoRI-AccIfragment (nt 1 to 827) of wild-type HBV DNA, respectively. For mutagenesis of cysteines 107, 121, 124, 137, 138, 139, 147, and 149, which are located in the major antigenic determinant, the 734-bp XhoI-AccI fragment of pBAP was cloned into HBVmp8, thereby replacing the 698-bp XhoI-AccI wild-type sequence; the construct is des-ignated HBsPOLmp8. Site-directed mutagenesis was per-formed with the following antisense oligonucleotides:
Cys48Ala, * 5'-ATF11lGGCCTAGAGCCACGGTAGT-3'; Cys48Ser, *
5'-ATTTTGGCCTAGAGACACGGTAGT-3';
Ser58Cys, *
5'-GGTGAGTGATTACATGTTGGGGACTG-3'; His6OAla,
5'-AGGTTGGTGATGCATTGGA-3';
His6O Gln,5'-ACAAGAGGTTGGTGAfJXATTGGA-3';
Cys65 Ala, *5'-GTTGGAGGAGCAGACGTCGGTGAGTGATT
G-3'; Cys65Ser, *
5'-GTTGGAGGACTAGACGTCGGTGA
GTGATT-3'; Cys69Ala, *
5'-CAGCGATATCCAGGAGCA
GTTGGAG-3'; Cys69Ser, *
5'-CAGCGATATCCAGGACT
AGTTGGAG-3'; Cys65/69Ser, * 5'-CAGCGATATCCAGG
ACTAGTTGGAGGACTAGAGGTTGG-3'; Cys76Ala,
5'-CGCCGCAGAGCCATCCAGC-3';
Cys76Tyr,5'-AACGCCGCAGAIACATCCAGCG-3'; Cys9OAla, 5'-AGAAGATGAG
GGCTAGCAGCAGG-3'; Cys9OPhe,
* 5'-CAACAAGAATATTAGGiAATAGCAGCAG-3';
CyslO7Ala, 5'-GAATTAGAGGAGCAACGGGCAAC-3';
Cysl21/124Ala, 5'-CAGTAGTCATGCjGGTCCGGGCTGGTCCCGTGC-3';
Cysl37/138/139Ala, 5'-GTCCGAAGG TTGGTAGCGGCAGG
AGGGATACATAGA3'; Asnl46Gln, * 5'-GGAATACATG
TGCACTGTCCGTCCGAAG-3';
Cysl47/149Ala, 5'-GGATGGGAATAGCGGTGGCAl-TICCGTCCG-3';
Cys221Ala,5'-ACCCAAAGAGCAAAGAAAAT-3'.
For designation of each mutation, the wild-type amino acid is indicated, and then itspositionandthemutantamino acid are listed. In the DNA sequences, the corresponding antisense codons areunderlined; lightface characters refer to thewild-type DNA sequence, and boldface characters refer to mismatches. Asterisks indicate theincorporationorremoval ofdiagnostic restrictionsites. Mutationsoutsidetheunderlinedsequences aregeneticallysilent.
Themutagenic oligonucleotideswere annealed to single-stranded DNAsobtained from the recombinant M13 phages described above. Mutants were isolated by the
phospho-rothioate method (16) asmarketedby Amershamand were screened directly by restriction site analysis or DNA se-quencing. Fragments containing a desired mutation were generated bycleavage withXbaIand AccIorwithXhoIand
SpeI andwereusedtoreplace thecorresponding wild-type
sequenceofplasmid pW;theexchanged regionwasverified
by DNAsequencing. Multiplemutationswereintroduced by combination ofappropriate fragments carrying single muta-tionsorbyrepeatingthemutagenesis withmutanttemplate
DNA.
Cell culture, transfection, and HBsAg detection. Human
hepatoma HepG2(14)orCOS-7cellswere seeded at -5 x
lm
per 6-cm dish in Dulbecco's modified Eagle's mediumplus 10% fetal calf serum 1 day prior to transfection with calciumphosphate precipitatesof DNA(16 ,ug) (29).HepG2 and COS-7 cellswereexposedtotheprecipitatesfor 20to24 h and for 8 to 14 h, respectively, washed twice, and then incubated with fresh Dulbecco'smodifiedEagle'smedium. Three days after transfection, the culture medium was collected. The cells were washed twice with
phosphate-buffered saline(PBS) andlysed byincubationoniceduring
15 minwith 1 ml of0.5% Nonidet P-40 in PBS. Thelysates were collected and vortexed three times for 20 s. Culture
medium and cellular lysates were clarified by centrifugation (10 min at 2,000 xg and 4°C in an Eppendorf Microfuge), andHBsAg reactivity wasdeterminedby anenzyme-linked immunosorbent assay (ELISA; Auszyme II ELISA; Ab-bott).
Density
and sedimentationvelocity analysis.
Mutant and wild-type HBsAgparticles secreted by transfected HepG2cells were concentrated by centrifugation for 4 h at 45,000 rpm and 4°C in a Beckman SW60 rotor. Thepellets were resuspended, layered onaCsClorsucrosestepgradient (10 to50% [wt/wt]), and centrifuged for 18 h at 35,000 rpm in an SW60rotor. Gradientswerefractionated, and eachfraction was diluted and analyzed for the presence of HBsAg by ELISA; thedensities of CsCl and sucrose were determined byrefractometry.
Metabolic labeling, immunoprecipitation, and gel electro-phoresis. Isotopic labeling was started 36 to 48 h after transfection. Culture medium was removed and samples
weresubjected toELISA asdescribedabove.The cells were washed and incubated for 40 min in 1.5 mlof methionine-free
minimal essential medium with1% dialyzedfetal calfserum. Then, 375 ,uCi of [35S]methionine (>1,000
Ci/mmol;
NewEnglandNuclear)was added. Aftera pulse-labeling period
of 2 to 4 h, a chase was performed byreplacement of the medium with 2 ml of Dulbecco's modified Eagle's medium plus 10% fetalcalfserum,which containsexcess unlabeled methionine.Atthe end of the chaseperiod, the mediumwas collected; the cellswerewashed twice in TN buffer(0.1 M
Tris-HCl [pH
8.0]-0.1
M NaCl, with or without 20 mMiodoacetamide), lysedwith 1 mlof 0.5%Nonidet P-40 in TN
buffer, and vortexed three times for 20 s. Except for the
experiments shown in Fig. 1 and 3A, B, and D, freshly prepared iodoacetamide (400 mM in water; Sigma) was added (1:20dilution) tothe TN buffer andto the collected culture medium. Forimmune precipitation, we usedrabbit antiserum to HBsAg particles from human serum (Calbio-chem) or ascitic fluid containing the monoclonal antibody C3, which recognizes the inserted poliovirus epitope. Ly-sates and mediawere cleared by centrifugation (10 min at 2,000xgand4°C inaMicrofuge) andwereincubatedonice
for 2 h with antibodies (1:100 dilution) in the presence of detergents(0.5% NonidetP-40,0.05%sodiumdeoxycholate,
and 0.01% sodium dodecyl sulfate [SDS]) and foran addi-tional hour with protein A-Sepharose CL-4B (Pharmacia),
with rotationat4°C. Immune complexeswerewashed three times in RIPA buffer(10 mM Tris-HCl [pH 7.5], 150 mM
NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS) andoncewith 0.1 MTris-HCl, pH6.8. Thiswashing procedure also removed excess iodoacetamide. Samples were prepared for SDS-12% polyacrylamide gel
electro-phoresis(PAGE)by the addition of Laemmli buffer with5% 2-mercaptoethanol and 2% SDS, boiling for 7 min, and incubationonicefor 15to30 min(standardconditions).To obtainnonreducingconditions, 2-mercaptoethanolwas omit-ted from thesamplebuffer. Thegelswerefixed, impregnated with Amplify (Amersham), and dried before exposure to a KodakXARSfilm at -70°C.
RESULTS
ThenonconservedcysteinesintheHBVenvelopeproteinare not essential for secretion of 20-nm particles. To
study
the contribution of the cysteine residues in the HBV smallenvelope proteintoassembly andsecretion of 20-nm
parti-cles, mutant S genes (subtype
ayw)
were constructedby
oligonucleotide-directed
mutagenesis.
Mutant genes were VOL.67,1993on November 9, 2019 by guest
http://jvi.asm.org/
A. sw a b c d e f g h
27 _ ____
24 14151
12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
B. a w C. a w
43.0-
29.0-18.4
1 2 3 4 1 2 3 4
D.
FIG. 1. Synthesis and secretion ofS proteins lacking
noncon-servedcysteines. COS-7cells transfected with plasmids encoding wild-typeormutant Sproteinswerepulse-labeledwith
[35S]methio-nine for 3 hand thenchasedfor 12(A),6(B),or18(C)h.Aliquots
of cellular lysates (odd-numbered lanes) or cellular supernatants (even-numbered lanes)weresubjectedtoimmunoprecipitationwith
anti-HBsAg antiserum followed by SDS-PAGE under standard
conditions.Arrowheads mark thepositionsofnonglycosylated (24)
andglycosylated (27) Sproteins.Themigrationofmarkerproteins (molecular masses in kilodaltons) is represented on the left (B).
Mutantandwild-type proteinsaredesignated bysmallletters(D).
expressed inCOS-7orHepG2cells fromthe human metal-lothionein IIA promoter by usingthe vectorpNI2 (13).
Initially, cysteine residues thatarenotconserved among
the mammalianhepadnaviruseswerestudied. WhereasHBV
carriesacysteineresidue at eachofthepositions 76, 90,and 221 of theSprotein,both WHVandGSHV havetyrosineat
position 76, leucine at position221, and an additional
cys-teine at position 58; cysteineis also found atposition 90of
the WHVSprotein,butphenylalanineisfound in GSHV(7, 25). To investigate whether the HBV S protein tolerates
similar variation in these positions, HBV Ser-58 was
re-placed by cysteine, Cys-76 was replaced by alanine or
tyrosine, Cys-90was replaced byalanineorphenylalanine,
and Cys-221wasreplaced byalanine.
Theamounts ofSproteinssecreted fromtransfectedcells weredeterminedbyELISA andappearedto beverysimilar
for the wild type, the sixsingle mutants, thedoublemutant
Cys76Ala;Cys9OPhe, and the triple mutant Cys76/221Ala; Cys9OPhe. Subsequently, the transiently expressed S
pro-teins were metabolically labeled, immunoprecipitated, and analyzed bySDS-PAGE(Fig. 1). Asshownbythe compar-ison with wild type (Fig. 1A, lanes 1 and 2), all mutants displayed the same pattern of glycosylated (27-kDa) and nonglycosylated (24-kDa) proteinboth inlysatesandcellular supernatants(Fig. 1A,lanes 3 to 18).TheSer58Cysmutant (lanes a) seemed to have a higher electrophoretic mobility
andto besecretedfasterthan wildtype.This isshownmore
clearlyinFig. 1BandCbyapulse-chase experimentwitha
short(Fig. 1B)and long (Fig. 1C)chase.The other mutants (lanes b to h) were synthesized and secreted like the wild type,consistent with theELISA data.
The finding that all mutants were easily detected by ELISA indicates that the structure of the major antigenic determinantwasnotgreatly disturbed. Moreover,
sucrose-and cesium chloride-gradient analyses (data not shown) revealedthat the secretedproteins formed particleswith the size and buoyant density (1.2 g/ml) characteristic of wild-type 20-nmlipoproteinparticles.
These results demonstrate that cysteines 76, 90, and 221
are dispensable for assembly and secretion of 20-nmHBV lipoprotein particles andsuggestthatan additionalcysteine
atposition58 enhances theefficiencyofsecretion.
Contribution of nonessential cysteines to disulfide
cross-linkingof HBVenvelope proteins.Toinvestigate whether the degreeof intermolecular disulfidecross-linkingwasaffected
by mutation of the nonessential cysteines, wild-type and mutant S proteins were compared by SDS-PAGE under reducingandnonreducingconditions (Fig. 2). Cell lysisand immunoprecipitation were performed in the presence of
iodoacetamidetoprevent the formation of disulfide bondsin vitro. Ser58Cys, the triplemutant Cys76/221Ala;Cys9OPhe, and the followingtwo additional mutantswere includedin this study: a sextuplemutant (i), whichwasobtained from
thetriplemutantbyreplacing cysteines 48, 65,and 69, and the mutantAsnl46Gln(v).Whereasthesextuplemutantwas
deficient for secretion (Fig. 2A, lanes 10 and 11), the nonglycosylated mutantAsnl46Glnwas secreted, although
slightlylessefficientlythanthe wild type(Fig. 2A,lanes 4to 7).
Underreducingconditions, the total amountof immuno-precipitated- S proteins migrates exclusively as monomers
(Fig. 2A). Whenthesameamountofproteinswasanalyzed
undernonreducingconditions, onlydimersandhigher-order oligomerswerefound(Fig. 2B).Three bandsweredetected
in the dimer region (lanes 7 and 9), two of which could tentativelybe ascribed to homodimers ofglycosylated (27/ 27)andnonglycosylated (24/24)Sprotein bycomparingthe nonglycosylatedmutant(Fig. 2B,lane5)with the wild type (Fig. 2B,lane7). Theintermediatebandmaycorrespondto heterodimers (24/27) and/oranalternative form ofthe24/24 homodimer(Fig. 2B, lane5). Inaddition, a diffuse band in
the80- to 90-kDaregion(Fig. 2B, lanes 5, 7, and9) which might represent tetrameric forms was observed.
Higher-orderoligomers apparentlydid not enterthegel. (Notethe relatively strong signalsin theupperpart andontopof the gel,forexamplein lane13 ofFig. 2B.) By comparisonwith cells transfected with the vector pNI2 lacking the S gene
(Fig. 2B,lane2),dimerswerealsodetected in lysatesofcells synthesizingSproteins (even-numbered lanes).
Todemonstrate thatoligomerizationwas duetodisulfide bonding, mutant and wild-type S proteins were analyzed
undermildly reducingconditions (Fig. 2C). Inthepresence
of 1 mM dithiothreitol, both monomers and dimers were detectable(lanes1 to12).Whentheconcentration of dithio-threitolwasgraduallyraisedfrom 0 to30 mM,the
conver-sion ofoligomersinto dimers andofdimers intomonomers wasobserved,as shownfor theSer58Cys mutant(Fig. 2C, lanes 13 to 18).
The finding that the secretion-deficient sextuple mutant formeddimers(Fig. 2B,lane10)indicatesthat theremaining eight cysteines located in the major antigenic region are
involved in dimerization. Both dimers and higher-order oligomers were detected for the triple mutant (Fig. 2B,
mutant h). However, the amounts of dimers under
nonre-ducingconditions (Fig. 2B, lanes 6 to 9) and ofmonomers under mildly reducing conditions (Fig. 2C, lanes 4 to 7) appeared to beslightlylargerforthe triplemutantthan for
HBVsmall Sproteinsanalyzed
a Ser58Cys b Cys76Ala
c Cys9OAla d Cys221 Ala e Cys76Tyr
f Cys9OPhe
g Cys76Ala;Cys9OPhe
h Cys76/221 Ala;Cys9OPhe
w wild-type
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.59.298.73.296.2]CYSTEINES IN THE HBV ENVELOPE 4591 REDUCING
M u v w h a
m. m _- wm m m
B. NONREDUCING
M u v w h a
m rn m m r---m m
200.00u-54!!| | | l l 4-mer[ 68.0-- w ss
43.0- * 27/27
24/27-
24/24-ea.e_
__ 0b
_m
wa,"~-18.4- S
1 2 3 4 5 6 7 8 9 10 11 12 13
w
1 2 3 4 5 6 7 8 9 10 11 1213
1 mM DTT 0 .2 1 5 30 mM DTT
u v w h a a w
s.In
.S.k ". - 0,"_..
4Imer
I
4-er[ i - s 3 3-mer
I
... * *
2-mer[ - 410.|lt tv
.~~
29.0-27_
24_- ..61.
[image:4.612.140.494.71.483.2]1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
FIG. 2. Analysisofoligomerizationof mutantSproteins.COS-7cells weretransfected withplasmidsencodingwild-type (w)ormutant
Sproteins (a, h, i,andv)orwith thevectorpNI2 (u).[35S]methionine-labeledproteins (3-h pulse,12-hchase)wereimmunoprecipitatedand
analyzed bySDS-PAGEinthepresenceof 5%2-mercaptoethanol (A),in the absence ofareducing agent (B),and in thepresenceof various
concentrations ofdithiothreitol (DTT) (C). Molecularmasses(in kilodaltons) of 14C-labeled markerproteins (M) (GIBCO-BRL) and the
positionsofnonglycosylated (24)andglycosylated (27)monomers(arrowheads)and ofputative oligomersofSproteinsareindicatedonthe
left of theautoradiograms. 24/24, 27/27,and24/27markpresumptivehomo- and heterodimers(B). (AandB)Even-numbered lanes,cellular
lysates;odd-numberedlanes,cellularsupernatants;(C)lanes1, 2, 4, 6, 8,and10,lysates;otherlanes,supernatants.In lanes 12 to17,only
one-third of the normalamountof cellularsupernatantwasused forimmunoprecipitation.
the wild type, although virtuallyidentical amounts of these proteins were immunoprecipitated (Fig. 2A, lanes 6 to 9). These results suggestthatcysteines 76, 90, and/or221may
be involved in intermoleculardisulfide bridges.
Asurprisinglysmallamountof dimers andlargeamount of high-molecular-weightmaterial werefound when Ser58Cys
was analyzed (compare lanes 13 of Fig. 2A and B). This indicates that the additionalcysteinepresentin this mutant favors the formation ofhigher-order oligomers. Curiously, both the glycosylated and nonglycosylated forms of Ser58Cyswere splitinto two bands at 30 mM dithiothreitol (Fig. 2C, lane 17). Thiswas also observed with 5% 2-mer-captoethanol (Fig. 2A, lane 13),but not when the proteins
were notalkylated (Fig. 1,mutant a).
Cysteines 48, 65, and 69 and histidine 60 in the HBV S protein are essential for secretion of 20-nm particles. The cysteinesatpositions 48, 65,and 69of theHBVSproteinare
strictlyconservedamong allhepadnaviruses (7, 17, 25, 26). Tostudytheir role in the assemblyand secretion of 20-nm particles, these residues were replaced by alanine and serine. Single and multiple mutants were constructed, in-cludingthetriplemutants(gandq), aquadruplemutant (h) carryinganadditional cysteine76substitution, anda
sextu-ple mutant (i) in which cysteines 90 and 221 were also
replaced (cf. Fig. 3). As shown in Fig. 3A through C, all
mutantswere clearlydetectable in cellular lysatesof
trans-fected COS-7 cells but not in the cell culture media. To
analyzewhether theblock to secretioncould be reversedby A.
29.0
-27
_-24
_-C.
HBVsmall S proteinsanalyzed
a Ser58Cys
h Cys76/221Ala;Cys9OPhe Cys48/65/69/76/221Ala;
Cys9OPhe
u noS proteins
v Asnl46Gln
w wild-type VOL. 67,1993
on November 9, 2019 by guest
http://jvi.asm.org/
4592 MANGOLD AND STREECK
A. M a b c d e f
43.0-
v
*'
._.i29.0
-27
P-24_ 18.4
-0 ...*...".,
1 2 3 4 5 6 7 8 9 10 11 12 13
C. k w m n o p q
_ 2 3 4x
1 2 3 4 5 6 7 8 9 10 111213141516
B. M g h w
D. u w .
_m '
4 ^,
1 2 3 4 5 6 7 8 91
E. a b c g
.
y--1-0r
[image:5.612.151.454.76.459.2]12345 6 7 8 9 10
FIG. 3. Analysisof Sproteins lackingconservedcysteines..COS-7cellstransfected with the vectorpNI2 (u)orwithplasmids encoding wild-type (w) or mutant S proteins (ato t) were pulse-labeled with [35S]methionine for 3 h and then chased for 20 h (A through D). Immunoprecipitated proteinsand markerproteins (M)weresubjectedtoSDS-PAGEunder standard conditions. Molecularmassesof marker
proteins (in kilodaltons) and thepositionsofglycosylated (27) andnonglycosylated (24) S proteins (arrowheads)areindicated. (Aand B) Even-numberedlanes,cellularlysates;odd-numberedlanes,cellular supernatants; (CandD)odd-numberedlanes,lysates;even-numbered
lanes,supernatants; (E)cellularlysatesafter3-h chase(odd-numbered lanes)or20-h chase(even-numbered lanes).
the introduction ofa cysteine residue at another position, Ser-58 was replaced by cysteine in the secretion-deficient single mutants (Fig. 3, mutants r, s, and t). However, no
effect of the Ser58Cyssubstitution onthesecretory pheno-typeof anyof these mutantscould be observed (Fig. 3D). Thisclearlydemonstrates that each of thecysteines 48, 65, and 69 is indispensablefor secretionofSprotein.
For comparison, histidine 60, which is also conserved
among allhepadnaviruses, wasmutatedtoeitherglutamine
oralanine. As shown inFig.3C(lanes1to4),noneofthese mutants was secreted, indicating that His-60 must be in-cluded amongtheessential amino acidsof the Sprotein.
Although the cysteine and histidine mutants described abovewerenotsecreted, theywerestillglycosylatedtothe
sameextentasthewild-typeprotein (Fig. 3).This indicates
thattranslocation across the membraneof the ERwasnot noticeablyaffected.Accordingtotheelectrophoretic mobil-ities of themutantproteins, theircarbohydratechainswere
not converted to the complex form found in secreted S proteins (see,e.g.,Fig. 3C,lanes 5 and6).Therefore, these mutantsweremostlikelyarrested priortothe medialGolgi compartment. Since allmutantswere also detectable in the cellularlysates byELISA(datanotshown),thestructureof the major antigenic determinant was probably not greatly disturbedbythemutations.
Toinvestigatewhether the differentamountsofSproteins found for some of the mutants should be attributed to differences in stability, pulse-chase experiments were
car-riedout (Fig. 3E).When the amountsofprotein incellular lysateswerecomparedaftera3-h chase(lanes 1, 3, 5, 7,and
9)anda20-h chase(lanes 2, 4, 6, 8,and10),adecreasewas
detectable. Thesinglemutants(a, b,andc)seemedtobe less stablethan the tripleand sextuplemutants.
Disulfide cross-linkingofsecretion-deficient mutant S
pro-teins. To analyze whether substitution of essential amino acids in the HBV S protein noticeablyaffects thedegree of
a Cys48AIa m Cys48Ser
b Cys65AIa n Cys65Ser
c Cys69AIa 0 Cys69Ser
d Cys48/65AIa p Cys65/69Ser
e Cys48/69AIa q Cys48/65/69Ser f Cys65/69AIa r Cys48AIa;Ser58Cys g Cys48/65/69Ala s Cys65AIa;Ser58Cys h Cys48/65/69/76Ala t Cys69AIa;Ser58Cys
Cys48/65/69f76/221Ala;Cys9OPhe
k His6OGln u no S proteins I His6OAla w wild-type
J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
CYSTEINES IN THE HBV ENVELOPE 4593 NONREDUCING
k w m n o p q u M
s:PX
4 64bg
a=s I% fi
27 -24~
1 2 3 4 5 6 7 8 9 10 11
FIG. 4. Disulfide cross-linking of secretion-deficient mutant S proteins. [35S]methionine-labeled proteins (3-h pulse, 20-h chase) wereimmunoprecipitated from COS-7 cells transfected with plas-mids encoding wild-type (w) or mutant S proteins (k to q) or with the vectorpNI2(u). Precipitated proteins and marker proteins (M) were subjected to SDS-PAGE under nonreducing conditions. The corre-sponding reducing gel is shown in Fig. 3C, which also specifies the mutations introduced. The positions of S monomers (P24 and GP27) and dimersareindicatedontheleft. Lanes1to 3 and 5 to10, cellular lysates;lane4, cellular supernatant; lane 11, markerproteins.
intermolecular disulfide cross-linking, SDS-PAGE was per-formedunder nonreducingconditions. The resultsobtained forfive mutants in which cysteines 48, 65, and/or 69 were replaced by serine and for thetwo secretion-deficientHis-60
mutantsareshown inFig.4; thecorrespondingreducing gel is shown in Fig. 3C. Small amounts of monomers were
detected for all mutants, and the signals obtained in the
dimer region were equally faint for the wild type and the
single and double mutants (Fig. 4, lanes 1 to 8). This indicates that the majority of polypeptides formed larger
disulfide-linked complexes. In contrast, the triple mutant accumulated in a dimeric form (Fig. 4, lane 9), as was
previously observed for the sextuple mutant(Fig. 2B, lane
10).
Interaction between secretion-deficient and secretion-com-petent S proteins.We next investigated whether the
secre-tion-deficientmutantsassociatewith thewild-typeSprotein
and whether this leads to cosecretion of the mutant or to intracellular retention of the wild type. To be able to
distinguishbetweenthe wildtype and mutants, we initially
chose aswild type thenonglycosylatedmutantAsnl46Gln,
which isefficientlysecreted(Fig.2A,lanes4to7) and forms
20-nm lipoprotein particles (data not shown). When the mutant genes were cotransfected with the phenotypically wild-type gene (v) intoCOS-7 cells at a molar ratio of3:1,
secretion ofAsnl46Glnwas
drastically
reduced(Fig. 5).Noglycosylated S protein could be detected in cellular super-natants, with the exception of
Cys48Ala;Ser58Cys,
which wascosecreted in atinyamount(Fig.5, lane15). Curiously,the secreted GP27 form of this mutant was split into two bands onelectrophoresis, as wasobserved for the
Ser58Cys
mutant.
Todemonstratemoreclearlyspecificinteraction between
wild-typeand mutant Sproteins, epitopetaggingof the wild
M v v+a v+b v+c v+g v+i v+r u
29.0O- i l l
27
1-
24-18.4-_
-
X4
a
[image:6.612.331.558.72.177.2]1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 FIG. 5. Intracellular retention ofasecretion-competentS protein bysecretion-deficientmutants.COS-7cellsweretransfected witha 1:3mixture of the secretion-competent Asnl46Glnmutant (v) and one of the following secretion-deficient mutants: Cys48Ala (a), Cys65Ala (b), Cys69Ala (c), Cys48/65/69Ala (g), Cys48/65/69/76/ 221Ala;C'ys9OPhe(i), andCys48Ala;Ser58Cys(r). In the control (v), pNI2 DNAwasused instead ofmutant DNA.Cellstransfected with thevectorpNI2 alone (u)wereusedfor lanes 16and 17.
[35S]me-thionine-labeled proteins (4-hpulse, 23-h chase)were immunopre-cipitated and analyzed by SDS-PAGE under standard conditions. Molecularmasses(in kilodaltons) of marker proteins(M) and the positions ofglycosylated (27) and nonglycosylated (24) S proteins (arrowheads)areindicated.Even-numberedlanes, cellularlysates; odd-numbered lanes,cellular supernatants.
type was used. In this approach, cysteine mutants were
coexpressed withamodified Sgeneencoding the
secretion-competent, hybrid protein HBsPolioAg. This polypeptide carriesan11-amino-acidpoliovirus epitope inserted between theduplicated glycine 50 residue ofthe HBVsmall Sprotein (5). Immunoprecipitation was performed by using either HBsAg-specificantiserum(Fig.6A andC)orthe poliovirus-specific monoclonal antibody C3(Fig. 6B andD). Cysteine
mutants, but not the wild-type S protein (w), reduced the
efficiency ofsecretion of the hybrid protein (wp).
Surpris-ingly, inhibition ofsecretion ofHBsPolioAg was less pro-nounced thanthat observed for theAsnl46Gln protein (Fig. 5). Inaddition, smallamountsofsecretion-deficient mutant
proteinsweresecreted under these conditions,as seenmost
clearly for
Cys48Ala;SerS8Cys
(Fig. 6A [wp+r, revealingtwobandsfor P24]). With the antibody C3,
coimmunopre-cipitation of the cysteine mutants with HBsPolioAg was found (see P24 in Fig. 6B and D), indicative of a stable
associationbetween these proteins. Theefficient
coprecipi-tation obtained from thecellularsupernatantssuggests that all of the secreted mutantpolypeptideswereassembled with thehybridprotein into mixed particles (compareP24inFig.
6Awith P24inFig. 6B,e.g., lanes6 or16).
Taken together, these results demonstrate that the
perti-nent cysteine mutations do not prevent association with either of the two phenotypically wild-type proteins (v and
wp). The interaction leads to an almost complete block to
secretion for the Asnl46Gln protein; the hybrid protein HBsPolioAg is retained to a lower extent and enables
inefficient secretion of thecysteinemutants.
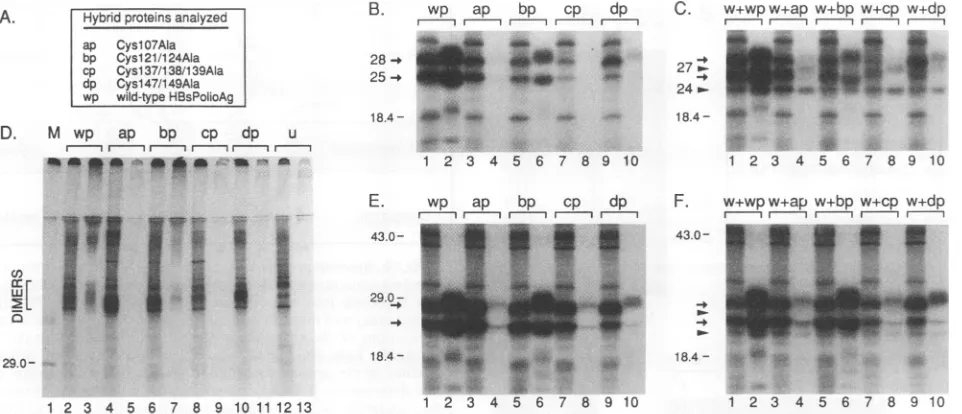
Contribution ofcysteines located in the antigenicregionto
oligomerization
and secretion ofHBV S proteins. Of the 14 cysteine residues in the HBV Sprotein, 8 arelocated in a hydrophilic region carryingthemajorantigenic determinant, HBsAg,which is aconformationalepitope(2). Substitutionofcysteineswas therefore carriedoutin the
hybrid protein
HBsPolioAg, which makes
immunoprecipitation
with the monoclonal antibodyC3 possible, asdescribed above. Thefollowing mutants were constructed:
CyslO7Ala, Cys121/
124Ala, Cysl37/138/139Ala,
andCysl47/149Ala.
VOL.67, 1993
on November 9, 2019 by guest
http://jvi.asm.org/
[image:6.612.109.260.74.273.2]A. wp wp+w wp+a wp+b wp+c wp+g wp+i wp+r u
w
wpw
wp+
wp+wp+
wp+ wp+wp+
u 2825
18.4-B. wp wp+w wp+a wp±bwp+cwp+g wp+qwpr u
2430
C. w
wpw
1wp
wp
wp*
wp+
wp+ u4.0
-18.4 P;rS;
D. wp wp+w wp+m wp±n wp+o wp+p wp±q u
I--I-- '- '1 1 --- l ----'
43.0
X
,.'WA.sAhX* ,
18.4
[image:7.612.59.296.71.485.2]-1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
FIG. 6. Interaction betweensecretion-deficientcysteinemutants
andthesecretion-competenthybridprotein HBsPolioAg. Wild-type (w) or secretion-deficient S proteins (a to r) were synthesized
togetherwiththesecretion-competent hybrid protein HBsPolioAg (wp)inCOS-7cellscotransfectedwiththecorresponding plasmids
mixedatamolarratioof3:1. Ascontrol,cellsweretransfectedwith thevectorpNI2alone(u).Thesecretion-deficientsingle (a, b,c, m,
n,ando), double (pandr,triple (gandq),andsextuple (i)mutants
arespecifiedin Fig.3. [3S]methionine-labeledproteins (4-h pulse,
23-h chase) were immunoprecipitated with HBsAg-specific
anti-serum (Aand C)orwith themonoclonal antibody C3 (B and D).
Precipitated proteinswereanalyzed by SDS-PAGE under standard conditions.Themigrationof markerproteins(molecularmasses in
kilodaltons)isrepresentedontheleft. Thepositions ofglycosylated
(28) and nonglycosylated (25) HBsPolioAg (wp) are indicated by
arrows,and thepositions of S proteins (P24 and GP27)areindicated byarrowheads.
Asexpected, none of thesemutants could be detected in supernatantsorlysatesoftransfected COS-7cells by ELISA with a mixture of HBsAg-specific monoclonal antibodies, indicating that the conformation of the antigenic loop was
disturbedby themutations.Asshown in Fig. 7, themutant
S proteins could be immunoprecipitated with rabbit
poly-clonal antiserum to HBsAg particles (Fig. 7B), but more efficiently so with C3 antibodies (Fig. 7E). The double mutant Cysl21/124Ala (bp) was synthesized, glycosylated, andsecreted like the corresponding wild-type protein (wp), which indicates that thesecysteines are not required for the secretion of S proteins. All other mutants were rather poorly secreted (Fig. 7E [ap, cp, and dp]). Interestingly, Cysl47/
149Ala, which had been mutated right next to the carbohy-drateattachment site, Asn-146, was glycosylated to a higher extent than thewild-type protein (Fig. 7 [dp]).
To study the interaction between each of these mutants and the wild-type S protein (w), the corresponding genes were coexpressed in COS-7 cells (Fig. 7C and F). With HBsAg-specific antibodies, partial retention of the wild type (w) was found (Fig. 7C). Moreover, coimmunoprecipitation ofwild-type S proteins was obtained with C3 antibodies (Fig. 7F [P24]), indicative of the formation of mixed protein aggregates between wild-type and mutant S proteins.
Finally, the mutants were analyzed by SDS-PAGE under nonreducing conditions (Fig. 7D). Dimers were found in the cellular lysates for the mutants CyslO7Ala, Cysl21/124Ala, andCysl47/149Ala (ap, bp, and dp) in amounts larger than those for the wild type (wp). Putative multimers were also detected on top of the gel and in secreted Cysl21/124Ala
particles.
Free sulfhydryl groups are present in HBV envelope pro-teins. In the course of this study, we observed that the electrophoretic mobilities of S proteins were reduced through addition of the alkylating agent iodoacetamide dur-ing cell lysis. This agent reacts with free sulfhydryl groups and was used toprevent the formation of disulfide bonds in vitro. Since excess iodoacetamide was removed upon wash-ing of the immune precipitates, the observed shifts in elec-trophoretic mobility upon alkylation should reflect the pres-ence ofcysteines carrying free SH groups at the moment of cell lysis (Fig. 8). Theretardation observed in this analysis was particularly strong for mutants carrying the Ser58Cys
mutation (Fig. 8B, lanes 1 to 4). A minor shift was noticed for thewild-type protein (Fig. 8B, lanes 5 and 6). However, alkylation did not affect migration of the sextuple mutant in which thecysteines 48, 65, 69, 76, 90, and 221 were replaced byeither alanine or phenylalanine (Fig. 8C, lanes 1 and 2). Thisfinding suggests that the remaining eight cysteines in the antigenic region are all disulfide linked. Moreover, it con-firms that the changes in electrophoretic behavior observed were not caused by alkylation of residues other than cys-teines. The corresponding triple mutants, lacking either cysteines 48, 65, and 69 or 76, 90, and 221,respectively, both exhibited a small shift in electrophoretic mobility on alkyla-tion, indicating the presence of free SH groups (Fig. 8C, lanes 5 to 8). Mostnoticeably, alkylation of secreted 20-nm particles also changed the electrophoretic mobility of the wild-type protein (Fig. 8D, lanes 3 and 4). These results strongly suggest that both intracellular and secreted wild-type S proteins contain one or more cysteines that are not involved in disulfide bonding.
DISCUSSION
This paper presents the results of anextensive mutational analysis of the 14 cysteines in the small envelope protein of HBV, subtype ayw. These residues are strictly conserved among all HBV subtypes known (17). To study their contri-bution to the assembly and secretion of 20-nm HBsAg lipoprotein particles, each cysteine was replaced by alanine. Alanine was chosen to minimize secondary effects on the
on November 9, 2019 by guest
http://jvi.asm.org/
CYSTEINES IN THE HBV ENVELOPE 4595
A. - Hybrid proteins analyzed
ap Cysl07AIa
bp Cysl2l/124Ala
cp Cysl37/138/139AIa
dp Cysl47/149AIa
wp wild-type HBsPolioAg
D. M wp ap bp cp dp u
m| rm-mmm----r---i 1l X
4-**a _ ^
B. wp ap bp cp dp
mm
m*
28-. a*
25 W 4
18.4- 440_t - -*
1 2 3 4 5 6 7 8 9 10
C. w+wpw+ap w+bpw+cp w+dp
,, r- r- I
27 _. We z
24_. _ _ _ w.w
18.4
-1 2 3 4 5 6 7 8 9 10
.iw
w
I
il,
U)
w
I'll
IE
29.0-1 2 3 4 5 6 7 8 9 10 11 1213
E.
43.0-
29.0-wp ap bp cp dp
r---
'
rII
_|fi~~~~~t
Pa....
18.4
-..*.
1 2 3 4
43.0
-18.4
-5 6 7 8 9 10
F. w+wpw+ap w+bp w+cp w+dp
m--- m---i m m m
ce
1 2 3 4
_
-+_
5. 8
5 6 7 8 9 10
FIG. 7. Analysis ofhybridSproteins lacking cysteines in the antigenic region. COS-7 cells were transfected with plasmids encoding the wild-type(wp) or one of the mutant hybrid proteins (ap to dp) (A) or with 3:1 mixtures of plasmids encoding a mutant and the wild-type S protein (w).[35S]methionine-labeledproteins (4-hpulse,20-hchase) wereimmunoprecipitated with rabbit antiserum against HBsAg particles (B and C) or with the monoclonal antibody C3 (D, E, and F). Precipitated proteins were subjected to SDS-PAGE under reducing (B, C, E, andF)ornonreducing conditions (D). The positions of glycosylated (28) and nonglycosylated (25) hybrid proteins are marked by arrows, and thepositions of wild-type S proteins (P24 and GP27) are marked by arrowheads. Migration of marker proteins is represented on the left; molecular masses are in kilodaltons. (B, C, E, and F) Odd-numbered lanes, cellularlysates; even-numbered lanes, cellularsupernatants;(D) even-numbered lanes,lysates;odd-numberedlanes,supernatants;lanes 12 and 13,control cellstransfected with the vector pNI2 (u). The positions of dimersareindicated.
proteinstructure (4), but some mutantsintroducing serine,
phenylalanine,ortyrosinewere alsoconstructed. Sincethe HBV middle and large envelope proteins carry no other
cysteinesbutthose present in the smallprotein,someofthe
resultsobtained for20-nmparticles shouldalso bepertinent
tothe assembly of whole virions.
Contribution ofcysteinestosecretionand
antigenicity
ofthe HBVenvelopeprotein. Each ofthecysteinesatpositions48,65, and 69 and histidine at position 60 were shown to be
indispensable for secretionof HBVsubviral particles.This is
consistent with the observation that these residues are
strictly conservedamong allhepadnaviruses (7, 17,25, 26), and it also explains why deletion mutants involving this
region of the S protein are secretion defective (23). In contrast,thenonconservedcysteinesatpositions76, 90, and 221 are completely dispensable, since neither single nor
multiple mutations
noticeably
affected the efficiency ofse-cretion of 20-nmlipoprotein particles. The SerS8Cys
muta-tion is perfectly tolerated, but an HBV subtype carrying cysteine 58 has not yet been found (17). It would be
interestingtoanalyzethe effect of theSer58Cys mutationon theassemblyandsecretionof thecompleteHBVvirion and of thehepatitis deltavirus (3).
Theeight cysteines located in the antigenic region were
studiedbyusing the
hybrid
Sprotein HBsPolioAgtoensure immunologic detection after mutagenesis of the HBV sur-face antigen. The additional 12 amino acids of poliovirus capsid protein VP1 inserted at position 50 of the HBV Sprotein contain no cysteine residues and do not affect the
efficiency of secretion or the HBs antigenicity (5). The double mutant Cysl21/124Ala was secreted like the wild type,as
previously
reportedfor thesinglemutantCysl21Ser
(1). Since the mutants
Cysl07Ala,
Cysl37/138/139Ala,
andCysl47/149Alawereratherpoorly secreted, atleast someof
thesixcysteinesinvolved shouldplay a role in the secretion process. Substitution of most of the antigenic region was
previously reportedtobe compatiblewithparticle assembly and secretion. Von Brunn et al. obtained hybrid 20-nm
particleswhen amino acids114 to 156 of the Sproteinwere
replaced byPlasmodium falciparum merozoite
gp190-spe-cificsequences(28). Moreover,the envelopeproteinsof the
avianHBVscompletelylack thesequencecorrespondingto HBV amino acids 105 to 155 (15, 27). This indicates that
assemblyandsecretion ofemptyenvelopeparticlesmay be
achievedwithoutdisulfidebonds involvingcysteinesof the
antigenic determinant. Itshould benoted, however, that the
morphologyof avian viralparticles is atypical(24).
The four mutants carrying cysteine substitutions within theantigenic regionreactedonlymarginallyif at all with the mixture of HBsAg-specific monoclonal antibodies used in theimmunoassay. Similarresults had beenobtainedfor the
singlemutants
Cysl24Ser
and Cysl47Ser (2), whereas theantigenic reactivity of
Cysl21Ser
waseithergreatly reducedor enhanced,
depending
onthe monoclonal antibody used (1).The structureof themajorantigenic determinantis mostlikely disturbedby theeliminationofdisulfidebonds. How-ever, authentic folding of the antigenic region is not a
prerequisite for secretion of the S protein, since both
Cysl21Ser (1)
andCysl21/124Ala
wereefficiently
secreted.Itisnoteworthythat mutantslackingcysteines48, 65,69, 76,
90, and/or 221 were easily detected by ELISA, which suggests that these residues do not interact withcysteinesin theantigenic region.
Mutationofcysteines 121and 124 shouldpresenta selec-tive advantage to HBV in vivo, helping to evade the host immune system. For in vitro studies, the mutant
Cysl2l/
124Ala could be useful for the selection of monoclonal antibodiesrecognizingother
epitopes
in theSprotein,
e.g., VOL.67,1993on November 9, 2019 by guest
http://jvi.asm.org/
[image:8.612.78.559.74.281.2]4596 MANGOLD AND STREECK
A.
B.
HBV small S proteinsanalyzed
a Cys48Ala;Ser58Cys b Ser58Cys
c Cys48/65/69/76/221 Ala;
Cys9OPhe 27
m-d Cys48/65/69AIa
e Cys76/221Ala;Cys9OPhe 24 >, w wild-type
C. c w d e
,, , m m,
Alkylation - + - + - + - +
29
0--a
_k*a
~~~~~~~~~~~~~~~~~~ip
b
w.r-* i
,, ;_,. . '
Ai& 'p", a
_ _
1 2 3 4 5 6
D.
wAl l:..
'..
.-_ f:.
_ 4sos
*_ _
18.4
[image:9.612.61.295.74.413.2]-1 2 3 4 5 6 7 8 1 2 3 4
FIG. 8. Analysis of free sulfhydrylgroupsin Sproteins.
Dupli-cate cultures ofHepG2 cells
synthesizing
wild-type or mutant S proteins (A)werelabeled with[ S]methionine for2h, chased for 6 h,andsubsequently lysed in thepresence(+)orabsence(-) of the alkylatingagent iodoacetamide.Proteinswereimmunoprecipitatedfrom the lysates (B and C) and analyzed by SDS-PAGE under standardconditions. (D)Immunoprecipitationofthewild-type (w)
fromlysate (lanes 1 and2) and supernatant(lanes3 and4),half of
which was treated with iodoacetamide (+). Migration of marker proteins is represented on the left (C); molecular masses are in
kilodaltons. Arrowheadsmarkthepositionsof thenonglycosylated (24)andglycosylated (27)S protein.
to improve detection in clinical diagnosis. Finally, these mutantsmightbeusedtofurthercharacterize the role of the various cysteines for the subtype-specific determinants of HBV.
Disulfide cross-linking of envelope proteins. It has been reported that the approximately 100 S protein molecules presentinHBsAg particles areextensively cross-linked by
disulfide bonds (10, 11) and do not contain palmitylated cysteine residues (20) or free sulfhydryl groups (10). In
contrast, the results of alkylation experiments presented heresuggestthepresenceoffree sulfhydrylgroups, evenin
secretedparticles. The disulfide-bonding pattern within the 20-nm particles seems to be heterogeneous, since both oligomersandpolymersweredetectedby gel electrophore-sisunder nonreducing conditions.The existence of hetero-geneityis alsosuggested bytheelectrophoreticbehavior of the SerS8Cys and Cys48Ala;Ser58Cys mutants, showing doublebandsunderreducingconditions.
FIG. 9. Secondary-structure model for the HBV S protein. A secondary-structuremodel(adaptedfromreference27)divides the HBV Sprotein into hydrophobic regions that interact with lipids (shadedarea) andmorehydrophilic regions. The numbers indicate thepositions of the cysteines in the protein. Cysteines in the first hydrophilic loop are initially located in the cytoplasm, whereas cysteines in the second hydrophilic loop carrying the major anti-genicdeterminant, HBsAg,arepositioned in the lumen of theER. After assemblyand secretion of Sproteins, the antigenic loopis exposedonthe surface of(sub)viral lipoprotein particles(outside), while the firstloop isproposedtobe locatedinside theparticles.
Role of cysteines in intracellular transport and
assembly
of S proteins. A secondary-structure model for the S protein(adaptedfrom reference27) is presented in Fig. 9. According tothismodel, specifichydrophobicregions in the S protein interact with lipids and separate two major hydrophilic
regions. Wheninitially synthesized as transmembrane
pro-tein,the secondhydrophilicloop of the Sproteinis translo-cated into the lumen oftheER, whereas the first hydrophilic loop carrying cysteines 48, 65, 69, and 76 remains in the
cytoplasm(6, 9).Assuggested bythepartialglycosylationof thewild-type protein and all mutants studied, the glycosy-lation attachment site asparagine 146 located in the second hydrophilic loop is only partially accessible. This may partly be duetodisulfide bondsinvolvingcysteines 147 and/or 149, since thedoublemutantCysl47/149Ala was more efficiently glycosylated than the wild type. As demonstrated by the
secretion-competent phenotype of the Asn146Gln mutant and aspreviously shown by tunicamycin experiments (18),
glycosylationofthe S protein is notessential. However, the
nonglycosylated mutant was secreted somewhat less
effi-ciently than the wild type. Glycosylation may speed up secretion by preventing the formation of wrong disulfide bonds.
Recently, evidencehas been obtained thatdisulfide-linked HBVS dimers,whichare formed early in the ER, are first sortedto apost-ER, pre-Golgicompartment andthen con-verted slowly into high-molecular-weight, disulfide-linked oligomers (11). Sincenoneofourcysteinemutants accumu-lated inamonomeric form, proper folding of the monomeric S protein is not a prerequisite for dimerization. Previous studies with other secretoryproteins have shown that aber-rantprotein foldinggenerally causes ER retention (12, 19). Failure of furtheroligomerizationof mutant S proteins may therefore be duetomisfoldingofthedimers.Thefinding that eventhesextuplemutant still formed dimers indicates that disulfidebondinginvolved one or more of the eightcysteines in theantigenic region. Thisconclusion isconsistent with the
secondary-structuremodel(Fig. 9)which postulates that the
antigenic loopistranslocated into the oxidizing environment of the ER lumen. Sincenoneof the mutations involving the
cysteinesintheantigenicregioneliminatedthe capacity for J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:9.612.312.552.74.194.2]CYSTEINES IN THE HBV ENVELOPE 4597 dimerization, the proteinsforming adimer were mostlikely
cross-linked byseveral disulfide bonds. However,itcannot be excludedthat the elimination ofcysteines causesshuffling of the remaining disulfide bonds.
According to the secondary-structure model, the first hydrophilic loop is initially located in the reducing environ-ment of the cytoplasm. The final disposition of this loop in secreted particles is still disputed (21, 27). It is remarkable that three of the four cysteines present in this loop are indispensable for secretion. If the high-molecular-weight aggregatesformed by the singlemutantsand a double mutant involving these essential residues resemble authentic high-order oligomers, then these residues should be dispensable for the intermolecular disulfide cross-linking within these structures. Cysteines 48, 65, and 69, together with the essential histidine 60, may therefore play a critical role in one of the subsequent steps of the maturation of 20-nm particles, e.g., in the budding from the membrane into the lumen of the intermediate compartment (9, 11).The question of whether cysteines 48, 65, and 69form disulfide bondsor carry free sulfhydryl groups is currently beingaddressedby analyzing the DHBV small S protein which exclusively carries these cysteineresidues.
ACKNOWLEDGMENTS
Wethank G. Lutfalla for the HepG2 cell line, B. Fleischer for the COS-7 cells, F. Delpeyroux forproviding plasmid pBAP and mono-clonal antibody C3, N. Israelfor plasmid pNI2, and B. Mechler for the synthesis of some of the oligonucleotides. We gratefully ac-knowledge thetechnical assistance of B. Zahn and M. Werr.
This work was supported by a grant from the Deutsche For-schungsgemeinschaft (Ma 1212/1-1).
REFERENCES
1. Antoni, B. A., and D. L. Peterson. 1988.Site-directed mutagen-esisof the hepatitis B surface antigen gene: creation of a free sulfhydryl group and modification of the protein in the 22-nm particlestructure, p. 313-317. In A. J. Zuckerman (ed.), Viral hepatitisand liver disease. Alan R. Liss, Inc., New York. 2. Ashton-Rickardt, P. G., and K. Murray. 1989. Mutants of the
hepatitis B virus surfaceantigen that define some antigenically essential residues in the immunodominant a region. J. Med. Virol. 29:196-203.
3. Bonino, F., K. H. Heermann, M. Rizetto, and W. H. Gerlich. 1986. Hepatitis delta virus: protein composition of delta antigen and itshepatitis Bvirus-derived envelope. J. Virol. 58:945-950. 4. Cunningham, B. C., and J. A. Wells. 1989. High-resolution epitopemapping ofhGH-receptorinteractions by alanine-scan-ningmutagenesis. Science244:1081-1085.
5. Delpeyroux, F.,E. vanWezel, B.Blondel,and R. Crainic. 1990. Structural factors modulate the activity of antigenic poliovirus sequences expressed on hybrid hepatitis B surface antigen particles. J. Virol. 64:6090-6100.
6. Eble,B.E., D. R.Macrae, V. R.Lingappa,and D.Ganem. 1987. Multiple topogenic sequences determine the transmembrane orientation of hepatitis B surface antigen. Mol. Cell. Biol. 7:3591-3601.
7. Galibert, F., T. N. Chen, and E. Mandart. 1982. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: com-parisonwith the hepatitis B virus sequence. J. Virol. 41:51-65. 8. Galibert, F.,E.Mandart,E.Fitoussi, P. Tiollais, and P. Char-nay.1979. Nucleotide sequence of the hepatitis B virus genome (subtypeayw)cloned in E. coli. Nature(London) 281:646-650.
9. Ganem,D.1991.Assembly ofhepadnaviral virions and subviral particles.Curr. Top. Microbiol. 168:61-83.
10. Guerrero, E.,F.Gavilanes,and D. L.Peterson.1988. Modelfor theprotein arrangementin HBsAg particles basedonphysical and chemical studies, p. 606-613. In A. J. Zuckerman (ed.), Viral hepatitis and liver disease. Alan R. Liss, Inc.,NewYork. 11. Huovila, A.-P. J., A. M. Eder, and S. D. Fuller. 1992. Hepatitis Bsurface antigen assembles in a post-ER, pre-Golgi compart-ment. J. CellBiol.118:1304-1320.
12. Hurtley, S. M., and A. Helenius. 1989.Proteinoligomerizationin theendoplasmic reticulum.Annu. Rev. Cell Biol. 5:277-307. 13. Israel, N.,N.Chenciner, C. Houlmann, and R. E. StreecL 1989.
An expressionvector forhigh-level protein synthesis in Vero cells. Gene 81:369-372.
14. LutfaUa, G.,L.Armbruster, S. Dequin,and R. Bertolotti. 1989. Construction ofan EBNA-producing lineof well differentiated human hepatoma cells and of appropriate Epstein-Barr virus-based shuttlevectors. Gene76:27-39.
15. Mandart, E., A. Kay, and F. Galibert. 1984. Nucleotide
se-quence ofacloned duck hepatitisBvirusgenome: comparison with woodchuck and human hepatitis B virus sequences. J. Virol.49:782-792.
16. Nakamaye, K. L., and F. Eckstein. 1986. Inhibition of restriction endonuclease NciI cleavage by phosphorothioategroups andits application to oligonucleotide-directed mutagenesis. Nucleic AcidsRes. 14:9679-9698.
17. Norder, H.,B.Hammas, S.Lofdahl, A.-M. Courouc6,and L.0. Magnius. 1992. Comparison of the aminoacid sequences ofnine different serotypes of hepatitis B surface antigen andgenomic classification ofthe corresponding hepatitisBvirus strains. J. Gen. Virol. 73:1201-1208.
18. Patzer, E. J., G.R. Nakamura, and A. Yaffe. 1984.Intracellular transportand secretion ofhepatitis Bsurface antigen in mam-malian cells. J. Virol. 51:346-353.
19. Pelham, H. R. B. 1989. Control of protein exit from the endoplasmic reticulum. Annu. Rev. Cell Biol. 5:1-23.
20. Persing, D. H., H. E.Varmus,and D.Ganem. 1987. The preSl protein of hepatitis B virusis acylated at itsamino terminus with myristic acid. J. Virol. 61:1672-1677.
21. Peterson, D. L. 1987. The structure of hepatitis B surface antigen and its antigenic sites. Bioessays6:258-262.
22. Peterson, D. L.,N. Nath, and F. Gavilanes. 1982. Structure of hepatitis B surface antigen: correlation of subtype with amino acid sequence andlocation of thecarbohydrate moiety. J. Biol. Chem. 257:10414-10420.
23. Prange, R., R. Nagel, and R. E. Streeck. 1992. Deletions in the hepatitisB virus small envelope protein: effectonassembly and secretionof surface antigenparticles. J.Virol. 66:5832-5841. 24. Robinson,W.S. 1990. Hepadnaviridae and theirreplication,p.
2137-2169. In B.N. Fields, D. M.Knipe, etal.(ed.), Virology, 2nd ed. RavenPress, Ltd., NewYork.
25. Seeger, C., D. Ganem, and H. E. Varmus. 1984. Nucleotide sequenceof an infectious molecularlyclonedgenomeofground squirrel hepatitisvirus. J. Virol. 51:367-375.
26. Sprengel, R., E. F. Kaleta, and H. Will. 1988. Isolation and characterization of a hepatitis B virus endemic in herons. J. Virol. 62:3832-3839.
27. Stirk, H. J., J. M. Thornton, and C. R. Howard. 1992. A topologicalmodel for hepatitis B surfaceantigen.Intervirology 33:148-158.
28. von Brunn, A., K.Frfh, H.-M.Muller, H.-W.Zentgraf,and H. Bujard. 1991. Epitopes of the human malaria parasiteP. falci-parum carried on the surface of HBsAg particles elicit an
immune response against the parasite. Vaccine 9:477-484. 29. Wigler, M., S. Silverstein, L. S. Lee, A. Pellicier, Y. C.Cheng,
and R. Axel. 1977. Transferofpurified herpesvirusthymidine kinase gene to cultured mouse cells. Cell 11:223-232.
VOL. 67,1993