Copyright © 2004, American Society for Microbiology. All Rights Reserved.
Use of a Synthetic Peptide Epitope of Asp f 1, a Major Allergen or
Antigen of
Aspergillus fumigatus
, for Improved Immunodiagnosis
of Allergic Bronchopulmonary Aspergillosis
Taruna Madan,
1Priyanka Priyadarsiny,
1† Mudit Vaid,
1Neel Kamal,
1Ashok Shah,
2Wahajul Haq,
3Seturam Bandacharya Katti,
3and P. Usha Sarma
1*
Molecular Biochemistry and Diagnostics, Institute of Genomics and Integrative Biology,1and Clinical Research
Centre, Vallabh Bhai Patel Chest Institute, University of Delhi,2Delhi, and Biopolymers Division, Central
Drug Research Institute, Lucknow,3India
Received 23 September 2003/Returned for modification 13 November 2003/Accepted 22 January 2004
Allergic bronchopulmonary aspergillosis (ABPA) is an immunologically complex allergic disorder caused by
the fungal pathogenAspergillus fumigatus. Elevated levels of total immunoglobulin E (IgE), specific IgE, and
IgG antibodies in sera are important immunodiagnostic criteria for ABPA. International reference standards or standardized immunodiagnostic assays are not available due to a lack of well-defined diagnostic antigens. The present study was carried out to identify and evaluate the immunodiagnostic relevance of synthetic
epitopic peptides of Asp f 1, a major allergen, antigen, or cytotoxin ofA. fumigatus. Five overlapping peptides
were synthesized from the N terminus of Asp f 1, one of the potential immunodominant regions predicted by algorithmic programs. The 11-amino-acid synthetic peptide (P1) significantly inhibited both IgG binding
(89.10% ⴞ 4.45%) and IgE binding (77.32% ⴞ 3.38%) of the standardized diagnostic antigen (SDA) (a
well-defined pool of diagnostically relevant allergens and antigens ofA. fumigatus). With a panel of sera of
ABPA patients, allergic patients with skin test negativity toA. fumigatus, and healthy individuals, P1 showed
a higher diagnostic efficiency than SDA (specific IgG, 100%; specific IgE, 98.3%). The diagnostic efficiency of P1 could be attributed to the presence of homologous epitopes in various immunodominant allergens or
antigens ofA. fumigatus. The ability of P1 to induce histamine release from sensitized mast cells and a Th2 type
of cytokine profile in peripheral blood mononuclear cells of ABPA patients suggests its potential for use in intradermal testing. P1 could be further explored for development of a standardized, specific, and sensitive immunodiagnostic test for aspergillosis.
Allergic bronchopulmonary aspergillosis (ABPA) is an im-munologically complex allergic disorder induced by the fungal pathogen Aspergillus fumigatus. ABPA is clinically character-ized by episodic bronchial obstruction; positive immediate skin reactivity; elevated total immunoglobulin E (IgE), specific IgG, and IgE antibodies; peripheral and pulmonary eosinophilia; central bronchiectasis; and expectoration of brown plugs (26). Various allergens and antigens ofA. fumigatus induce type I and type III hypersensitivity reactions in ABPA patients. The humoral response in ABPA patients includes polyclonal acti-vation, and the cellular response is predominantly Th2 type with elevated interleukin 4 (IL-4) and IL-5 cytokines and ele-vated eosinophilia similar to that of asthma. In view of the progression of ABPA leading to irreversible damage of the bronchi and fibrosis in the lungs, an early diagnosis of ABPA is emphasized. ABPA also needs to be differentially diagnosed from tuberculosis, pneumonia, bronchiectasis, lung abscess, and bronchial asthma (1, 8, 17, 28, 31). Various groups have reported the utility of antigen-based serodiagnostic methods for the determination of specific IgG and IgE antibodies (28),
but no World Health Organization or international reference diagnostic antigens or standardized assays are yet available. Intradermal testing, another supportive diagnostic test for ABPA, also suffers from a lack of well-characterized antigens. Some of the recombinantly expressed allergens and antigens
ofA. fumigatus, viz., Asp f 1, Asp f 2, Asp f 3, Asp f 4, and Asp
f 6, showed relevance in immunodiagnosis and intradermal testing (12, 14, 15, 16, 19). However, limitations of posttrans-lational modifications and folding during purification of re-combinant proteins can lead to loss of immunoreactivity. The presence of cross-reactivity among allergens and antigens of several fungi further necessitated the characterization of their immunodominant regions. Probable sequential epitopic re-gions of the allergenic and antigenic proteins with known pro-tein and nucleotide sequences can be identified using algorith-mic programs (9). Synthesis of peptides based on the epitopic regions is simple and has been useful for the diagnosis of various allergic disorders, such as milk allergy and birch pollen allergy, and infectious diseases, such as human immunodefi-ciency virus (HIV) disease, influenza, hepatitis, and malaria (34).
Asp f 1 was the firstA. fumigatusallergen or antigen to be cloned, sequenced, and produced by recombinant DNA tech-nology (2, 24). Our group sequenced Asp f 1 isolated from an Indian clinical strain ofA. fumigatus(GenBank accession no. AF181859 and SwissProt accession no. P04389). The diagnos-tic relevance of Asp f 1 has been indicated by the presence of * Corresponding author. Mailing address: Molecular Biochemistry
and Diagnostics, Institute of Genomics and Integrative Biology, Mall Rd., Delhi, India. Phone: 91-011-27666158. Fax: 91-011-27667471. E-mail: u_sarma@hotmail.com.
† Present address: Division of Molecular Pharmacology, Ranbaxy Laboratories Ltd., Gurgaon-122001, India.
552
on August 17, 2020 by guest
http://cvi.asm.org/
specific IgE antibodies in 85% of allergic aspergillosis patients and the absence of its homologous proteins in other fungi (3). Asp f 1-specific IgE antibodies appeared during the early phase of ABPA (15, 32). Identification of immunodominant regions of Asp f 1 may facilitate the development of specific and standard peptide-based diagnoses. Some of the epitopes of Asp f 1 have been identified by using T-cell clones and periph-eral blood mononuclear cells (PBMCs) ofA. fumigatus -sensi-tized patients, but they have not been evaluated for their di-agnostic relevance (10, 18).
In the present study, the identification of probable immu-nodominant regions of Asp f 1 has been attempted using epitope prediction algorithms. Most of the algorithms pre-dicted the N-terminal region of Asp f 1 as the potential im-munodominant region. Five overlapping peptides from the N-terminal region were synthesized, purified, and analyzed by inhibition enzyme-linked immunosorbent assay (ELISA). P1 showed significant inhibition of IgE and IgG antibody binding of standardized diagnostic antigen (SDA; a well-defined pool of diagnostically relevant allergens and antigens ofA. fumiga-tus). P1 was further examined for its serodiagnostic potential using a panel of sera from 20 clinically confirmed ABPA pa-tients, 20 controls (allergic patients with skin test negativity to
A. fumigatus), and 25 healthy individuals. The ability of P1 to
induce histamine release from sensitized mast cells of ABPA patients and a Th2 type of cytokine profile in PBMCs of ABPA patients suggests its potential for use in intradermal testing.
MATERIALS AND METHODS
SDA.A. fumigatus(strain 285, isolated from the sputum of an ABPA patient visiting Vallabh Bhai Patel Chest Institute, Delhi, India) was grown in a synthetic broth (L-asparagine medium) for 3 weeks at 37°C in a stationary culture (4, 5). The filtrate obtained after separating the mycelium was dialyzed extensively against deionized water. The dialysate was subjected to ammonium sulfate pre-cipitation (80% [wt/vol]) and lyophilized to get the protein-enriched antigenic fraction. The fraction obtained was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (⬎30 proteins in the range of 12 to 100 kDa). The immunoreactivity of the fraction was analyzed by immunodiffusion, ELISA, and Western blotting (comprising most of the re-ported immunodominant allergens and antigens ofA. fumigatus[see Fig. 4, lanes 2 and 4]). This fraction is routinely used in our laboratory for quantitating specific IgG and IgE antibodies in serum samples from patients with suspected allergic aspergillosis (⬎3,200 serum samples have been screened so far, including confirmed and suspected cases of aspergillosis, allergic patients, and healthy individuals) and is referred to as SDA in the present study.
Human sera.The sera of ABPA patients were obtained from clinically con-firmed cases (satisfying the criteria of Rosenberg et al.), and control sera were obtained from A. fumigatusskin test-negative allergic patients registered at Vallabh Bhai Patel Chest Institute (26). The normal sera were obtained from healthy donors without an indication of pulmonary disease. The study was ap-proved by the institute’s Human Ethics Committee, and the serum samples were taken with the written consent of the subjects.
Purified Asp f 1 and MAb against Asp f 1.Asp f 1 was purified from the SDA as described in an earlier communication (23). Monoclonal antibody (MAb) raised against Asp f 1, MAb 4A6 (ammonium sulfate precipitated), was a kind gift from L. Karla Arruda, Department of Clinical Allergy and Immunology, University of Virginia.
Identification of immunodominant regions.Ten algorithmic programs were used to identify the immunodominant regions of Asp f 1 (the protein sequence of Asp f 1 used was AAB22442 of the National Center for Biotechnology Information). They were Hopps and Woods (hydrophilicity), Fraga global scale (hydrophilicity), Kyte and DoLittle (hydropathy), Novotny large sphere (acces-sibility), Welling (antigenicity), Parker (hydrophilicity; retention times in re-verse-phase high-performance liquid chromatography), Janin (accessibility), bulk hydrophobic scale (hydrophobicity), Fauchere and Pliska (hydrophobicity), and Hopp scale (acrophilicity) (9, 35). Rothbard and Taylor’s predictions for T-cell
epitopes and prediction of amphipathic helices were used manually to identify the potential T-cell epitopic domains in Asp f 1 (27).
Synthesis of overlapping peptides.The N-terminal region was one of the immunodominant regions predicted by all 10 algorithmic programs and also showed the presence of potential T-cell epitopes in two manual predictions. Five overlapping peptides (Table 1) from this region were synthesized by solid-phase peptide synthesis using standard 9-fluorenylmethoxy carbonyl chemistry and purified by reverse-phase high-performance liquid chromatography on an ana-lytical CE-18 column (Applied BioSystems). The synthetic peptides were char-acterized by fast atom bombardment-mass spectrophotometry (Jeol JMS-360). (The sequences of the peptides used in the present study have been patented under the following numbers: Indian patent no. 184440, 189314, 189176, 751/ Del/98, and 754/Del/98; U.S. patent no. 6262231; and European patent no. EP1104768.)
Diagnostic relevance of synthetic peptides. (i) Immunoreactivities of peptides with MAbs.The immunoreactivities of the synthetic peptides (P1 to P5) and Asp f 1 with the MAb raised against Asp f 1 were assayed by dot blotting. In brief, 1 g of peptide or Asp f 1 in 1l of phosphate-buffered saline (PBS) was applied to a Hybond C nitrocellulose membrane (Amersham Life sciences, Little Chal-font, United Kingdom). Five micrograms ofA. fumigatus3-week culture filtrate was used as a positive control, while 1g of purified bovine serum albumin was used as a negative control (since the culture filtrate is a mixture of a number of allergens and antigens, five times more was used than for the purified proteins). The dot blot was further processed according to the method for immunoblotting described in an earlier communication using MAb (1 mg/ml) and anti-mouse IgG peroxidase (1:1,000 dilution in PBS) (4).
(ii) Inhibition ELISA.To evaluate the diagnostic relevance of the synthetic peptides, the pooled sera of 10 ABPA patients (diluted 1:100) were preincubated with various peptides (P1 to P5; concentrations ranged from 50 ng to 1g). The preincubated sera were centrifuged at 10,000 rpm for 5 min to remove any insoluble complexes. The immunoreactivities (specific IgE and IgG binding) of the preincubated sera with SDA (1g/well) were analyzed by indirect ELISA as described in an earlier communication (6). Briefly, microtiter plates were coated with SDA (1g/well) in 100 mM sodium bicarbonate (pH 9.2) overnight at 4°C. The plates were washed with PBS containing 0.05% Tween 20 and blocked with PBS containing 1% bovine serum albumin for 1 h at 37°C. Serum samples (preincubated with 100 ng of various peptides) were diluted 1/100 (for IgG) and 1/25 (for IgE) in PBS with 0.05% Tween 20 and 1% bovine serum albumin. After 2 h of incubation with the diluted serum samples, the plates were washed and incubated with 100l of peroxidase-conjugated anti-human IgG (for IgG) or anti-human IgE (for IgE) (appropriately diluted in PBS with 1% bovine serum albumin) for 3 h at room temperature. The plates were washed and incubated with substrate solution containing H2O2(1l/ml) and orthophenylenediamine
dihydrochloride (1 mg/ml) for 20 min at room temperature. The optical densities at 490 nm were measured with an ELISA reader (Nunc Immunoreader).
Serodiagnostic efficiency of P1.The binding of P1 with specific IgE and IgG antibodies in the sera of 20 clinically confirmed ABPA patients, 20 controls, and 20 healthy individuals was compared with that of SDA (1g/well) by indirect ELISA as described in an earlier communication (6). The optimum amount of P1 for indirect ELISA was observed to be 80 ng/well in a dose-response curve. A 16-amino-acid sequence from gp120 of HIV (QIINMWQKVGKAMYAP) was used as a control peptide during the studies.
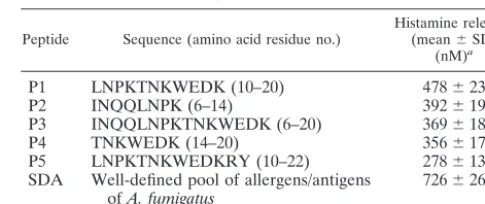
TABLE 1. Overlapping synthetic peptides from the N-terminal region of Asp f 1 and histamine release
Peptide Sequence (amino acid residue no.) Histamine released(mean⫾SD) (nM)a
P1 LNPKTNKWEDK (10–20) 478⫾23
P2 INQQLNPK (6–14) 392⫾19
P3 INQQLNPKTNKWEDK (6–20) 369⫾18
P4 TNKWEDK (14–20) 356⫾17
P5 LNPKTNKWEDKRY (10–22) 278⫾13
SDA Well-defined pool of allergens/antigens
ofA. fumigatus 726⫾26
aThe values are averages of histamine release stimulated by the peptides (10
g/well) and SDA (10g/ well) from sensitized basophils of three clinically confirmed ABPA patients by a whole-blood assay. For each patient, incubation with the peptide or SDA was carried out in triplicate. The SD for the values were within⫾5%.
on August 17, 2020 by guest
http://cvi.asm.org/
Data analysis.The cutoff for positive scores was calculated for SDA and P1 from the mean absorbance value obtained for the 20 healthy individuals plus 3 standard deviations (SD). The following definitions were used to calculate the corresponding diagnostic parameters: true-positive values (tp), sera from pa-tients with clinically confirmed ABPA showing positive readings; false-negative values (fn), sera from patients with clinically confirmed ABPA showing negative readings; false-positive values (fp), sera from controls and healthy individuals showing positive readings; true-negative values (tn), sera from controls and healthy individuals showing negative readings; sensitivity, tp⫻100/(tp⫹fn); specificity, tn⫻100/(tn⫹fp); and diagnostic efficiency, (tn⫹tp)⫻100/(tp⫹ fp⫹tn⫹fn). All values in the study are expressed as the mean⫾SD of triplicate values for each sample. The immunoreactivities of P1 and SDA were compared by the one-population analysis of variance test in the MicroCal Origin version 3.0 statistical package.
Cytokine profile induced by P1 in PBMCs.PBMCs (from three ABPA pa-tients, three controls, and three healthy individuals) were fractionated, and 2⫻ 106cells/well were incubated with SDA (10g/ml) and P1 (10g/ml) for 6 days
in RPMI 1640 medium with 10% complement-inactivated fetal calf serum. A 16-amino-acid sequence from gp120 of HIV (10g/ml) was used as a control peptide during the studies (see above). The supernatant medium was collected for cytokine analysis, and the proliferation response was measured colorimetri-cally. 3-[4,5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (to a final concentration of 0.5 mg/ml) was added to each well for 30 min, followed by extraction of the dye from the cells by acidified isopropanol. The absorbance was measured at 590 nm (23). Cytokine assays were carried out in supernatants of PBMCs by ELISA kits for human gamma interferon (IFN-␥), IL-2, IL-4, IL-6, and IL-10 (Pharmingen, San Diego, Calif.) according to the manufacturer’s instructions.
In vitro histamine release assay.To evaluate histamine release induced by various peptides (10g) and SDA (10g) from the sensitized basophils of ABPA patients (present in 100 l of 1:7-diluted whole blood), a histamine immunoassay kit (Immunotech, Marseille, France) was used according to the protocol for whole-blood assay. A standard curve was drawn with histamine standards, and the corresponding histamine release by various peptides and SDA was calculated from the standard curve.
Purification of P1-specific antibodies.In order to obtain P1 bound to the solid support, the peptide P1 was synthesized by solid-phase synthesis using a 9-flu-orenylmethoxy carbonyl chemistry protocol in a separate experiment. After the completion of synthesis, the alpha-amino-protecting group and the side chain-protecting groups were selectively removed, leaving the peptide bound to the solid support. An aliquot of the support-bound peptide was cleaved, and the peptide thus obtained was confirmed by fast atom bombardment-mass spectro-photometry. For purification of P1-specific antibodies, P1 bound to the solid support was incubated with pooled sera from 10 ABPA patients (diluted 1:1 with 1 M phosphate buffer, pH 9.0, at 4°C). The bound antibodies (P1-specific anti-bodies) were eluted with 0.1 M citric acid buffer (pH 3.0) at 4°C, neutralized with 1.5 M Tris-HCl (pH 7.5), and dialyzed against PBS.
Binding of SDA with P1-specific antibodies.SDA was electrophoresed by SDS-PAGE (12% acrylamide) and electroblotted onto a nitrocellulose mem-brane. The membrane was blocked with 3% defatted milk in PBS, washed, and further incubated with pooled sera of ABPA patients (diluted 1:100 in PBS) or P1-specific antibodies (0.5 mg/ml) for 2 h at 37°C. The washed membranes were incubated with human IgG peroxidase (diluted 1:1,000 in PBS) and anti-human IgE peroxidase (diluted 1:5,000 in PBS) separately for 2 h at 37°C. The immunoblots were washed and developed with diaminobenzidine (12.5g) and H2O2(25l) in 25 ml of 0.25 M sodium acetate buffer, pH 6.0.
RESULTS
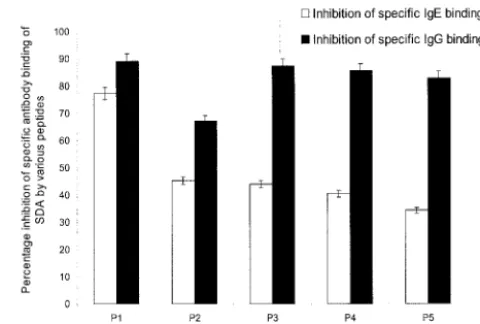
The N-terminal region of Asp f 1 was one of the immuno-dominant regions predicted by all 10 algorithmic programs. The region showed the presence of an amphipathic helix and potential T-cell epitopes according to the criteria of Rothbard and Taylor (27). Five overlapping peptides (Table 1) from this region were synthesized and purified. All five peptides reacted with the MAb raised against Asp f 1 on the dot blot, indicating the epitopic nature of the peptides (result not shown). The diagnostic relevance of these peptides is evident from an inhi-bition ELISA that showed significant inhiinhi-bition of specific IgE (34 to 77%) and IgG (67 to 89%) antibody binding of SDA by
the peptides (Fig. 1). Peptide P1 exhibited maximum inhibition of the binding of SDA to both specific IgE antibodies (77.32%⫾ 3.38%) and specific IgG antibodies (89.1%⫾4.45%) and was selected for further analysis of its immunological and antigenic properties.
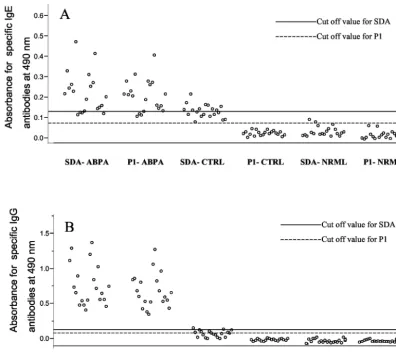
In order to explore the diagnostic potential of P1, we exam-ined its binding to specific IgE and IgG antibodies in sera of ABPA patients, controls, and healthy individuals by indirect ELISA (Fig. 2). All ABPA sera showed significant IgE and IgG binding to P1 in comparison to those of controls and healthy individuals. The mean absorbance value of P1 binding with specific IgE antibodies in the sera of ABPA patients was 5.907-and 7.807-fold higher than for the controls 5.907-and healthy indi-viduals, respectively. The mean absorbance value of P1 binding with specific IgG antibodies in the sera of ABPA patients was 15.30- and 10.22-fold higher than for the controls and healthy individuals, respectively. There was no significant difference between the absorbance values for P1 and SDA with respect to their binding with specific IgE and IgG antibodies in the sera of ABPA patients (P⬍0.05). The reactivity of P1 with the IgE and IgG antibodies in the sera of controls was significantly lower than that of SDA, suggesting a higher specificity of P1 than SDA (P⬍0.05). The ELISA absorbance values (mean⫾ SD) forA. fumigatus-specific IgG binding of control peptide with the sera of 20 ABPA patients and with the sera of 20 controls were 0.078⫾0.007 and 0.051 ⫾0.011, respectively. The ELISA values (mean⫾SD) forA. fumigatus-specific IgE binding of control peptide with the sera of 20 ABPA patients and with the sera of 20 controls were 0.058⫾0.009 and 0.037 ⫾ 0.004, respectively. The diagnostic efficiencies of P1 and SDA to detect specific IgG and IgE antibodies in the sera are compared in Table 2.
In order to investigate the utility of P1 for intradermal test-FIG. 1. Inhibition of binding of SDA to specific IgE and IgG anti-bodies in pooled sera of ABPA patients by the five synthetic peptides. To evaluate the diagnostic relevance of the synthetic peptides, the patient sera (diluted 1:100) were preincubated with various peptides (concentrations ranging from 50 ng to 1g). The immunoreactivities of the preincubated sera with SDA (1 g/well) were analyzed by indirect ELISA. The inhibition percentages obtained with various pep-tides (100 ng/well) are presented here. The values represent the mean values of three readings, and the SD for each value was within a⫾5% range.
on August 17, 2020 by guest
http://cvi.asm.org/
ing, it was subjected to lymphoproliferation and histamine release assays. On incubation with P1 and SDA, the PBMCs of ABPA patients showed 5.132- and 7.217-fold proliferation, respectively. PBMCs of controls incubated with P1 and SDA showed 1.239- and 2.375-fold proliferation, respectively. PBMCs
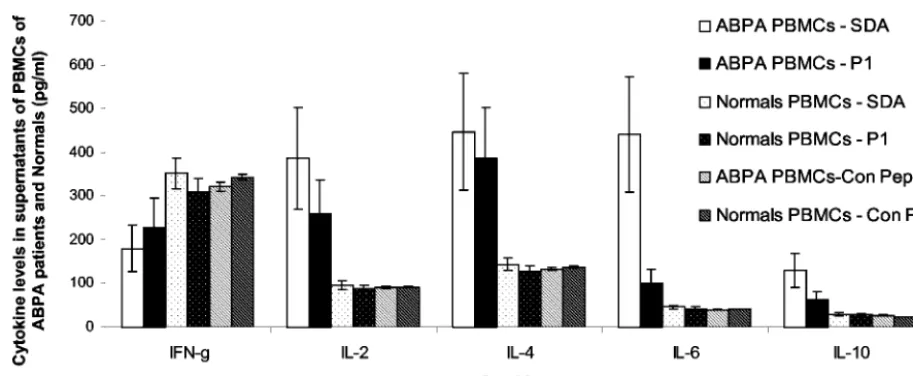
of healthy individuals incubated with P1 and SDA showed 1.072- and 1.517-fold proliferation, respectively. The cell su-pernatants from the lymphoproliferation assay were analyzed for IFN-␥, IL-2, IL-4, IL-6, and IL-10, and the results are shown in Fig. 3. The PBMCs of ABPA patients incubated with SDA showed decreased levels of IFN-␥but increased levels of IL-2, IL-4, IL-6, and IL-10 in comparison to those of healthy individuals. The PBMCs of ABPA patients incubated with P1 showed a cytokine profile similar to that of SDA, although at a lower magnitude. The P1-induced decrease in the IFN-␥ level was 1.440-fold lower than that of SDA, while the in-creases in the levels of IL-2, IL-4, IL-6, and IL-10 induced by P1 were 1.365-, 1.034-, 4.024-, and 1.969-fold, respectively, less than those induced by SDA. PBMCs of ABPA patients and healthy individuals incubated with the control peptide did not show stimulation of IFN-␥, IL-2, IL-4, IL-6, and IL-10 cyto-kines.
P1 induced maximum histamine release from the sensitized basophils of ABPA patients in comparison to the other four peptides; however, the histamine release induced by P1 was 34.15% less than the histamine release induced by SDA (Table 1). FIG. 2. Binding of peptide (80 ng/well by a dose-response curve) and SDA (1g/well) with specific IgE (A) and IgG (B) antibodies in sera of 20 clinically confirmed ABPA patients (ABPA), 20 controls (CTRL) (allergic patients with skin test negativity toA. fumigatus), and 25 healthy individuals (NRML) by indirect ELISA. The values represent the mean ELISA absorbance values for 20 ABPA patients, 20 controls, and 25 healthy individuals. The ELISA absorbance for each patient was the mean of three readings, and the SD for each value was in the⫾5% range. The cutoff for each assay was calculated for SDA and P1 from the mean absorbance value obtained for the 20 healthy individuals plus 3 SD.
TABLE 2. Diagnostic performances of SDA ofA. fumigatusand P1 in the immunodiagnosis of ABPA by ELISAa
Antigen-antibody Sensitivity% Specificity% % Diagnosticefficiency
SDA-IgG 100 90.9 93.75
P1-IgG 100 100 100
SDA-IgE 95.2 76.92 82.19
P1-IgE 100 97.5 98.3
aThe following definitions were used to calculate the corresponding diagnostic
parameters: true-positive values (tp), sera from patients with clinically confirmed ABPA showing positive readings; false-negative values (fn), sera from patients with clinically confirmed ABPA showing negative readings; false-positive values (fp), sera from controls and healthy individuals showing positive readings; true-negative values (tn), sera from controls and healthy individuals showing true-negative readings; sensitivity, tp⫻100/(tp⫹fn); specificity, tn⫻100/(tn⫹fp); diagnostic efficiency, (tn⫹tp)⫻100/(tp⫹fp⫹tn⫹fn).
on August 17, 2020 by guest
http://cvi.asm.org/
The absorbance values of P1 and SDA with sera of ABPA patients for both specific IgE and IgG antibodies were com-parable and suggest that the epitope represented by P1 (se-quentially or conformationally) may be present in multiple allergens and antigens ofA. fumigatus. BLAST analysis of the amino acid sequence of P1 with the genome sequence of A.
fumigatus (TBLASTN analysis of the peptide sequence with
the whole genome assembly of the shotgun sequence of A.
fumigatus at The Wellcome Trust Sanger Institute website
[http://www.sanger.ac.uk]) showed that the complete sequence appeared only once (with a score of 65) but partially matched with 38 sequences in the whole genome (with a score range of 31 to 40). To investigate the presence of epitopes similar to P1 in allergens and antigens other than Asp f 1, P1-specific anti-bodies were purified from pooled sera of 10 ABPA patients. P1-specific IgG antibodies recognized six allergens and anti-gens (with molecular masses of 24, 29, 55, 66, 77, and 88 kDa) (Fig. 4, lane 5) besides Asp f 1 (18 kDa). P1-specific IgE antibodies recognized three allergens and antigens with masses of 27, 34, and 43 kDa besides Asp f 1 (Fig. 4, lane 3).
DISCUSSION
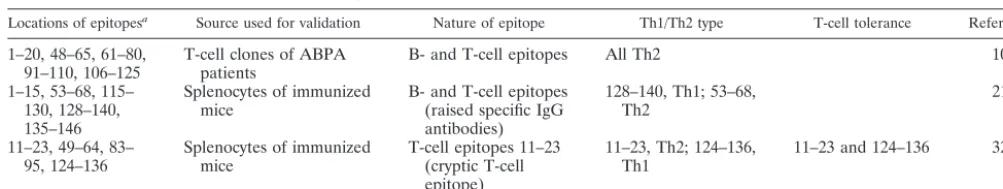
Significant levels of Asp f 1-specific antibodies are present in a majority of ABPA patients in the early stages of the disease (3, 15, 32). The present study attempted to analyze the diag-nostic relevance of the N-terminal region of Asp f 1 (6 to 22 amino acid residues), which has been predicted to be an im-portant epitopic region by 12 algorithmic programs used in the present study and earlier studies (Table 3) (10, 22, 32). The N-terminal region has been shown to be important for the allergenicity of a number of other allergens, like hevein pre-protein, rPhl p 6, and Bet v4 (7, 33, 36).
All five peptides, P1, P2, P3, P4, and P5, reacted with MAb on dot blots and showed significant inhibition of IgE (34 to 77%) and IgG (67 to 89%) antibody binding of SDA, indicat-ing that the N-terminal region of Asp f 1 is diagnostically
FIG. 3. Cytokine levels in the PBMCs of ABPA patients incubated with SDA and P1. PBMCs of the ABPA patients and healthy individuals (Normals) were incubated with SDA, P1, and control peptide (Con Pep; HIV epitopic peptide) (each at a concentration of 10g/well). The supernatants were subjected to ELISA-based cytokine assays for human IFN-␥, IL-2, IL-4, IL-6, and IL-10. The values represent the mean values of three readings each from three ABPA patients and three healthy individuals, and the SD for each value was within a⫾5% range.
FIG. 4. P1-specific IgG and IgE antibody binding to electropho-resed allergens or antigens (SDA) on immunoblots (SDS–12% PAGE). Lane 1, molecular mass markers; lane 2, IgE binding of SDA with pooled sera of ABPA patients; lane 3, IgE binding of SDA with P1-specific antibodies; lane 4, IgG binding of SDA with pooled sera of ABPA patients; lane 5, IgG binding of SDA with P1-specific antibod-ies.
on August 17, 2020 by guest
http://cvi.asm.org/
relevant and has both IgE and IgG binding domains. Further, the ability of synthetic peptides to induce histamine release from the sensitized basophils suggested the presence of IgE binding epitopes in the N-terminal region of Asp f 1. Kurup et al. reported an N-terminal Asp f 1 peptide (1 to 15 amino acid residues) that induced specific IgE and IgG antibodies in mice (22). Another N-terminal Asp f 1 peptide (1 to 20 amino acid residues) reacted with T-cell clones of ABPA patients (10). In a recent study, Svirshchevskaya et al. reported that 11 to 23 amino acid residues of Asp f 1 comprise a T-cell epitope (32). However, Kurup et al. showed that three peptides from the C terminus of Asp f 1 significantly inhibited IgE binding of Asp f 1 (inhibition ranged from 80 to 100%) (18). In an earlier study, a recombinant N-terminal fragment of Asp f 1 (8 to 45 amino acid residues), which comprised the immunodominant region of the present study, showed binding to specific IgG and IgE antibodies in ABPA patients by immunoblotting and ELISA (29).
In view of its maximum inhibition of specific IgE (77.32%⫾ 3.38%) and IgG (89.1%⫾4.45%) binding of SDA, the peptide P1 was further analyzed for its diagnostic potential. Compari-son of the diagnostic efficiency of P1 with that of SDA showed that the diagnostic performance of P1 in ELISA was superior to that of SDA, although it could not completely inhibit the IgG and IgE binding of SDA. A synthetic peptide of antigen B
ofEchinococcus granulosuswas also observed to be of higher
diagnostic value than the parent protein (13). This improve-ment in the diagnostic efficiency of P1 was attributable to its increased specificity and comparable sensitivity compared to that of SDA. The increased specificity of P1 may be due to its lower cross-reactivity than SDA, since SDA comprises a large number of allergenic and antigenic proteins that may be ho-mologous to other fungal allergens and antigens. It is impor-tant to note that P1 lacks significant sequential homology with other fungal proteins available in the public domain. The Na-tional Center for Biotechnology Information protein BLAST tool for short sequences in fungi showed that P1 has complete sequence similarity only to Asp f 1 and related ribotoxins of otherAspergillusspecies (E value, 1e-04) and partial similarity to elongation factor 2 of various species ofSaccharomycesand
Candida(only 7 out of 11 amino acid residues were identical,
with an E value of 4.3). The comparable sensitivity of P1 to that of SDA may be due to the presence of homologous IgG bind-ing epitopes in six immunodominant allergens and antigens (24, 29, 55, 66, 77, and 88 kDa) and homologous IgE binding
epitopes in three immunodominant allergens and antigens (27, 34, and 43 kDa) besides Asp f 1 (28).
P1 stimulated proliferation of PBMCs of ABPA patients with a stimulation index lower than that of SDA observed in the present study but similar to that of the crude antigen (a mixture of culture filtrate and mycelial extract ofA. fumigatus), as reported by Kurup et al. (18). The stimulation index of P1 was⬃2.5-fold higher than that of another Asp f 1 peptide (6 to 21 amino acid residues, comprising the amino acid region of P1) (18).
ABPA patients show a Th2 response toA. fumigatus aller-gens and antialler-gens, manifested by elevated levels of IL-4, IL-5, IL-10, and IL-13 and low levels of IFN-␥(20, 21). A similar cytokine profile was observed with the PBMCs incubated with SDA in the present study (the ratios of IFN-␥, IL-2, IL-4, IL-6, and IL-10 levels in supernatants of PBMCs of ABPA patients to those of healthy individuals incubated with SDA were 0.511, 4.027, 3.12, 9.687, and 4.347, respectively). Mice exposed to purified Asp f 1 also showed expression of regulatory cytokines characteristic of a Th2 response (32). The majority of the T-cell clones specific for Asp f 1 isolated from the ABPA patients, including a T-cell clone identifying the N terminus (1 to 20 amino acid residues), showed a Th2 type of cytokine profile (10). Lymph node cells stimulated by a T-cell cryptic epitopic peptide of Asp f 1 (11 to 23 amino acid residues, comprising the region of P1) also demonstrated a Th2 type of response with increased IL-4 mRNA but no IFN-␥ mRNA (32). The cytokine profile of P1-incubated PBMCs of ABPA patients was characterized by an increase in IL-4 levels and a decrease in levels of IFN-␥, suggesting that P1 also induces a Th2 type of response (the ratios of IFN-␥, IL-2, IL-4, IL-6, and IL-10 levels in supernatants of PBMCs of ABPA patients to those of healthy individuals incubated with P1 were 0.738, 2.95, 3.015, 2.407, and 2.207, respectively). However, the ratio of IL-4 levels to IFN-␥levels in supernatants of PBMCs of ABPA patients incubated with P1 (1.693) was significantly lower than the ratio observed with SDA (2.481), suggesting that the Th2 response induced by P1 is lower than that induced by SDA. IL-6 and IL-10 levels in supernatants of PBMCs incubated with P1 were also 4.024- and 1.969-fold lower, respectively, than the levels observed with SDA, suggesting that P1 can induce cyto-kine secretion from the sensitized PBMCs at a lower level than SDA. The ability of P1 to stimulate histamine release from ABPA patients and cytokines of Th2 type, although at a lower TABLE 3. Identified T-cell and B-cell epitopes of Asp f 1 using human T-cell clones isolated from ABPA patients and synthetic peptides
inducing proliferation of PBMCs ofA. fumigatus-sensitized mice
Locations of epitopesa Source used for validation Nature of epitope Th1/Th2 type T-cell tolerance Reference
1–20, 48–65, 61–80,
91–110, 106–125 T-cell clones of ABPApatients B- and T-cell epitopes All Th2 10 1–15, 53–68, 115–
130, 128–140, 135–146
Splenocytes of immunized
mice B- and T-cell epitopes(raised specific IgG antibodies)
128–140, Th1; 53–68,
Th2 21
11–23, 49–64, 83–
95, 124–136 Splenocytes of immunizedmice T-cell epitopes 11–23(cryptic T-cell epitope)
11–23, Th2; 124–136,
Th1 11–23 and 124–136 32
aThe amino acid residue number is given according to the protein sequence of Asp f 1 in AAB22442 of the National Center for Biotechnology Information.
on August 17, 2020 by guest
http://cvi.asm.org/
magnitude than SDA, suggests that it could be further ex-plored for intradermal skin testing.
There have been numerous reports of diagnostically relevant native and recombinant antigens for serodiagnosis of aspergil-losis (12, 14, 15, 16, 19, 25, 28, 30). The reported diagnostic sensitivity and specificity vary greatly among the different re-ports, even for similar antigen preparations. The higher diag-nostic efficiency of P1 than SDA in ELISA may lead to the development of a sensitive and specific serodiagnostic assay for aspergillosis. Furthermore, P1 constitutes a standardized re-agent that can be obtained by chemical synthesis in any labo-ratory. Another advantage of P1 is that it binds to ELISA plates by passive adsorption on the plastic surface and does not need conjugation to a carrier protein. However, the relevance of P1 for serodiagnosis of allergic aspergillosis patients other than ABPA patients remains to be examined. The present study has shown that P1 is diagnostically relevant for ABPA patients in the Indian population. Since there is a probability of heterogeneous recognition of an IgE epitope by sensitive pa-tients from various ethnic groups, the peptide P1 needs to be further evaluated in other populations for universal diagnostic application (11).
ACKNOWLEDGMENTS
We are grateful to the Institute of Genomics and Integrative Biol-ogy, a laboratory of the Council of Scientific and Industrial Research, Government of India, for financial support.
We acknowledge D. N. Rao, Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India, for his help in algo-rithmic analysis.
REFERENCES
1. Al Moudi, O. S.2001. Allergic bronchopulmonary aspergillosis mimicking pulmonary tuberculosis. Saudi Med. J.22:708–713.
2. Arruda, L. K., B. J. Mann, and M. D. Chapman.1992. Selective expression of a major allergen and cytotoxin Asp f 1 inA. fumigatus. J. Immunol. 149:3354–3359.
3. Arruda, L. K., T. A. E. Platts-Mills, J. W. Fox, and M. D. Chapman.1992.
Aspergillus fumigatusallergen I, a major IgE binding protein, is a member of the mitogillin family of cytotoxins. J. Exp. Med.172:1529–1532.
4. Banerjee, B., A. Chetty, A. P. Joshi, and P. U. Sarma.1990. Identification and characterisation of diagnostically relevant antigens ofAspergillus fumiga-tus. Asian Pac. J. Allergy Immunol.18:13–18.
5. Banerjee, B., M. Mukherjee, S. V. Gangal, and P. U. Sarma.1990. Studies on
Aspergillus fumigatusantigens for serodiagnosis. Serodiagn. Immunother. Infect. Dis.4:183–191.
6. Banerjee, B., T. Madan, G. L. Sharma, H. K. Prasad, I. Nath, and P. U. Sarma.1995. Chracterisation of glycoprotein antigen (45kD) ofAspergillus fumigatus. Serodiagn. Immunother. Infect. Dis.7:147–152.
7. Beezhold, D. H., D. A. Kostyal, and G. L. Sussman.1997. IgE epitope analysis of the hevein preprotein; a major latex allergen. Clin. Exp. Immunol. 108:114–121.
8. Behera, D., R. Guleria, S. K. Jindal, A. Chakrabarti, and D. Panigrahi.1994. Allergic bronchopulmonary aspergillosis: a retrospective study of 35 cases. Indian J. Chest Dis. Allied Sci.36:173–179.
9. Carter, J. M.1994. Epitope prediction methods. Methods Mol. Biol.36:193– 206.
10. Chauhan, B., A. P. Knutsen, P. S. Hutcheson, R. G. Slavin, and C. J. Bellone. 1996. T cell subsets, epitope mapping and HLA-restriction in patients with ABPA. J. Clin. Investig.97:2324–2331.
11. Costa, M. A., G. Duro, V. Izzo, P. Colombo, M. G. Mirisola, G. Locorotondo, R. Cocchiara, and D. Geraci.2000. The IgE-binding epitopes of rPar j 2, a major allergen ofParietaria judaicapollen, are heterogeneously recognized among allergic subjects. Allergy55:246–250.
12. Crameri, R.2002. Molecular cloning ofAspergillus fumigatusallergens and their role in allergic bronchopulmonary aspergillosis. Chem. Immunol.81: 73–93.
13. Gonzalez-Sapienza, G., C. Lorenzo, and A. Nieto.2000. Improved immuno-diagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein,Echinococcus granulosus
antigen B. J. Clin. Microbiol.38:3979–3983.
14. Hemmann, S., C. Ismail, K. Blaser, G. Menz, and R. Crameri.1998. Skin-test reactivity and isotype-specific immune responses to recombinant Asp f 3, a major allergen ofAspergillus fumigatus. Clin. Exp. Allergy28:860–867. 15. Hemmann, S., W. H. Nikolaizik, M. H. Schoni, K. Blaser, and R. Crameri.
1998. Differential IgE recognition of recombinantAspergillus fumigatus al-lergens by cystic fibrosis patients with allergic bronchopulmonary aspergil-losis orAspergillusallergy. Eur. J. Immunol.28:1155–1160.
16. Hemmann, S., G. Menz, C. Ismail, K. Blaser, and R. Crameri.1999. Skin test reactivity to 2 recombinantAspergillus fumigatusallergens inA. fumigatus -sensitized asthmatic subjects allows diagnostic separation of allergic bron-chopulmonary aspergillosis from fungal sensitization. J. Allergy Clin. Immu-nol.104:601–607.
17. Kothari, K., V. Singh, R. Sharma, and R. Khandelwal.2000. Diagnostic dilemma: aspergillosis. J. Assoc. Physicians India48:445–447.
18. Kurup, V. P., B. Banerjee, P. S. Murali, P. A. Greenberger, M. Krishnan, V. Hari, and J. N. Fink.1998. Immunodominant peptide epitopes of allergen Asp f 1 from the fungusAspergillus fumigatus. Peptides19:1469–1477. 19. Kurup, V. P., B. Banerjee, S. Hemmann, P. A. Greenberger, K. Blaser, and
R. Crameri.2000. Selected recombinantAspergillus fumigatusallergens bind specifically to IgE in ABPA. Clin. Exp. Allergy30:988–993.
20. Kurup, V. P., B. W. P. Seymour, H. Choi, and R. L. Coffmann.1994. Par-ticulateAspergillus fumigatusantigens elicit a TH2 response in BALB/c mice. J. Allergy Clin. Immunol.93:1013–1020.
21. Kurup, V. P., J. Q. Xia, R. Crameri, D. A. Rickaby, H. Y. Choi, S. Fluckiger, K. Blaser, C. A. Dawson, and K. J. Kelly.2000. Purified recombinantA. fumigatusallergens induce different responses in mice. Clin. Immunol.98: 327–336.
22. Kurup, V. P., V. Hari, J. Guo, P. S. Murali, A. Resnick, M. Krishnan, and J. N. Fink.1996.Aspergillus fumigatuspeptides differentially express Th1 and Th2 cytokines. Peptides17:183–190.
23. Madan, T., N. Arora, and P. U. Sarma.1997. Cytotoxicity of Asp f 1, a major allergen/antigen ofA. fumigatus. Mol. Cell Biochem.167:89–97.
24. Moser, M., R. Crameri, G. Menz, T. Schneider, T. Dudler, C. Virchow, M. Gmachi, K. Blaser, and M. Suter.1992. Cloning and expression of recom-binantA. fumigatusallergen I/a (rAspf I/a) with IgE binding and type I skin test activity. J. Immunol.149:454–460.
25. Nigam, S., P. V. Sarma, P. C. Ghosh, and P. U. Sarma.2001. Characteriza-tion ofAspergillus fumigatusprotein disulfide isomerase family gene. Gene 281:143–150.
26. Rosenberg, M., R. Patterson, M. Roberts, and J. Wang.1978. The assess-ment of immunologic and clinical stages occurring during corticosteroid therapy for ABPA. Am. J. Med.64:599–607.
27. Rothbard, J. B., and W. R. Taylor.1988. A sequence pattern common to T cell epitopes. EMBO J.7:93–100.
28. Sarma, P. U., T. Madan, and V. P. Kurup.2003. Immunodiagnosis of ABPA. Front. Biosci.8:S1187–S1198.
29. Sarma, P. V. G. K., S. Purkayastha, T. Madan, and P. U. Sarma.1999. Expression of an epitopic region of Asp f 1, an allergen/antigen/cytotoxin of
Aspergillus fumigatus. Immunol. Lett.70:151–155.
30. Saxena, S., T. Madan, K. Muralidhar, and P. U. Sarma.2003. cDNA clon-ing, expression and characterization of an allergenic L3 ribosomal protein of
Aspergillus fumigatus. Clin. Exp. Immunol.134:86–91.
31. Shah, A., and C. Panjabi.2002. Allergic bronchopulmonary aspergillosis: a review of a disease with a worldwide distribution. J. Asthma39:273–289. 32. Svirshchevskaya, E., E. Frolova, L. Alekseeva, O. Kotzareva, and V. P.
Kurup.2000. Intravenous injection of major and cryptic peptide epitopes of ribotoxin, Asp f 1 inhibits T cell response induced by crudeAspergillus fumigatusantigens in mice. Peptides21:1–8.
33. Twardosz, A., B. Hayek, S. Seiberler, L. Vangelista, L. Elfman, H. Gronlund, D. Craft, and R. Valenta.1997. Molecular characterization, expression in
Escherichia coli, and epitope analysis of a two EF-hand calcium-binding birch pollen allergen, Bet v 4. Biochem. Biophys. Res. Commun.239:197–204. 34. Van Regenmortel, M. H.1992. Protein antigenicity. Mol. Biol. Rep.16:133–
138.
35. Van Regenmortel, M. H., and J. L. Pellequer.1994. Predicting antigenic determinants in proteins: looking for unidimensional solutions to a three-dimensional problem? Pept. Res.7:224–228.
36. Vrtala, S., S. Fischer, M. Grote, L. Vangelista, A. Pastore, W. R. Sperr, P. Valent, R. Reichelt, D. Kraft, and R. Valenta.1999. Molecular, immunolog-ical, and structural characterization of Phl p 6, a major allergen and P-particle-associated protein from Timothy grass (Phleum pratense) pollen. J. Immunol.163:5489–5496.