Interventions for Occluded Central
Venous Catheters: A Meta-analysis
Ana Cristina Carvalho da Costa, RN, PhD,aNayara Narley Pires Vieira, RN, MSc,aChristiane Inocêncio Vasques, RN, PhD,a
Elaine Barros Ferreira, RN, PhD,aEliete Neves Silva Guerra, DDS, PhD,bPaula Elaine Diniz dos Reis, RN, PhDa
abstract
CONTEXT:Thrombotic occlusion is 1 of the most frequent complications in catheters implanted inchildren.
OBJECTIVE:To identify the interventions used to treat thrombotic events in long-term central venous catheters in pediatric patients with cancer.
DATA SOURCES:Electronic searches were performed in the Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, Latin American and Caribbean Health Sciences Literature, LIVIVO, PubMed, Scopus, Web of Science, Google Scholar, OpenGrey, and ProQuest databases. There were no restrictions on language or publication period.
STUDY SELECTION:This systematic review was performed in 2 phases and included clinical trials
and observational studies on drugs used to treat thrombotic catheter events in pediatric patients with cancer. The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist, and the protocol was registered at
PROSPERO (identifier CRD42018083555).
DATA EXTRACTION:The authors evaluated the quality of included studies using the Methodological
Index for Nonrandomized Studies and Grading of Recommendations Assessment, Development and Evaluation methods. The meta-analysis was performed by using Stata software.
RESULTS:Ten studies were included. The drugs used to restore catheter function were alteplase,
urokinase, and streptokinase. A meta-analysis of 6 studies revealed an overall restoration rate of 88% for alteplase.
LIMITATIONS:Reference studies were excluded when it was not possible to reliably extract data
that met the inclusion criteria of this review. Sampling issues (absence of randomization, blinding, or a control group) were the main methodologic concerns for the included articles.
CONCLUSIONS:On the basis of the evidence obtained, thrombolysis is effective and potentially safe in this population.
a
Interdisciplinary Research Laboratory Applied to Clinical Practice in Oncology andbLaboratory of Oral Histopathology, Faculty of Health Sciences, University of Brasília, Brasília, Brazil
Drs Costa and Ferreira conceptualized and designed the study, drafted the initial manuscript, and reviewed the manuscript; Ms Vieira and Dr Guerra designed the data collection instruments, collected data, conducted the initial analyses, and reviewed the manuscript; Drs Vasques and Reis conceptualized and designed the study, coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content; and all authors approved thefinal manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered with PROSPERO (https://www.crd.york.ac.uk/prospero/) (identifier CRD42018083555).
DOI:https://doi.org/10.1542/peds.2018-3789 Accepted for publication Sep 6, 2019
Central venous catheters (CVCs) have been used for.3 decades in pediatric oncology. These devices are
considered appropriate in the care of children with cancer and are
commonly used for drug
administration, blood sampling, and nutritional support.1–3However, they are also associated with a variety of complications, including catheter occlusion.4
CVC occlusion may be due to thrombus formation or nonthrombotic causes, such as catheter malposition or intraluminal drug precipitation. The most frequent causes of catheter dysfunction in pediatric patients are wall thrombi, intraluminal and CVC tip thrombi, or thrombi caused byfibrin sheaths.5 CVC occlusion may lead to inability to draw blood and/or infusefluids.6
CVC thrombotic occlusion is 1 of the most frequent complications, occurring in 20% to 40% of the catheters implanted in children.7This type of CVC occlusion can lead to infection, thromboembolism, catheter dysfunction, or thrombus
propagation, resulting in deep venous thrombosis.3
Replacement or removal of an occluded CVC in a child who is sick may lead to discontinuation of therapy, need for sedation or general anesthesia, repeated surgical
interventions, and significant cost increases associated with prolonged treatment and additional
procedures.8The treatment of CVC occlusion is less costly and faster than the surgical removal and replacement of the catheter, reducing the risk of adverse events (AEs) to the patient.9
Fibrinolytic therapy has been used for .20 years in the treatment of CVC thrombotic occlusion. Thefibrinolytic system in children is a dynamic, age-dependent system with unique characteristics that markedly influence the response to
thrombolytic agents. In addition, the pathophysiological mechanisms of
thrombosis in children are different from those in adults.10Despite this, protocols for the administration of thrombolytic agents for restoration of catheter function in children with cancer are generally empirical and are extrapolated from adult guidelines; moreover, thrombolytic therapies differ in the type of drug used, dosing, and the duration of treatment.5,10
A systematic review to evaluate interventions to restore patency of occluded CVCs has been previously published.11However, the authors of this review examined a small number of randomized clinical trials that generally addressed the management of thrombotic occlusion, regardless of the type of catheter or the patient age and clinical condition. In addition, new technologies and studies have been developed since its publication, and there is a need to update the
findings.
Although alteplase is the only drug approved by the US Food and Drug Administration (FDA) for restoration of catheter function in the United States, urokinase and streptokinase are available in other countries and continents for this purpose, despite established recommendations. Thus, the use of different types of
thrombolytic agents for the treatment of CVC thrombotic occlusion around the world justifies the need for such a systematic review and meta-analysis.
In this systematic review, we aimed to identify the interventions used to treat thrombotic events involving long-term use of CVCs in pediatric patients with cancer.
METHODS
Protocol and Registration
This systematic review was
performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist.12The protocol was
registered at the International Prospective Register of Systematic Reviews (PROSPERO) under identifier CRD42018083555.13
Eligibility Criteria
Inclusion Criteria
This review included articles about clinical trials or observational studies involving pediatric patients with cancer (children and adolescents younger than 18 years) with partially or completely occluded long-term CVCs (totally implanted catheters [TICs] and tunneled catheters [TCs]), including all treated lumens and all types of cancer; in addition, this review included articles about interventions to treat thrombotic events by using pharmacologic and nonpharmacologic substances. Restoration of catheter function was defined as the ability to instill 5 mL of saline solution and to aspirate 3 mL of blood in patients weighing$10 kg or the ability to infuse 3 mL of saline solution and to aspirate 1 mL of blood in patients weighing,10 kg.8There were no restrictions on language or publication period.
Exclusion Criteria
The studies were assessed in 2 phases. In phase 1 (title and abstract review), the following exclusion criteria were applied: (1) studies with use of a short-term CVC, hemodialysis catheter, peripherally inserted central catheter, apheresis catheter, umbilical catheter, or arterial catheter or with use of different types of catheters in the same study; (2) studies in adults or in mixed populations in which it was not possible to perform reliable extraction of data referring to children with cancer; (3) studies in which the authors evaluated interventions to prevent thrombotic events; and (4) reviews of the literature, letters, case reports, or protocols.
did not investigate treatment of catheter occlusion, (6) duplicated data, (7) studies in which authors did not include patients with cancer, and (8) studies with incomplete data on the treated population or catheter type used.
Search Methods
We developed search strategies for each of the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), Latin American and Caribbean Health Sciences Literature (LILACS), LIVIVO, PubMed, Scopus, and Web of Science. A gray literature search was
performed by using Google Scholar, OpenGrey, and ProQuest Theses and Dissertations. The ending search date was February 16, 2018, across all databases. Hand searching of the reference lists of included studies was also performed. In addition, e-mails were sent to experts to inquire about additional studies relevant to the review.
Appropriate truncation and word combinations were selected and adapted for each database search (Supplemental Tables 3 through 12). All references were managed by using reference manager software
(EndNote X7; Thomson Reuters, New York), and duplicates were removed.
Search Outcomes
The selection was completed in 2 phases. In phase 1, 2 reviewers (A.C.C.d.C. and N.N.P.V.) independently reviewed the titles and abstracts of all citations identified from databases. Articles that did not appear to meet the inclusion criteria were discarded. In phase 2, the same reviewers applied the inclusion criteria to the full text of the articles. The reference lists of selected studies were critically assessed by both examiners. Any disagreement in phase 1 or 2 was resolved by discussion until an agreement between the 2 authors was
attained. When consensus was not reached, a third author (C.I.V.) was included for afinal decision.
Data Extraction and Synthesis
Two reviewers independently collected data from the selected studies. A third reviewer assessed the accuracy of the information collected. For all included studies, the following characteristics were recorded: study characteristics (author, year, country of publication, and study design), sample characteristics (size, catheter type, and number of occluded CVCs), intervention characteristics (drug type, doses, infusion time, and follow-up time), outcome characteristics (efficacy [restoration of catheter patency] and safety), and the main conclusion. If the required data were not complete or the data presented could not be extrapolated, attempts were made by e-mail to contact the authors to retrieve missing
information.
The efficacy outcome was expressed as the percentage of catheter function restored from the total sample of included studies.
After heterogeneity between the studies was assessed, a meta-analysis was performed for the data by using Stata software version 14 (Stata Corp, College Station, TX). Heterogeneity was calculated by usingI2, following the appropriate Cochrane
guidelines.14A value.50% was considered an indicator of substantial heterogeneity among studies, enabling the use of a random-effects model. When I2was,50%, afi xed-effects model was used. The significance level was set at 5%.
Quality Appraisal
Risk of bias of selected studies was evaluated by using the
Methodological Index for
Nonrandomized Studies (MINORS)15 for nonrandomized clinical trials and observational studies. Two reviewers independently assessed the quality of each study. For judgment of risk of
bias, items were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The ideal overall score was 16 for noncomparative studies and 24 for comparative studies. Disagreements between both reviewers were resolved by a third reviewer.
A summary of the overall strength of evidence available was performed by using the Grading of
Recommendations Assessment, Development and Evaluation (GRADE) method.16A summary of
findings table was produced by using GRADEpro software (McMaster University, Hamilton, Canada).
RESULTS
Study Selection
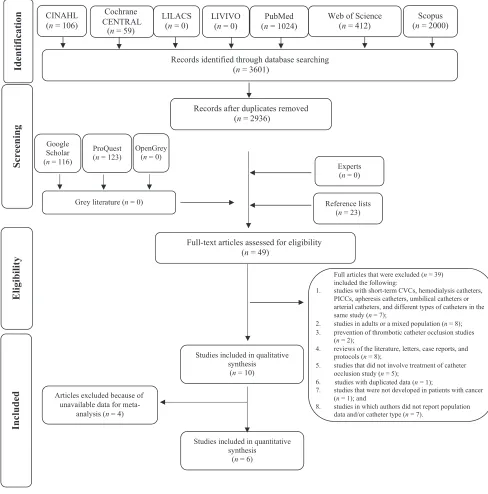
In phase 1, 3601 articles were found across the 7 databases. After duplicates were removed, 26 of the 2936 studies were selected for phase 2. A gray literature search was used to identify 242 articles, but none met the inclusion criteria. The reference lists of included studies were screened, and 23 articles were included. After 3 consecutive attempts in a period of 1 month, we did not obtain answers from the experts, and articles were not included through this type of search. Subsequently, 49 articles were obtained for full-text reading, and 39 articles were excluded (Supplemental Table 13). Therefore, only 10 studies fulfilled the
eligibility criteria and were included in the qualitative synthesis. Of these, 6 matched the criteria used for the meta-analysis. Aflow diagram of the process of identification, inclusion, and exclusion of studies is shown in Fig 1.
Study Characteristics
Of the 10 selected studies, 6 were clinical trials8,17–21; of 4
based on evaluation of patient charts,22–24and 1 was a prospective cohort study.25CVC sample sizes ranged from 417to 1608among a total of 447 long-term CVCs studied in 747 patients. Among the long-term CVCs used, 218 (48.3%) were TCs and 233 (51.7%) were TICs. Partial occlusion was evaluated in 4 studies (40%), and
partial or total occlusion was evaluated in 6 studies (60%).
Partial occlusion was defined as the inability to withdraw 3 mL of blood, with a retained ability to infuse 5 mL of saline through the catheter. Complete occlusion was defined as the inability to withdraw 3 mL of
blood and the inability to infuse 5 mL of saline through the catheter.26
The drugs used for restoration of catheter patency were
alteplase8,17,19,22–24(n= 6), urokinase18,20,21 (n= 3), and streptokinase25(n= 1). In all trials, investigators evaluated the efficacy of
FIGURE 1
Flow diagram of literature search and selection criteria. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items
drugs without using a control group. In only 1 study20was the same drug (urokinase) used in 3 different groups with 2 infusion regimens.
The aim of the studies was to evaluate the efficacy and safety of the drugs in restoring catheter patency by using different doses and different infusion times. The restoration rate ranged from 50%25to 97.5%,19and the administration time ranged from 30 minutes8,20,22,23to 48 hours.18The authors of 2 studies reported AEs related to alteplase, including sepsis,8 catheter rupture,22and blood dyscrasia (characterized as an abnormality of either prothrombin or partial thromboplastin time, or of
fibrinogen, with increased bleeding events within 48 hours).22There were no AEs reported in the other articles. The descriptive
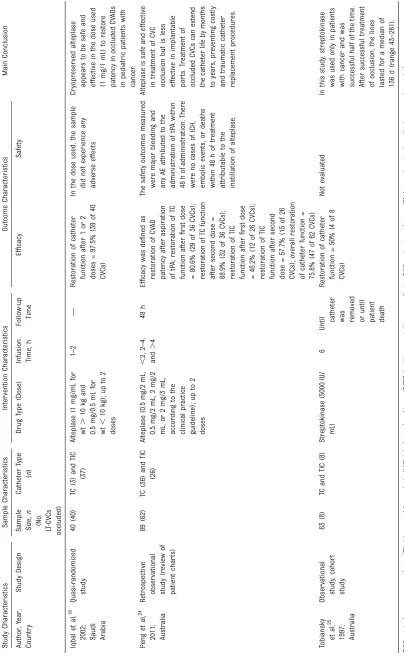
characteristics of the included articles are summarized in Table 1.
Results of Individual Studies
The Atkinson et al17study included the smallest number of catheters, and alteplase was used at the standard 2-mg dose, achieving a success rate of 75%. It should be noted that the CVC used had already been treated with a urokinase bolus (10 000 IU) without success. The Blaney et al8 study included the largest number of catheters, with the same 2-mg dose of alteplase, and achieved a restoration rate of 83.1%.
Chesler and Feusner22achieved a high success rate (88.1%) despite using a minimum dose of alteplase (0.5 mg). Fisher et al23also used a lower dose of alteplase (1 mg) but achieved an even higher rate of restoration (92.8%). Iqbal et al19 used alteplase doses between 0.5 and 1 mg, depending on patient weight, and had the highest success rate among the included studies (97.5%). Peng et al24used alteplase in doses ranging from 0.5 to 2 mg, achieving a restoration rate of 75.8%. Bagnall et al18used urokinase at a dose of 200 IU/kg per hour in a prolonged
infusion, achieving a success rate of 91.7%. The catheters used in this study had already undergone unsuccessful thrombolysis with consecutive urokinase boluses at 5000 and 10 000 IU.
Molinari et al20used urokinase in 3 different doses and in 2 infusion protocols. Partially occluded CVCs were divided into 2 groups: 1 by using urokinase bolus administration of 5000 IU and another by using urokinase bolus administration of 25 000 IU. Fully occluded CVCs, or those refractory to the previously described treatments, received low-dose systemic urokinase at a low-dose of 1000 IU/kg per hour in a prolonged infusion that varied from 1 to 3 hours because low-dose systemic urokinase could be administered up to 4 times. Restoration rates in these 3 groups were 89.4%, 97%, and 77.8%, respectively.
The Winthrop and Wesson21study had the earliest publication date, and the authors used a urokinase dose of 5000 IU, achieving a success rate of 66.7%. Tobiansky et al25alone used streptokinase for CVC thrombolysis, in a dose of 5000 IU, and showed the lowest rate of restoration among the included studies (50%).
Synthesis of Results
Six of the 10 studies included were used in the meta-analysis. One study25was excluded as unique in using streptokinase, and another20 was excluded because the catheters were treated with multiples doses of urokinase, making it impossible to identify the dose received through each catheter. In addition, 2 studies18,21were excluded because the authors addressed the use of urokinase in totally different doses and infusion regimens, with considerable heterogeneity among them, not allowing for a reliable comparison. Because the
heterogeneity among the studies was significant (59.22%; confidence interval 1.41–83.65), the
random-effects model was chosen for the statistical analysis. After
consideration of the alteplase intervention, the results revealed an overall CVC function restoration rate of 88% (total sample = 322 catheters) (Fig 2).
Risk of Bias of Individual Studies
Three studies17,19,21were classified as having a high risk of bias because they scored#8 points (50% of the total score), and 3 other studies8,20,25 were classified as having a low risk of bias because they scored between 13 and 18 points. The other 4 studies were classified as having an unclear risk of bias because they scored between 9 and 11 points.18,22–24More information about the MINORS15 scores is provided in Fig 3.
Risk of Bias Within Studies
Although the studies had different designs, the main methodologic problem concerned the sample. In most of the studies, including the clinical trials, convenience samples were used, without randomization of participants and without use of a control group.
Confidence in Cumulative Evidence
Using the GRADE summary of
findings table, we found that the quality of evidence ranged from very low to low. This variation was directly related to the risk of bias as well as to heterogeneity among the studies (Table 2).
DISCUSSION
TABLE 1 Summary of Descriptive Char acteristics of Included Studies ( n = 10) Stu dy Char acte ristics Sam ple Cha racteristics Int ervention Char ac teristics Out come Char acte ristics Main Conclu sion Aut hor , Year ; Cou ntry Stu dy Design Sample Size, n (No . LT -CV Cs occlu ded) Cathe ter Type ( n ) Drug Type (Dose) Infusion Time , h Follow-u p Time Ef fi ca cy Safety Bag
nall etal,
TABLE 1 Continued Stu dy Char acte ristics Sam ple Cha racteristics Int ervention Char ac teristics Out come Char acte ristics Main Conclu sion Aut hor , Year ; Cou ntry Stu dy Design Sample Size, n (No . LT -CV Cs occlu ded) Cathe ter Type ( n ) Drug Type (Dose) Infusion Time , h Follow-u p Time Ef fi ca cy Safety Iqb al et al, 19 2002 ;
Saudi Arabia
approach to restore catheter patency.5
In this systematic review, we investigated the available evidence for the interventions used in the treatment of thrombotic events in long-term CVCs in pediatric patients with cancer. Among interventions used to restore catheter function, alteplase was the most frequent, followed by urokinase. No
nonpharmacologic intervention was identified.
Alteplase is a recombinant tissue plasminogen activator and is considered thefirst option in the noninvasive treatment of CVC thrombotic occlusion. The benefit of alteplase is its high specificity for
fibrin, low immunogenicity, and short systemic half-life (5 minutes). This protease converts plasminogen to plasmin when in contact with
fibrinous material, promoting thrombolysis.27
The use of alteplase in the restoration of catheter function was approved in September 2001 by the FDA after 2 clinical trials,28,29but the population used in these studies consisted predominantly of adult patients, prompting the need for a phase IV study to clarify the safety and efficacy of the drug in the general pediatric population. The results of this research8corroborated the results of previous studies regarding the safety and efficacy of alteplase in restoring
CVC function in children, regardless of age, weight, or type of
catheter used.
The alteplase dose for restoration of catheter patency depends on patient weight and thefilling volume of the occluded lumen. The FDA,28,29the manufacturer,30and the American College of Chest Physicians31
recommended administration of up to 2 doses of 2 mg (2 mL) for patients weighing$30 kg and up to 2 doses of 1 mg/1 mL for patients weighing,30 kg; the dose should be up to 110% of the volume of the occluded catheter, not exceeding 2 mL. However, in this systematic review, large dose variations were observed, ranging from 0.5 mg/0.5 mL to 2 mg/3 mL, FIGURE 2
with different dose standardizations in relation to the child’s weight, differing from the recommendations described previously.
There are some differences in the literature related to the drug infusion time; however, the FDA and the manufacturer recommend that
alteplase remain within the catheter for 30 minutes and that if function is not restored within that time, 90 minutes be added for a total time of 120 minutes for thefirst dose. If the CVC remains occluded, a second intraluminal dose should be administered for an equal period of time, extending the infusion time to 240 minutes.32With some slight variations, in this review, most studies in which alteplase was used followed the recommended
treatment times.
Alteplase was generally well tolerated in clinical trials in pediatric
populations, with a reported low incidence of AEs such as sepsis, minor bleeding (gastrointestinal bleeding, hematomas), fever, and venous thrombosis.32The studies included in this review reported AEs considered serious and related to the drug, including 1 case of sepsis and 1 case of catheter rupture. Particular attention should be paid to sepsis because a positive correlation was observed between the use of alteplase for treatment of occluded catheters and the development of CVC-associated bloodstream infection. This correlation probably reflects the potential of the thrombus to contain
biofilm, making alteplase a vehicle for bacterial dissemination when infused into the catheter.33
Although variations in doses and infusion times were found, the authors of the studies considered alteplase to be safe and effective in restoration of CVC patency. However, one should always consider the risk of bleeding associated with
thrombolytic therapy, especially when the drug is used in children younger than 2 years and those weighing ,10 kg because there are few studies in this population. The risk of catheter thrombosis and associated complications was significantly higher in young children and those with low weight. Higher risk was associated with the relatively greater size of the catheter compared with that of the small vessel lumen, reduced plasma levels of plasminogen and antithrombin III, and low catheterflow when compared with that in older children.26In addition, there was a risk of systemic infusion of alteplase because of reduced priming of pediatric catheters.
Until 1999, urokinase was used worldwide in pediatric centers for treatment of CVC occlusion. However, in January 1999, the FDA withdrew FIGURE 3
Methodologic appraisal of selected studies based on MINORS.
TABLE 2GRADE’s Summary of Findings Table
Certainty Assessment Certainty
No. Studies Study Design Risk of
Bias
Inconsistency Indirectness Imprecision Other Considerations
Restoration of catheter function with thrombolytic therapy
9 Clinical trials and
observational studies
Seriousa Seriousb Not serious Seriousc None ⨁◯◯◯very low Restoration of catheter function with
alteplase
6 Clinical trials and
observational studies
Seriousa Seriousd Not serious Not serious None ⨁⨁◯◯low
Restoration of catheter function with urokinase
2 Clinical trials and
observational studies
Seriousa Seriouse Not serious Not serious None ⨁⨁◯◯low
Question: What is the efficacy of interventions used to treat thrombotic catheter occlusion in pediatric patients with cancer?
aAll included studies are nonrandomized with the absence of blinding and a control group. bI2
= 62.86%.
cUse of different doses and different infusion times. dI2
= 59.86%.
urokinase from the market because of suspected transmission of infectious agents associated with its use.34 Urokinase is a type of plasminogen activator that also converts plasminogen to plasmin and has a half-life of∼12 minutes. Although urokinase derived from human cells was withdrawn from the market in the late 1990s, this form is still available for use in Europe, having been replaced by the recombinant form derived from nonhuman cells in North America on the basis of FDA recommendations.10
Studies have reported the use of urokinase in different doses, both for intraluminal bolus administration and for extended systemic infusion, with varying infusion times.5The Italian Association of Pediatric Hematology and Oncology recommends use of urokinase for treatment of CVC occlusion at a bolus dose of 5000 IU/ mL, with an infusion time of 15 to 60 minutes (recommendation IIB). In the case of persistent CVC occlusion that is resistant to intraluminal thrombolysis, and in the absence of catheter-related thrombosis, systemic urokinase infusion is indicated at 1000 IU/kg per hour for 3 hours, and may be repeated for a maximum of 12 hours, or at a dose of 200 IU/kg per hour for up to 24 hours (recommendation IIB).5In most of the studies in this systematic review, doses were used in accordance with the literature.
A study34conducted in several pediatric oncology and hematology centers revealed a low incidence of complications with use of urokinase (9%). Allergic reactions, fever and tremors, generation of microemboli, and minor hemorrhagic events were reported. According to the
manufacturer, urokinase is contraindicated in patients with central nervous system neoplasms because of the risk of intracranial bleeding.34However, no reports of this restriction of use or of severe bleeding events were found in the
studies included in this review. In addition, no drug-related AEs were reported in the studies.
Studies conducted in the 1990s revealed that some catheters might have undergone multiple treatments with urokinase. This partial treatment of occlusion might have led to inadequate lysis of the thrombus and formation of a nidus for infection, thus increasing the risk of CVC-associated bloodstream infection and associated complications.34Infectious complications related to the use of urokinase were not reported in the studies in this systematic review.
Streptokinase, a thrombolytic agent produced byb-hemolytic group C streptococci, is a single-chain polypeptide that reacts with
circulating plasminogen, converting it to plasmin and forming
streptokinase-plasmin complexes. The streptokinase-plasmin complex has increased thrombolytic activity compared with that of plasmin because it is not inactivated bya2
-antiplasmin anda2-macroglobulin.
Streptokinase has a half-life of 18 to 30 minutes, with an associated lytic effect ranging from 82 to
184 minutes.10Streptokinase was widely used in the 1970s and 1980s; however, it induced the formation of antibodies, which led to resistance to treatment, as well as allergic
reactions, such as fever, hypotension, urticaria, and bronchospasm, after repeated infusions.10Thus,
streptokinase is not considered to be a good alternative for treatment of CVC occlusion because of FDA warnings about the serious risk of anaphylaxis and the possibility of death.26
A qualitative data analysis revealed that similar restoration rates were observed between studies in which alteplase and urokinase were used, with a marked decrease in efficacy with use of streptokinase. However, when the data were evaluated in
a meta-analysis, alteplase appeared to be superior to other interventions.
Because of high heterogeneity in the doses of the thrombolytics studied, the duration of infusion of these drugs should be considered in clinical practice because a shorter infusion time optimizes the use of medical resources and avoids delays in treatment, increased costs, and associated complications.
Moreover, the safety factor is fundamental, especially in the pediatric population. Although the authors of the studies included in this review considered thrombolytic therapy as safe for restoration of CVC patency, more vigilance and follow-up protocols should be instituted when these drugs are used in children. The lack of well-established clinical protocols, the use of varied concentrations of drugs (often with no adequate correlation with child weight or occluded CVC intraluminal volume), and the paucity of studies in more vulnerable pediatric
populations (low weight and young) induces an increased risk of AEs (especially hemorrhagic events) associated with therapy.
Another important factor to be taken into account in the use of
cost-effective,finding a high success rate in restoration of CVC patency (98%), with the estimated cost for catheter replacement.20 times greater than that for use of the drug.
As a methodologic limitation of this review, we highlight the exclusion of reference studies with representative samples and the use of other thrombolytics not described here that met the main outcome of this review (restoration of CVC function). However, some of these studies had a mixed population, and others had different types of catheters, and it was not possible to reliably extract the necessary data for the analysis; even after attempting to contact the authors by e-mail, it was not possible to aggregate this data for the review.
All included studies had a risk of bias related to the study population and the high heterogeneity between the doses used and infusion times; moreover, the quality of the evidence generated was not substantial. Although the evidence that was found tends to reiterate the success of thrombolytic therapy in the pediatric population, there are insufficient data
for use in the preparation of robust drug protocols, including
concentration and infusion time, to standardize and guide clinical practice for restoration of catheter function.
The authors of only 2 studies8,22 reported AEs related to thrombolytic therapy; this is an important item to consider in designing future studies.
CONCLUSIONS
In view of the evidence obtained, the most common interventions used for treatment of thrombotic catheter occlusion in pediatric patients with cancer were alteplase and urokinase. The studies included in this
systematic review revealed that thrombolysis is effective and potentially safe in this population, with slight superiority of alteplase when compared with other interventions.
Although thrombolytic therapy is considered safe for restoration of patency in occluded catheters, more vigilance and follow-up protocols should be instituted when these
drugs are used in children, especially in more vulnerable populations (low weight and very young). The impact of our results may affect catheter management, safety, and quality of life in pediatric patients with cancer.
ABBREVIATIONS
AE: adverse event
CENTRAL: Central Register of Controlled Trials CINAHL: Cumulative Index to
Nursing and Allied Health Literature CVC: central venous catheter FDA: US Food and Drug
Administration
GRADE: Grading of Recommendations Assessment Development and Evaluation
LILACS: Latin American and Caribbean Health Sciences Literature MINORS: Methodological Index for
Nonrandomized Studies TC: tunneled catheter
TIC: totally implanted catheter
Address correspondence to Ana Cristina Carvalho da Costa, RN, PhD, Interdisciplinary Research Laboratory Applied to Clinical Practice in Oncology, Faculty of Health Sciences, University of Brasília, Rua 36 Norte, 3350, bloco D, apartamento 102 Águas Claras, Brasília-DF 71919-180, Brazil. E-mail: anacristina_costa@yahoo.com.br
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2019 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose. FUNDING:No external funding.
POTENTIAL CONFLICT OF INTEREST:The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
1. Anttila VJ. Central venous catheter care for children with cancer should focus on early infections.Acta Paediatr. 2019; 108(2):204–205
2. Camp-Sorrell D. State of the science of oncology vascular access
devices.Semin Oncol Nurs. 2010;26(2): 80–87
3. Galloway M. Insertion and placement of central catheters in the oncology
patient.Semin Oncol Nurs. 2010;26(2): 102–112
4. Pektas¸ A, Kara A, Gurgey A. Cohort study: central venous catheter-related complications in children with hematologic diseases at a single center. Turk J Haematol. 2015;32(2):144–151
5. Giordano P, Saracco P, Grassi M, et al; Italian Association of Pediatric Hematology and Oncology (AIEOP).
6. Baskin JL, Reiss U, Wilimas JA, et al. Thrombolytic therapy for central venous catheter occlusion. Haematologica. 2012;97(5):641–650
7. Fratino G, Molinari AC, Mazzola C, et al. Prospective study of indwelling central venous catheter-related complications in children with broviac or clampless valved catheters.J Pediatr Hematol Oncol. 2002;24(8):657–661
8. Blaney M, Shen V, Kerner JA, et al; CAPS Investigators. Alteplase for the treatment of central venous catheter occlusion in children: results of a prospective, open-label, single-arm study (The Cathflo Activase Pediatric Study).J Vasc Interv Radiol. 2006;17(11, pt 1):1745–1751
9. Gilarde JA, Chung AM, Vidal R, Falkos S. Efficacy and economic evaluation of a volume-based cathflo activase protocol versus afixed-dose alteplase protocol for catheter occlusions in pediatric patients.J Pediatr Pharmacol Ther. 2006;11(4):237–244
10. Albisetti M. Thrombolytic therapy in children.Thromb Res. 2006;118(1): 95–105
11. van Miert C, Hill R, Jones L.
Interventions for restoring patency of occluded central venous catheter lumens.Cochrane Database Syst Rev. 2012;(4):CD007119
12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.PLoS Med. 2009;6(7):e1000097
13. da Costa ACC, Narley N, Vasques CI, da Silva Guerra EN, dos Reis PED. Interventions for treatment of thrombotic occlusion in long-term central venous catheters in pediatric cancer population: a systematic review. Available at: www.crd.york.ac.uk/ PROSPERO/display_record.php?ID= CRD42018083555. Accessed August 5, 2018
14. Higgins JPT, Green S, eds.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, United Kingdom: The Cochrane Collaboration; 2011. Available at: https://training.cochrane.org/ handbook/current/chapter-10
15. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument.ANZ J Surg. 2003;73(9): 712–716
16. Schünemann H, Bro_zek J, Guyatt G, Oxman A, eds. GRADE handbook. 2013. Available at: http://
guidelinedevelopment.org/handbook. Accessed May 2, 2018
17. Atkinson JB, Bagnall HA, Gomperts E. Investigational use of tissue plasminogen activator (t-PA) for occluded central venous catheters. JPEN J Parenter Enteral Nutr. 1990; 14(3):310–311
18. Bagnall HA, Gomperts E, Atkinson JB. Continuous infusion of low-dose urokinase in the treatment of central venous catheter thrombosis in infants and children.Pediatrics. 1989;83(6): 963–966
19. Iqbal Y, Al-Katheri A, Al-Sedairy R, et al. Cryopreserved recombinant tissue plasminogen activator for the restoration of occluded central venous access devices in pediatric oncology patients.Ann Saudi Med. 2002;22(5–6): 300–302
20. Molinari AC, Haupt R, Saracco P, et al. Urokinase for restoring patency of malfunctioning or blocked central venous catheters in children with hemato-oncological diseases.Support Care Cancer. 2004;12(12):840–843
21. Winthrop AL, Wesson DE. Urokinase in the treatment of occluded central venous catheters in children.J Pediatr Surg. 1984;19(5):536–538
22. Chesler L, Feusner JH. Use of tissue plasminogen activator (rt-PA) in young children with cancer and dysfunctional central venous catheters.J Pediatr Hematol Oncol. 2002;24(8):653–656
23. Fisher AA, Deffenbaugh C, Poole RL, Garcia M, Kerner JA Jr. The use of alteplase for restoring patency to occluded central venous access devices in infants and children.J Infus Nurs. 2004;27(3):171–174
24. Peng C, Monagle P, Newall F. Clinical outcomes of management of CVAD occlusions.Arch Dis Child. 2011;96(9): 885–887
25. Tobiansky R, Lui K, Dalton DM, et al. Complications of central venous access devices in children with and without cancer.J Paediatr Child Health. 1997; 33(6):509–514
26. Ragsdale CE, Oliver MR, Thompson AJ, Evans MC. Alteplase infusion versus dwell for clearance of partially occluded central venous catheters in critically ill pediatric patients.Pediatr Crit Care Med. 2014;15(6):e253–e260
27. Ponec D, Irwin D, Haire WD, et al; COOL Investigators. Recombinant tissue plasminogen activator (alteplase) for restoration offlow in occluded central venous access devices: a double-blind placebo-controlled trial–the
Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12(8):951–955
28. Deitcher SR, Fesen MR, Kiproff PM, et al; Cardiovascular Thrombolytic to Open Occluded Lines-2 Investigators. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial.J Clin Oncol. 2002; 20(1):317–324
29. Genentech.Package Insert. Cathflo Activase (Alteplase). South San Francisco, CA: Genentech; 2001
30. Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic Therapy in Neonates and Children: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. In:Chest, vol. 141. 2012:p e737S–e801S
31. Anderson DM, Pesaturo KA, Casavant J, Ramsey EZ. Alteplase for the treatment of catheter occlusion in pediatric patients.Ann Pharmacother. 2013;47(3): 405–409
32. Rowan CM, Miller KE, Beardsley AL, et al. Alteplase use for malfunctioning central venous catheters correlates with catheter-associated bloodstream infections.Pediatr Crit Care Med. 2013; 14(3):306–309
patients.J Pediatr Hematol Oncol. 2003; 25(1):38–45
34. Kellerman S, Chan J, Jarvis W. Use of urokinase in pediatric hematology/ oncology patients.Am J Infect Control. 1998;26(5):502–506
35. Ernst FR, Chen E, Lipkin C, Tayama D, Amin AN. Comparison of hospital length of stay, costs, and readmissions of alteplase
versus catheter replacement among patients with occluded central venous
catheters.J Hosp Med. 2014;9(8): 490–496
DOI: 10.1542/peds.2018-3789 originally published online November 22, 2019;
2019;144;
Pediatrics
dos Reis
Vasques, Elaine Barros Ferreira, Eliete Neves Silva Guerra and Paula Elaine Diniz
Ana Cristina Carvalho da Costa, Nayara Narley Pires Vieira, Christiane Inocêncio
Interventions for Occluded Central Venous Catheters: A Meta-analysis
Services
Updated Information &
http://pediatrics.aappublications.org/content/144/6/e20183789 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/144/6/e20183789#BIBL This article cites 31 articles, 4 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/therapeutics_sub Therapeutics
http://www.aappublications.org/cgi/collection/pharmacology_sub Pharmacology
_sub
http://www.aappublications.org/cgi/collection/hematology:oncology Hematology/Oncology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2018-3789 originally published online November 22, 2019;
2019;144;
Pediatrics
dos Reis
Vasques, Elaine Barros Ferreira, Eliete Neves Silva Guerra and Paula Elaine Diniz
Ana Cristina Carvalho da Costa, Nayara Narley Pires Vieira, Christiane Inocêncio
Interventions for Occluded Central Venous Catheters: A Meta-analysis
http://pediatrics.aappublications.org/content/144/6/e20183789
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2019/11/19/peds.2018-3789.DCSupplemental Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.