0022-538X/87/030883-06$02.00/0
Copyright©) 1987, American Society for Microbiology
Adenovirus-Dependent Changes in Cell Membrane Permeability:
Role of
Na+,K+-ATPase
PREM SETH,t IRAPASTAN, AND MARKC. WILLINGHAM*
Laboratory of Molecular Biology, Division ofCancerBiology and Diagnosis, National Cancer Institute,
Bethesda, Maryland 20892
Received 21 August 1986/Accepted 19 November 1986
Adenovirus-dependent release of choline phosphate from KB cells at pH 6.0 was partially blocked by
ouabain. In K+-containing medium, maximum inhibition of release was obtained by i0-5 M ouabain and half-maximal inhibitionwasachieved by about 0.5x 10-6Mouabain. Ouabain didnotblockeither the binding or theuptake ofadenovirus byKB cells. Without K+, about 25% of cell-associated choline phosphate was
released by adenovirus,whereas with 1 mMK+about 50% wasreleased. This activationbyK+wasblocked by 0.1 mM ouabain. HeLa cells behaved likeKB cells, butamutantof HeLa cells resistanttoouabain (D98-OR)
releasedmuch loweramountsof choline phosphate inresponsetohuman adenovirustype2 (Ad2). Wild-type D98-ORcells bound nearly thesameamountof adenovirus asdid normal HeLa cells. Ad2alsoincreased the
activity ofNa+,K+-ATPaseinKBcells,with maximum activationat50pgof Ad2perml. InD98-ORcells,Ad2
failed to activate Na+,K+ ATPase activity. Ad2-dependent lysis of endocytic vesicles (receptosomes) was assayed by measuring Ad2-dependent enhancement of epidermalgrowthfactor-Pseudomonasexotoxin toxicity. Thisactionofadenoviruswasincreased whenK+ waspresentinthemedium. Under the conditions used,K+ hadno effectonthe amountof Ad2orepidermal growth factor takenupby the cells. On thebasis ofthese
results, itis suggested that Ad2-dependentcellulareffluxof choline phosphate and adenovirus-dependent lysis of receptosomes mayrequire Na+,K+-ATPase activity.
Humanadenovirus type 2 (Ad2) is a nonenveloped virus that enters the cell through a receptor-mediated process (4,
8, 25, 29). After initial binding to its cell surface receptor,
Ad2is sequestered in coatedpits onthe plasma membrane
and then internalized into uncoated endocytic vesicles termed receptosomes (18) or endosomes (13). Ad2 appears to escapefrom receptosomesinto the cytoplasmby
ruptur-ingthe receptosome membrane (8). Lysis of a receptosome appears to befavored by its acidic pH (pH 5 to 5.5) (24).
Fusionof enveloped viruses with the membrane of endocytic vesicles is also promoted by an acidic pH (16, 32). Ad2 also
increases the permeability of the plasma membrane toward small moleculessuch as5tCr-labeledpeptides, choline
phos-phate, deoxyglucose phosphos-phate, and ot-aminobutyric acid
when the cells are exposed to anacidic environment (26, 27).
This low-pH-dependent increase in cell membrane
perme-ability allows one to study the mechanism by which Ad2
causeslysis of thereceptosome membrane during itsescape
into the cytoplasm. We investigated here the role of a
membrane-associated ion pump regulated by Na+,K+-ATPase, since it islikelythatendocyticvesiclescontainthis
ionpump(7, 10, 33). We studied this by usingan inhibitor
(ouabain) and an activator (K+) of Na+,K+-ATPase. Ad2
effects were also studied in HeLacells and in a mutant of
HeLacells(D98-OR) with altered Na+,K+-ATPase (30).
MATERIALSANDMETHODS
Cellsandvirus. KB, HeLa, andHeLaD98-OR cells were maintained as monolayers inDulbecco modified Eagle
me-diumcontaining
penicillin-streptomycin
and10% calfserum. KB cells were obtained from the American Type CultureCollection
(Rockville, Md.).
Ad2waspropagated
inKBcells*
Corresponding
author.tPresent address: Laboratory of Developmental Oncology,
BioneticResearch, Inc., Frederick,MD 20871.
grownin suspension culture. Ad2 and 35S-labeled Ad2 were prepared by the procedure described earlier (11, 28). All of the experiments were conducted by using
UV-light-inactivated virus asdescribed earlier(23).
Choline release assays. Ad2-dependent release of choline fromcells was measured as described previously (26). (We have shownpreviously [26]that mostofthecholinereleased
fromthe cellsisintheform of choline phosphate.) Inbrief,
KB, HeLa,orD98-OR cellswereplatedat adensityof 0.5 x
106
cells per 35-mm(diameter) dish and were used24h later.Cellswerelabeled with
[3H]choline
(10 ,uCi/ml) for30 mininDulbeccomodifiedEagle mediumcontaining2 mgof bovine
serumalbumin perml(mediumA). Cells were washedfour
times with mediumA and once with basal Eagle medium,
without bicarbonate, containing2mgofbovine serum albu-min per ml (medium B) adjusted to pH 7.0. Cells were
exposed to Ad2 (1 to 50
jig/ml)
at 4°C for 60 min; 1,ug/ml
correspondsto anaverageof9 x 103 particlesadded per cell.
Choline releasewasmonitoredat37°C for60min,usually in
medium B adjusted to pH 6.0 with 25 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid). In
cer-tain experiments, isotonic sucrose-HEPES (225 mM su-crose, 5 mM glucose, 25 mM HEPES; pH 6.0) orisotonic
saline (125 mM NaCl, 5 mM glucose, 25 mM
HEPES; pH
6.0) was used. If
required,
various concentrationsof other ions were also included in these buffers. The effect of ouabain was studied byadding
various concentrations ofouabain
(10-9
to 10-4 M)tothe medium.Receptosomelysisassay.Lysis ofthe receptosome
by
Ad2wasmonitoredbymeasuringenhancementof the
toxicity
ofahybridtoxin of
epidermal growth
factor(EGF)
and Pseu-domonasexotoxin (PE)asdescribedpreviously (23,
24).
Inbrief,cellswereincubated in medium
B,
pH
7.0,
for 60 min with EGF-PE(0.5jig/ml)
andvariousconcentrationsof Ad2(0.01to 10
,g/ml).
Atthe endofincubation,
protein
synthe-883
on November 10, 2019 by guest
http://jvi.asm.org/
+Ad2
Na+,K+-ATPase activity wasassayed in the crudeplasma
membrane preparation as described earlier (6). Adenylate cyclase activity was measured by the procedure described earlier (21). The enzyme activities were monitored for the
required lengths of time in the absence or presence of 5 x
10-4
Mouabain.EGF-PE. EGF-PE hybrid toxin was prepared as
previ-ously described(8).
Chemicals. [3H]choline was obtained from New England
NuclearCorp.(Boston, Mass.).Mouse EGF was purchased
from Bethesda Research Laboratories(Gaithersburg,Md.). Medium constituents were from GIBCO Laboratories
(GrandIsland, N.Y.). All other chemicals were from Sigma Chemical Co. (St. Louis, Mo.).
,. -Ad2
RESULTS
O7oL
--10-
1 1Effect
of ouabain on Ad2-dependent choline release. To0-9 10-8 10-7 10-6 i0-5 i0-4 investigate the role of Na+,K+-ATPase in Ad2-dependent OUABAIN [M] release of choline from cells, we tested the effectof ouabain, 1. Effect of ouabain on Ad2-dependent choline release. KB an inhibitor of plasma membraneNa+ ,K+-ATPase. Increas-,eled with[3H]cholinewere washed four times with medium ing the concentration of ouabain blocked theAd2-dependent once with medium B, pH 7.0. The cells were exposed to 10lg choline released from cells (Fig.
1).
Choline released by per ml at4°Cfor 60min and then washed twice with medium control cells was not affected. Maximum inhibition (40 to 6.0. Choline release was monitored in medium B in the 50%) ofAd2-dependent release ofcholine was obtained byor presence of various concentrations of ouabain. The using l0-5 Mouabainand 50% inhibition by using ca. 0.5 x of choline released in the presence of Ad2 alone was
10-6
M ouabain. Similar amounts of Ad2 were bound to or ,red maximum release (100%). The percentage of choline internalized by the cellswhether ornotouabain
was presentI in the presence of Ad2 and various concentrations of (Table
1).
This indicated that the presence ofouabain doesis
represented
by
thesymbolS.
Thepercentageof choline notinterferewiththeassociationofAd2 with cells. Since the I inthepresenceofouabain aloneisshownbythesymbol0. concentration ofouabain required to inhibitAd2-dependentalcell-associated cholinecountwas 20,260cpm. choline release appears to be comparable to that requiredto inhibit Na+,K+-ATPase in these cells (30), it seems likely estimated by monitoringincorporation of[3H]leucine that Ad2-dependent choline release involves
Na+,K+-iloroacetic acid-precipitable material. ATPase activity.
ling and uptake of Ad2 by KB cells. Ad2 binding and Effect of K+ on Ad2-dependent choline release. Since were measured as described previously (23, 24). For Na+,K+-ATPaseactivityrequiresthe presence ofK+ionsin studies, cells grown in monolayer were incubated the external medium (1, 15), we tested the effect ofvarying
he
required
concentration of Ad2 (usually 1 to 100 the K+ concentrationon Ad2-dependent cholinerelease. To containing 35S-labeled Ad2 as a carrier. Incubations dothis, we placed the cells in a medium containing various[oneat4°Cfor 60min with 2 ml of medium B, pH 7.0. concentrations ofK+. In the absence of K+, Ad2 released end ofincubation, the cells were washed five times about 25% of total cell-associated choline (Fig. 2). At 0.1
tedium B andlysed in 2 ml of0.1 N NaOH. Samples mM K+, the amount of choline released was increased to tounted to estimate the amounts of Ad2 associated 35%. At 0.5 mM K+ or above, about48%ofcell-associated ie cells. To study Ad2 uptake, cells were exposed to choline was released into the medium. Increasing the con-r 60minat4°C and, after being washed five times with centration of K+ above 2 mM did not further increase the
tedium
B, the cellswere left at37°C for 60minin2 ml amount of choline released (data not shown). In the sameliumB, pH 6.0. At the end ofincubation, the medium experiment, addition of 0.1 mMouabainprevented enhance-wascollectedand
radioactivity
wascountedto estimate theamount of Ad2 dissociated from the cells. The amount of Ad2boundtothecellsurfacewasestimated
by
releasing
thesurfacevirus
by
trypsin.
Theamountof Ad2internalizedby
the cellswas estimated
by
lysing
the cells in2 mlof 0.1 NNaOHasdescribed
previously (23, 24).
['25
IEGF uptake.Uptake
of[125I]EGF
was measuredby
the
procedure
described earlier(24).
Inbrief,
cells wereexposed
to[125I]EGF
(50ng/ml)
for 60 min, usually in abuffer
containing
120 mMNaCl,
1 mMCaCl2,
1 mMMgCl2,
1 mM
KCI,
5 mMglucose,
and 25 mMHEPES,
pH 7.0. At the endofincubation,
the cells werewashedfive times with the buffer. Surface-bound EGF was released byusing
anacidic buffer
(0.2
N acetic acid in 0.5 MNaCl),
and the amount of[1251I]EGF
taken upby
the cells was estimated afterdissolving
the cells in 0.1 N NaOH.Enzyme
assays. Plasma membranes were prepared from [image:2.612.61.300.54.269.2]the cells
by
thepreviously published
procedure (22).TABLE 1. Effect of ouabainonbindinganduptake of Ad2 by KB cellsa
Amt(cpm) of Ad2:
Ouabain
concln Dissociated Associated
(M)
frmclswith
cell Internalizedsurface
0 1,107 2,275 2,562
10-6 1,032 2,432 2,629
10-5 1,116 2,315 2,718
10-4 1,265 2,493 2,652
10-3 1,416 2,102 2,490
aKB cells wereexposedtoAd2(5,ug/ml; specific activity, 7,260 cpm/,ug of
protein) for60minat4°Cinmedium B,pH 7.0.Atthe end ofincubation, the
cells were washed andfurtherincubatedat37°C for60minin the absenceor presence of the concentrations of ouabainshown. Amounts of adenovirus dissociatedfrom the cells, attachedtothe cellsurface, and internalizedbythe cells were estimated as described inMaterialsand Methods.
8
c])
0
0-w C') w -J wcr:
I
Ct) 6
4
2
-~~ ~ ~ ~ ~
.n-0
FIG. cells
lat
Aando of Ad2 I
B,
pH
absence
amount
conside
releasec
ouabain
releasec
The
tot;
siswas
in trici
Bind
uptake
bindinh with ti,ug/ml)
wered
At the withm
were c
with
th
Ad2
fo:
coldmofmed
I
-I/
ON
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.612.317.558.581.675.2]mentof Ad2-dependent release of choline by K+. However, Ad2-dependent choline release that occurred in the absence of K+ was not blocked by ouabain. Basal choline release in the absence of Ad2 was not affected by either ouabain or K+. These results indicate that the amount of choline released is significantly enhanced by K+, although some Ad2-dependent release of choline does take place in the absence of K+. Ouabain appears to block K+-activatable
Ad2-dependentcholine release. Therefore, it seems that K+ and ouabain compete on Ad2-dependent choline release. These results are expected from a reaction which requires
Na+,K+-ATPase activity.
Ad2-dependent release of choline in ouabain-resistant cells. IfNa+,K+ ATPase is involved in the Ad2 effect, then cells with a genetically altered Na+,K+-ATPase might have an
alteredresponse to Ad2. KB cells are thought to be closely related to HeLa cells, and the HeLa cell line D98-OR is about 100 times more resistant to ouabain than is its parental HeLa cell line. This cell line is known to have an altered
Na+,K+-ATPase (30). A comparison of the dependence on Ad2 concentration of choline release from D98-OR cells with thatofcholine release from KB and HeLa cells is shown in
Fig. 3. In wild-type KB or HeLa cells, there was a corre-sponding increase in the amount of choline released with
increasing concentrations of Ad2. As expected, KB and
HeLa cells were very similar. Up to 55% of cell-associated choline wasreleased by 10 ,ug of Ad2 per ml in KB cells, and
20
16
12(
8
+Ad2
+Ad2+Ouabain
0 EL..
0.1 0.5 1.0 1.5 2.0
KCI (mM)
FIG. 2. Activation ofAd2-dependentcholine releaseby K+.KB cells were labeled with [3H]choline, washed, and incubated with
Ad2 described in thelegendtoFig.1. Choline releasewasmonitored
inabuffercontaining125 mMNaCl,5 mMglucose, 1 mMCaC12, 1
mMMgCl2, 25 mM HEPES, pH 6.0, at 37°C fo 60min. Different
concentrations of KCl with and withoutouabain
(10'-
M)werealsoincluded in the buffer ifrequired.Cholinerelease from cellsexposed
to Ad2 and increasing concentrations of KCI with or without
ouabain(10' M) is shownbythesymbols0 and0, respectively.
Choline releasedfrom control cells which received buffer alone is
shown by the symbol A, and that from the cells which received
ouabain aloneis indicatedbythesymbolA. The total cell-associated
cholinecountwas44,626cpm.
a1)
al)
CIO a)0-0
-Cl) 40
30
20
104
KB
Hela
D-980R
10 20 50.0
Ad2
(gug/ml)
FIG. 3. Effect ofincreasing concentrations of Ad2 on choline release from KB, HeLa, and D98-OR cells. KB, HeLa, and D98-OR cellswerelabeled with choline and washedwith buffer as described inthelegendtoFig.1.Cells were exposed to various concentrations ofAd2, and choline releasewasmonitored in medium B, pH 6.0. The percentageof the total cell-associated choline releasedfromKB cells is shownby the symbol 0, that fromHeLacells is shown by thesymbolA,and that fromD98-OR cells is shown by the symbol 0. Total cell-associated choline counts were 53,990, 51,572, and 46,285 for KB,D980R, and HeLa cells, respectively.
up to 50% was released in HeLa cells. On the other hand, D98-OR cells released very little choline compared with HeLa or KB cells. Even20
jig
ofAd2 per ml releasedonly about 15% of cell-associated choline from these cells.In-creasing thelength ofincubation to up to 2 h or measuring cholinerelease inawidepHrange(pH5 to7.5)did not result in therelease ofany more Ad2-dependent choline in these cells(datanotshown). Althoughweconcluded thatinability of D98-OR cellstoreleasecholineisprobablyduetoaltered
Na+,K+-ATPase, there is a possibility that it could be
because D98-OR cellsbindandinternalize reducedamounts
of Ad2 compared with KB cells. To investigate this, we
measuredthebindinganduptakeof Ad2byD98-OR and KB
cells. Both KB and D98-OR cells bound nearly the same
amountsofAd2 when the cellswereexposedtoAd2at4°C for 60min
(Table
2). When bound Ad2was allowed to beinternalized at 37°C for 60 min, the percentages of Ad2
internalized by the cells or
remaining
on the cell surface were nearly equal.Therefore,itseemsthat the defect in therelease of choline from D98-OR cells is not due to the
inability of D98-ORcells tobindorinternalizeAd2. ActivationofNa+,K+-ATPasebyAd2.If
Na+,K+-ATPase
activity is
directly
involved inAd2-dependent
membranepermeability changes,it ispossiblethat Ad2actsby altering Na+,K+-ATPase
activity.
Toinvestigate this,
we treatedbothwild-typeKBand ouabain-resistant D98-ORcells with
various amounts ofAd2. The
activity
ofNa+,K+-ATPase
was measuredin crude membranes made from the cells in
the absence or presence of ouabain. The enzyme
activity
obtainedin control cells without ouabainwasconsidered to CY)
0
LLJ
C)
0
I
cv
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.313.560.68.320.2] [image:3.612.72.288.359.607.2]TABLE 2. Binding and uptake of Ad2 by KB and D98-ORcellsa
% of Ad2: Amt(cpm)
Cell line of Ad2bound Dissociated Associated
at4°C fromcells with cell Internalized surface
KB 6,255 22 31 46
D98-OR 6,492 27 29 44
aKB orD98-OR cells wereexposed to Ad2 (5,ug/ml;specific activity,5,965
cpm/,ugof protein) for 60 minat4°C, and theamountof Ad2 boundtothe cells wasdetermined as described in Materials and Methods. Duplicate dishes were washed and incubated further at37°C for 60 min. The percentages of total virusdissociated from the cells, attached to the cell surface, and internalized wereestimating as described in Materials and Methods.
be 100%. Na+,K+-ATPase activityobserved in the absence of ouabain increased with the increasing concentration of Ad2(Fig. 4). Up to 40% activation was observedat50,ug of
Ad2per ml.Ontheother hand,Ad2 did not seem to activate the enzyme activity when assayed in the presence of ouabain, suggesting that Ad2 enhances specifically ouabain-sensitiveNa+,K+-ATPase. InD98-OR cells therewas
insig-nificant activation of Na+,K+-ATPase whenassayed either
with orwithout ouabain. Activation of Na+,K+-ATPase by Ad2in KB cells was also found to be time dependent, with
maximum activation observedataround 30min (Fig. 5B). As acontrol, anotherplasma membrane enzyme was measured.
Adenylate cyclase activity was not affected by Ad2 (Fig. SA). These results suggested that Ad2-dependent activation ofNa+,K+-ATPasein KB cells was at leastpartially
respon-150
0
c 0 -o
en
a z
1301
110
90
70
A~~~~
0 d A A
c% 0
a-0c
_ 2.
llJ 1
l
z
Co 1. cc
0
(n
m 0.
15 30 45 60 75 90 105 120 TIME (Minutes)
FIG. 5. Effectof Ad2onmembrane-boundadenylate cyclase and Na+,K+-ATPase. KB cellswereexposedto10,ug of Ad2permlat
4°C. After being washed, cellswerefurtherincubatedat37°Cforthe
indicatedperiods oftime. At the endofincubation,the cellswere homogenized, and plasma membraneswerepreparedasdescribed in
Materials and Methods. Portions (100 ,ug) of the protein samples
wereusedtoassayadenylate cyclase andNa+,K+-ATPase
activi-tiesasdescribedinMaterials andMethods.(A)Countsperminute
produced, whichrepresenttheamountsofcAMPproduced by the membranespreparedin thepresence(0)orabsence(0)of virus.(B) Difference inA340betweenthesamples incubatedwith and without
membranes, which represents the Na+,K+-ATPase activity. Na+,K+-ATPase activitiesproduced inthepresenceorabsenceof
Ad2arerepresented bythesymbols0and0,respectively.
sible for the changes in the membrane permeability
ob-served.
IonrequirementofAd2-dependent membranepermeability changes. We also investigated the effect of varying the
concentrations ofNa+, K+, and Ca2+ on Ad2-dependent release of choline(Table 3). When isotonic sucrose-HEPES
50
/I
10 100
[image:4.612.335.547.66.285.2]ADENOVIRUS (jug/ml)
FIG. 4. Effects of various concentration of Ad2 on Na+,K+-ATPase activity in KB and D98-OR cells. One-day-old KB or
D98-ORcellsgrownin 100-mm dishesataninitialcelldensityof 106
cellsperdishwereexposedtovarious concentrations of Ad2for 60
minat4°C in medium B, pH 7.0. The cellswerethen washedtwice
withmedium B, pH 6.5, and incubated further inthis medium for 60
min at37°C. The cells were scraped and homogenized, and crude
membranepreparationsweremade. Portions(100,ug)of the samples wereused for assaying Na+,K+-ATPase activityin the absenceor
presenceof0.5x 10-3 M ouabain. Theenzymeactivity obtainedin the absence of ouabain in untreated cells was considered to be
100%. Shown is the Na+,K+-ATPase activity obtained in each
experiment relativetocontrol cells. Theenzymeactivitiesobtained
in KB andD98-OR cells in the absence of ouabainarerepresented
bythe symbols0 and A, respectively, and those obtained in the
presence of ouabain are represented by the symbols 0 and A,
[image:4.612.67.304.85.151.2]respectively.
TABLE 3. Effect of Na+, K+, andCa2l onAd2-dependent choline releasea
Choline released:
Buffer Without Ad2 With Ad2
cpm % cpm %
Sucrose-HEPES 752 4 3,561 19
+ 0.1 mMCaCl2 746 4 1,529 8
+ 1.0 mMCaCl2 685 4 1,429 8
+ 1mMCaCl2-1mMKCl 1,326 7 1,509 8
NaCl-HEPES 1,021 5 6,693 33
+ 0.1 mMCaC12 1,006 5 6,406 31
+ 1 mMCaC12 1,329 6 4,862 24
+ 1 mMCaClI-1mMKCI 1,617 8 9,529 47
aKBcellswerelabeled with[3Hlcholine,washed, and exposed to 5
pg
ofAd2perml.Choline release was monitored in either 225 mM sucrose-25 mM
HEPES-5 mMglucose, pH 6.0 (sucrose-HEPES) or 125 mMNaCI-25 mM HEPES-5mMglucose, pH 6.0(NaCl-HEPES).Ifrequired,CaC12(0.1or 1 mM)andKCI (1 mM)werealso included in the buffers. The actualcounts released in each case are shown. Thepercentages of total cell-associated choline calculatedareshown.
10
0 B
.0
_
.5
n s __ ' '
.0.
.0-
-4
2
l
1
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.65.305.390.570.2] [image:4.612.322.562.538.668.2]medium was used as the basal medium, about 19% of the
cell-associated choline was released by the cells. The
addi-tion of 0.1 or 1 mM CaCl2 reduced the amount of choline
releasedto about 8%. Further addition of 1 mM
KCI
did notincrease the amount of choline released by the cells,
sug-gestingthat choline released in this medium does not require
Na+,K+-ATPase activity. When
NaCl-HEPES
buffer was used alone, adenovirus caused about 33% of cell-associatedcholine to be released. Addition of 0.1 mM
CaC12
did notblockthe Ad2-dependent release of choline, but 1 mM
CaC12
reduced the amount released to about25%. Further addition
of 1 mM KCI increased the amount of choline released to
about 47%. These data
are
consistent with the notion thatcholine released in a medium containing
Na+
involvesNa+,K+-ATPase activity.
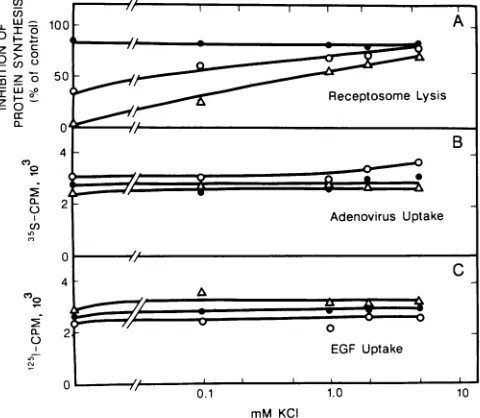
Activation of Ad2-dependent lysis of receptosomes by
K+.
Since the
Na+,K+-ATPase
of receptosomes is probablyderived from the plasma membrane (7, 33), we anticipated
thatthis enzyme activity also plays a role during the lysis of
receptosomes by Ad2. We therefore hypothesized that the
enhancement of EGF-PE toxicity by adenovirus, which
appearsto be due to vesicle lysis, would also be dependent on the K+ concentration.
K+
effects were studied in cellswhichwere either not preincubated or incubated for 30 or 60 min in K+-free buffer. Preincubation in
K+-free
buffer isnecessary to bring down the cellular content of
K+
to a lowlevel. In cells preincubated with
K+-free
buffer for 60min,
there was no Ad2-dependent enhancement of the inhibition
ofprotein synthesis by EGF-PE (Fig. 6A). However, with
increasing
concentrations ofK+,
there was an increase in theAd2-dependent enhancement of EGF-PE toxicity. For
ex-ample,additions of 0.1, 1, 2, and 5 mM
K+
to the incubationbuffer resulted in about 25, 60, 65, and 75% inhibition of
protein synthesis, respectively. In cells preincubated in
K+-free buffer for 30 min, about 35% inhibition of protein
synthesis
was observed in the absence of addedK+.
Therewas afurther increase in the inhibition of protein synthesis
with increasingconcentrations of
K+;
about 80% inhibitionwas observed at 5 mM
K+.
Cells not preincubated withK+-free buffer showed about 80% inhibition of protein
synthesis.
In these cells, there was no further increase in theinhibition of protein synthesis by Ad2. Cells which are not
extensively depleted of their cellular
K+
probably haveenough K+ present toallow Ad2 to lyse endocytic vesicles.
Themost plausible explanation for
K+
activation ofAd2-dependent
lysis of receptosomes is thatK+
is required for the normal functioning ofNa+,K+'-ATPase,
and thus thisenzyme participates in the lytic reaction. However, there
was a possibility that the removal of
K+
from the mediumreduced the amounts of Ad2 or EGF-PE internalized by the
cells. To investigate this possibility, we tested the uptake of Ad2 and EGF (which goes through the same route as
EGF-PE)
under the conditions used to study the effect ofK+
on Ad2-dependent receptosome lysis as discussed above. Theuptakeof Ad2 and EGF was not affected by the absence orpresenceofK+ in the medium, even in cells preincubatedwith K+-free buffer for 60
min
(Fig. 6B and C).DISCUSSION
The majornew finding reported here is that Ad2-mediated
release of choline from intact cells is partially blocked by
ouabain.
The concentration of ouabain required to inhibitcholine releaseparallels the concentration required toblock
Na+,K+-ATPase
in these cells (30). These results suggestthat Ad2-mediatedincrease in cell membrane permeability is
cn
U-)
C,)
'r0
Z Z C
O >- 0
mnZ°
Z
CCo
0Cl:
C)
f)I
cl
11-100
50
ReceptosomeLysis
4
~~~~~~~~~~B
4
2
i
AdenovirusUptake
4
2 0
EGFUptake
-0.1 1.0 10
mM KCI
FIG. 6. Effect of
K+
onAd2-dependent
enhancement of thetoxicity
ofEGF-PE
andon Ad2 and
EGF
uptake
in KB cells.One-day-old
KBcellsgrown
in35-mm dishesplatedat an initial celldensity
of 3 x105
cellsper
dish werepreincubated
in aK+-free
buffer
containing
120 mMNaCl,
1mM
MgCl2,
1 mMCaCl2,
5mM
glucose,
and 25 mMHEPES,
pH
7.0,
for0,
30, or 60min.
These cells were then usedto monitor(i)
Ad2-dependent
enhancement ofEGF-PE
toxicity
measuredby
monitoring
the inhibitionof proteinsynthesis
with,ug
1 of Ad2per
mland 0.5,ug
of EGF-PEper ml (A),(ii)
Ad2uptake
with 5jig
of Ad2per
ml (B), and (iii) [125]EGF
uptake
with50ng
of EGFper
ml(C).
Experimnents
weredone in thepresence
of various concentrations ofKCI,
as describedin Materialsand Methods. In all of the
panels,
results obtained in the cellspreincubated
inK+-free
buffer for
0,
30,
or 60
min
are
representedby
the
symbols
*,
0,
and A, respectively.
atleast
partly
duetoNa+,K+-ATPase
activity. As predictedfrom a reaction
involving
Na+,K+-ATPase,
Ad2-dependentrelease
ofcholine
is enhanced whenK+
ions are present inthe
medium,
and thisactivation
by
K+
is prevented byouabain
(Fig.
2). Moreover,
HeLa cells resistant to ca.100-fold concentrations of ouabain
(D98-OR
cell line)
releasesignificantly
lower amounts of choline as compared withwild-type
cells.
We have
shown
in
this study
that Ad2activates
Na+,K+-ATPase
present
in KBcell
membranes, andthis
activation seems to be absent in ouabain-resistantD98-OR
cells. Theinability
of Ad2 to release choline oractivate
Na+,K+-ATPase
in D98-OR cells, along with thefact
that these cellshave
an alteredNa+,K+-ATPase
(30),strongly suggests
a role of thisenzyme
in Ad2-dependentmembrane
permeability
changes.We also found that
lysis
of
endocytic
vesicles byAd2 wasenhanced
by
thepresence
ofphysiological
concentrations ofK+
(Fig.
6). Since,
in KBcells,
K+
depletion
did not affectthe amount of Ad2 taken
up
by
the cells, we favor thehypothesis
thatK+
functions via the activation ofNa+,K+-ATPase of
theendocytic
vesicles. This activation would berequired
for
lysis
of
endocytic
vesiclesby
Ad2, as wehaverecently postulated
(Fig.
1; 25).Results
presented
here
also showthat,
under conditions inwhich
Na+,K+-ATPase
activity
is considerably
suppressed(e.g.,
inthe
presence
of10-4
Mouabain or in the absence ofK+
in the
medium),
there is still asignificant
amount ofcholine
released fromthe cellsby
Ad2. Itisdifficulttoknow
the exact
nature of this alternate mechanism of cholineon November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.319.559.71.280.2]efflux,
but there are severalpossibilities
which deserve furtherinvestigation.
Forexample,
choline couldpassively
diffuse from the cells as a result of increased membrane
fluidity;
anincreasein membranefluidity
has been observed in cells infected with a fewenveloped
viruses(9, 14, 17).
Addition of 100
,uM
CaCl2
blocks theAd2-dependent
cholinerelease in the absence of
K+.
This result may indicate apassive
mode ofcholinerelease,
asCa2+ canbind with thenegatively
charged
components ofthe membrane(12, 20)
and makethem
impermeable
forpassive
diffusion(5, 19, 31).
Other
possible
waysby
which Ad2 could release choline isby opening
new channels(3)
orby
depolarizing
the cell membrane(2),
assuggested
for certainenveloped
viruses andtoxins.The biochemical mechanism
by
which adenovirus in-creases theactivity
ofNa+,K+-ATPase
has not yet beensttdied. It could be a direct effect on the enzyme or an
indirect effect. If it is an indirect
effect,
it is not ageneral
change
in theplasma membrane,
becauseadenylate cyclase
activity
wasunaffected.ACKNOWLEDGMENTS
We thank Michael Gottesman for providingHeLa and D98-OR
cells,MariaGallo forpreparingEGF-PE, andS. A. Lepplafor the
giftofPE.
LITERATURE CITED
1. Anner, B. M. 1985. The receptor function of the Na+,
K+-activatedadenosinetriphosphatesystem. Biochem.J.227:1-11. 2. Bashford,C.L.,G. M.Alder,M. A.Gray,K.J.Micklem,C. C.
Taylor,P.J.Turek, and C. A. Pasternak. 1985.Oxonoldyesas
monitors of membranepotential:the effect of viruses and toxins ontheplasmamembranepotentialof animal cells inmonolayer
culture and in suspension.J. Cell. Physiol. 123:326-336. 3. Bhakdi,S.,andJ.Tranum-Jensen. 1983.Membranedamageby
channel-forming proteins.Trends Biochem. Sci. 8:134-136. 4. Dales, S. 1973. Early events in cell-animal virus interactions.
Bacteriol.Rev. 37:103-135.
5. De Mello, W. C. 1984. Modulation ofjunctional permeability.
Fed. Proc. 43:2692-2696.
6. Dickson,R.B.,L.Beguinot,J.A.Hanover,N. D.Richert,M.C.
Willingham,and I. Pastan. 1983. Isolationand characterization ofa
highly
enrichedpreparationofreceptosomes(endosomes)from a human cell line. Proc. Natl. Acad. Sci. USA 80: 5335-5339.
7. Dickson,R. B.,M. C. Willingham,and I. Pastan.1982.
Recep-tor-mediated endocytosis of a2-macroglobulin: inhibition by
ionophores
and stimulation by Na+and HCO3-. Ann. N.Y. Acad. Sci. 401:38-49.8. Fitigerald, D. J. P., R. Padmanabhan, I. Pastan, and M. C.
Willingham. 1983. Adenovirus-induced release of epidermal
growthfactorandPseudomonas toxin into the cytosolofKB cellsduring receptor-mediatedendocytosis.Cell32:607-617. 9. Fuchs,P.,M.Spiegelstein,M.Haimsohn,J.Gitelman,J.,and A.
Kohn. 1978. Early changes in the membrane ofHeLa cells
absorbing Sendai virus under conditions of fusion. J. Cell.
Physiol.95:223-234.
10. Galloway, C. J., G. E. Dean, M. Marsh, G. Rudnick, and I. Mellman. 1983. Acidification of macrophage and fibroblast
endocytic vesicles in vitro. Proc. Natl. Acad. Sci. USA 80: 3334-3338.
11. Green, M.,and M. Pina. 1963.Biochemical studieson adeno-virus multiplication. IV. Isolation, purification, and chemical
analysis
ofadenovirus. Virology20:199-207.12. Hauser, H.,and M.Philips. 1975. Ionbindingtophospholipids. Interaction of calcium and lanthanide ions with phosphatidyl choline. Eur. J. Biochem. 58:133-144.
13. Helenius, A., I. Mellman, D. Wall, and A. Hubbard. 1983.
Endosomes. Trends Biochem. Sci. 8:45-250.
14. Impraim, C. C., K. A. Foster, K. J. Micklem, and C. A. Pasternak. 1980. Natureofvirally mediated changes in mem-brane permeability to small molecules. Biochem. J. 186: 847-860.
15. Jorgensen,P. L. 1982. Mechanism of theNa+,K+ pumpprotein structure and conformations of the pure (Na++K+)-ATPase. Biochim. Biophys.Acta 694:27-68.
16. Lenard, J.,and D. K. Miller. 1983. Entryofenvelopedviruses into cells,p. 301-330. In P. Cuatrecasas and T. F. Roth(ed.), Receptor-mediated endocytosis (receptors and recognition, se-riesB), vol. 15. Chapman&Hall, Ltd.,London.
17. Levanon, A., A. Kohn, and M. Inbar. 1977. Increase in lipid
fluidityof cellular membranes induced by adsorption of RNA and DNA virions. J. Virol.22:353-360.
18. Pastan, I., and M. C. Willingham. 1983. Receptor-mediated endocytosis: coated pits,receptosomes, and theGolgi. Trends Biochem. Sci.8:250-254.
19. Pasternak, C. A., and K. J. Micklem. 1974. The biochemistryof virus-induced cell fusion. Changes in membrane integrity. Biochem. J. 140:405-411.
20. Portis, A., C. Newton, W. Pangborn, and D. Papahadjopoulos. 1979. Studiesonthemechanismof membrane fusion: evidence for anintermembrane Ca2'-phospholipid complex, synergism withMg2+,andinhibitionby spectrin. Biochemistry 18:780-790. 21. Salomon, Y., C. Londos, and M. Rodbell. 1974. A highly sensitiveadenylate cyclaseassay. Anal. Biochem.58:541-548. 22. Schaefer, A., J. Kuhne, R. Zibirre, and G. Koch. 1982. Poliovirus-induced alteration in the HeLa cell membrane func-tions. J. Virol. 44:444 449.
23. Seth, P.,D.J.P.FitzGerald,H.Ginsberg, M.Willingham,and I. Pastan. 1984.Evidence that thepentonbase of adenovirusis involved inpotentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol. Cell. Biol. 4: 4:1528-1533.
24. Seth, P.,D.J. P.FitzGerald,M.C.Willingham,andI. Pastan. 1984.Roleofalow-pH environment in adenovirus enhancement of the toxicity ofaPseudomonas exotoxin-epidermal growth factorconjugate.J.Virol.51:650-655.
25. Seth, P., D. FitzGerald, M. Willingham, and I. Pastan. 1986. Pathway of adenovirus entry into cells, p. 191-195. In R. L. Crowell and K. Lonberg-Holm (ed.), Virus attachment and entryinto cells. AmericanSociety for Microbiology, Washing-ton,D.C.
26. Seth, P., I.Pastan, and M. C.Willingham. 1985. Adenovirus-dependent increase in cell membrane permeability. J. Biol. Chem. 260:9598-9602.
27. Seth, P., M. C. Willingham, andI. Pastan. 1984.
Adenovirus-dependentrelease of51CrfromKBcellsat anacidicpH. J.Biol. Chem.259:14350-14353.
28. Seth, P., M. C. Willingham, and I. Pastan. 1985. Binding of adenovirus and its external proteins to Triton X-114: depen-denceonpH.J. Biol. Chem.260:14431-14434.
29. Svensson, U.,and R.Persson. 1984.Entry of adenovirus2into HeLacells. J.Virol. 51:687-694.
30. Weissman, F.,andE. J.Stanbridge. 1980. Characterization of ouabain resistant, hypoxanthine phosphoribosyl transferase-deficienthumancells and their usefulnessas a general method for the production of human cell hybrids. Cytogenet. Cell Genet. 28:227-239.
31. Wenner, C., and J. Hackney. 1976. Inhibition of16Rb+ and 22Na+ efflux of Ehrlich Lettre tumor cells by Ca". Arch. Biochem.Biophys. 176:37-42.
32. White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by SemlikiForest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679.
33. Willingham, M. C., and I. Pastan. 1983. Formation of receptosomes from plasma membrane coated pits during endocytosis: analysis by serial sections with improved mem-branelabeling and preservation techniques. Proc. Natl. Acad. Sci. USA 80:5616-5621.