Prophylactic Ibuprofen for the Prevention of Intraventricular Hemorrhage
Among Preterm Infants: A Multicenter, Randomized Study
Carlo Dani, MD*; Giovanna Bertini, MD*; Marco Pezzati, MD*; Chiara Poggi, MD*; Pietro Guerrini, MD‡;
Claudio Martano, MD§; Firmino F. Rubaltelli, MD*; and the IntraVentricular Ibuprofen Study Group
ABSTRACT. Objective. Ibuprofen enhances cerebral blood flow autoregulation and was shown to protect neurologic functions after oxidative stresses in an animal model. For these reasons, we hypothesized that the pro-phylactic use of ibuprofen would reduce the occurrence of intraventricular hemorrhage (IVH) and its worsening toward grades 2 to 4 among preterm infants. To confirm this hypothesis, we planned the present prospective study.
Methods. This was a double-blind, randomized, con-trolled trial in which preterm infants with gestational ages of<28 weeks received ibuprofen or placebo within the first 6 hours of life. The infants were assigned ran-domly, at 7 neonatal care units, to receive ibuprofen (10 mg/kg, followed by 5 mg/kg after 24 and 48 hours) or placebo. Serial echoencephalography was performed 24 and 48 hours after the initial cerebral ultrasound study, on postnatal days 7, 15, and 30, and at 40 weeks’ postcon-ceptional age. Grade 1 IVH or no IVH was considered a successful outcome, whereas grade 2 to 4 IVH repre-sented failure. The rates of ductal closure, side effects, and complications were recorded.
Results. We studied 155 infants. Grade 2 to 4 IVH developed for 16% of the ibuprofen-treated infants and 13% of the infants in the placebo group. The occurrence of patent ductus arteriosus was less frequent only on day 3 of life in the ibuprofen group. There were no signifi-cant differences with respect to other complications or adverse effects.
Conclusions. Our study demonstrated that prophylac-tic ibuprofen is ineffective in preventing grade 2 to 4 IVH and that its use for this indication cannot be recommended.
Pediatrics 2005;115:1529–1535; ibuprofen, intraventricular hemorrhage, patent ductus arteriosus, infants.
ABBREVIATIONS. IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus; CBF, cerebral blood flow; iRDS, infant respiratory distress syndrome; ROP, retinopathy of prematurity; OR, odds ratio; CI, confidence interval; PVL, periventricular leukomalacia; PPHN, persistent pulmonary hypertension of the newborn.
D
espite improvements in the assistance and
treatment of preterm infants, intraventricular
hemorrhage (IVH) remains a frequent
com-plication among these patients.
1This point is crucial
because the most severe IVH cases are related to a
high risk of neurodevelopmental handicaps. In fact,
mental retardation, seizures, and cerebral palsy have
been reported for 45% to 86% of preterm infants with
parenchymal IVH involvement.
2–5Previous clinical trials demonstrated that
indo-methacin, a cyclooxygenase inhibitor of
prostaglan-din synthesis that is used commonly for the closure
of patent ductus arteriosus (PDA), decreased the
cidence of IVH among very low birth weight
in-fants.
6–10Indomethacin was shown to decrease
base-line cerebral blood flow (CBF), to modulate CBF
changes in response to hypercarbic insults, to
de-crease serum prostaglandin levels, and to promote
germinal matrix maturation in animal models.
11–14However, indomethacin prophylaxis for IVH has
never been used widely, because of the adverse
ef-fects of indomethacin on renal function and the
gas-trointestinal tract.
10,15–19In contrast, experimental
20and preliminary
clini-cal
21–24studies demonstrated that ibuprofen (another
cyclooxygenase inhibitor of prostaglandin synthesis)
was effective in closing PDA without reducing CBF
25or affecting cerebral vasoreactivity to arterial carbon
dioxide tension
26or intestinal
27,28or renal
29hemody-namics. Furthermore, ibuprofen enhances CBF
auto-regulation
30and was shown to protect neurologic
functions after oxidative stresses in an animal
mod-el.
31For these reasons, we hypothesized that the
pro-phylactic use of ibuprofen would reduce the
occur-rence of IVH and its worsening toward grades 2 to 4
among preterm infants. To confirm this hypothesis,
we planned a double-blind, randomized, controlled
trial in which preterm infants with gestational age of
⬍
28 weeks received ibuprofen or placebo within the
first 6 hours of life.
METHODS Patient Population
A multicenter, double-blind, prospective study, approved by local ethics committees, was conducted in 7 tertiary neonatal care units (Careggi University Hospital of Florence, Sant’Anna Univer-sity Hospital of Ferrara, Clinica Mangiagalli of Milan, Children’s Hospital “V. Buzzi” of Milan, San Gerardo Hospital of Monza, Regional Hospital of Bolzano, and Sant’Anna Hospital of Turin, all in Italy). Inclusion criteria were gestational age of⬍28 weeks From the *Department of Surgical and Medical Critical Care, Section of
Neonatology, Careggi University Hospital of Florence, Florence, Italy; ‡Di-vision of Neonatology, Sant’Anna University Hospital of Ferrara, Ferrara, Italy; and §Division of Neonatology, Sant’Anna University Hospital of Turin, Turin, Italy.
Accepted for publication Oct 4, 2004. doi:10.1542/peds.2004-1178 No conflict of interest declared.
Reprint requests to (C.D.) Division of Neonatology, Careggi University Hospital, University of Florence School of Medicine, Viale Morgagni, 85 Firenze, Italy. E-mail: cdani@unifi.it
and postnatal age of⬍6 hours. Exclusion criteria were the pres-ence of major congenital malformations; hydrops fetalis; persistent pulmonary hypertension of the newborn (PPHN); grade 2 to 4 IVH; platelet count of ⬍50 000 platelets per mm3; tendency to
bleed, as revealed by hematuria, blood in the endotracheal aspi-rate, gastric aspiaspi-rate, or stools, or oozing from puncture sites; or serum creatinine concentration of ⬎1.5 mg/dL. Neonates were enrolled after written informed consent was obtained from their parents.
Study Design
The infants in each unit were assigned randomly to a treatment group with the sealed-envelope technique; envelopes were pre-pared at Careggi University Hospital of Florence and then distrib-uted to participating centers. Each infant received 3 doses of ibuprofen lysine (Arfen; Lisapharma, Erba, Italy; 10 mg/kg within 6 hours after birth, followed by 5 mg/kg after 24 and 48 hours) or indistinguishable placebo. The medications were infused contin-uously in a period of 15 minutes. The doses and dosing intervals were the same as those used for newborn infants in previous studies.21–23
When the ductus arteriosus was still patent after the randomly assigned treatment for a patient of either group, ibuprofen was administered as a nonrandomized rescue treatment. If this ther-apy also failed to promote ductal closure or if there was a contra-indication to repeated pharmacologic treatment, then surgical li-gation of the ductus arteriosus was performed.
Echographic Study
All patients for whom study permission was requested under-went cerebral ultrasonography within the first 6 hours of life, to exclude grade 2 to 4 IVH. Serial echoencephalography was per-formed 24 and 48 hours after the first study, on postnatal days 7, 15, and 30, and at 40 weeks’ postconceptional age. The images were obtained with a high-resolution (7.5-MHz), real-time, sector scanner, in both coronal and sagittal projections through the an-terior fontanel.
The grading system for hemorrhage was adapted from that described by Papile et al,32 as follows: grade 1, blood in the
periventricular germinal matrix regions or germinal matrix hem-orrhage; grade 2, blood within the lateral ventricular system with-out ventricular dilation; grade 3, blood acutely distending the lateral ventricles; grade 4, blood within the ventricular system and parenchyma. Hemorrhage was considered to have extended if an intraventricular or parenchymal component developed from a germinal matrix hemorrhage or a second hemorrhage was not in the hemisphere opposite that with an existing hemorrhage. The echoencephalographic studies were also evaluated for the pres-ence of periventricular leukomalacia (PVL). All infants identified as having PVL had cystic areas on the 40-week cerebral ultrasound scans; earlier scans of the same infants showed parenchymal echodensities. PVL was graded from grade 1 to grade 3 according to the classification described by De Vries et al.33
Echocardiographic examinations were performed for all pa-tients before enrollment and on postnatal days 2, 3, 5, 7, and 21 (or more frequently, if indicated). The initial evaluation included 2-dimensional, M-mode, pulsed Doppler and color flow evalua-tions of cardiac flow dynamics, performed to ascertain the nor-mality of cardiac anatomic features and to rule out the possibility of congenital heart disease with “ductus-dependent” pulmonary or systemic blood flow or PPHN. Two-dimensional studies were performed for direct observation of the PDA, and left-to-right shunts were documented with pulsed Doppler echocardiography and color flow mapping. A diagnosis of significant PDA was made with echocardiographic demonstration of a ductal left-to-right shunt, with a left atrium/aortic root ratio of⬎1.3 or a ductal size of⬎1.5 mm.21All echographic studies were performed by
physicians who were unaware of the infants’ treatment assign-ments.
Concomitant Treatment
Daily clinical care was performed by attending physicians, in accordance with common practices. Fluid intake was based essen-tially on changes in body weight, serum electrolyte concentra-tions, and serum osmolality; we started with⬃70 mL/kg, which was increased 10 to 20 mL each day to reach 150 mL/kg at the end
of the first week of life. Infants could receive human milk from donors (and then from their own mothers) from the first day of life. When dextrose infusion was indicated, its concentration was set to maintain appropriate plasma glucose levels. Electrolytes were added after the first day of life, whereas intravenous admin-istration of amino acids and lipids was initiated generally by the second day of life.
When hypotension was refractory to fluid-replacement therapy (with plasma, packed red cells, or more rarely saline), dopamine and/or dobutamine infusion was started. For infant respiratory distress syndrome (iRDS), infants received oxygen therapy, respi-ratory support (nasal continuous positive pressure, synchronized mechanical ventilation, or high-frequency oscillatory ventilation), and early rescue surfactant treatment (Curosurf; Chiesi, Parma, Italy; 200 mg/kg plus 100 mg/kg after 12 hours). The objective of assisted ventilation was to maintain an arterial partial pressure of oxygen of 50 to 70 mm Hg, arterial partial pressure of carbon dioxide of⬍65 mm Hg, pH of⬎7.20, and oxygen saturation of 90% to 95%.
Infants who underwent mechanical ventilation, had signs of sepsis, or were predisposed to infection because of maternal fac-tors were given antibiotics after appropriate studies. Antibiotic therapy was stopped after 3 or 4 days if the bacterial cultures (of blood, tracheal aspirate, and urine) remained negative. Postnatal steroid treatment (12 days of tapering doses of dexamethasone34)
was started for infants with severe respiratory failure who were receiving maximal ventilatory and oxygen support and for infants who, after 2 weeks of life, were still undergoing mechanical ven-tilation and were considered at high risk of developing chronic lung disease.
Clinical Courses and Outcomes
For each newborn infant, gestational age, birth weight, gender, type of delivery, Apgar scores at 1 and 5 minutes, pH of umbilical artery blood, prenatal and postnatal steroid treatment, main ma-ternal pregnancy pathologic conditions, length of stay in the hos-pital, and death were recorded. To evaluate the severity of iRDS, initial (at the first blood gas analysis) and highest values of the oxygenation index (mean airway pressure⫻fraction of inspired oxygen⫻100/arterial partial pressure of oxygen) and the venti-latory index (oxygenation index ⫻ arterial partial pressure of carbon dioxide) were measured for patients undergoing mechan-ical ventilation.
Serum creatinine levels and platelet counts were measured at 1, 3, and 5 days of life. Urine output was measured every day during the first 5 days of life, by collecting urine in adhesive bags, and oliguria was defined as a urinary output of⬍1 mL/kg per hour during a 24-hour collection period; fluid intake was recorded every day during the first week of life. To evaluate bleeding tendencies, hematuria, gastric bleeding, blood in the endotracheal aspirate or stools, and oozing from puncture sites were assessed. Our patients were also monitored for complications such as sepsis, bronchopulmonary dysplasia, necrotizing enterocolitis, and retinopathy of prematurity (ROP). Diagnoses of sepsis were based on clinical and laboratory data (total neutrophil counts, immature/total neutrophil ratios, and C-reactive protein concen-trations) and confirmed with positive blood cultures.35Diagnosis
of bronchopulmonary dysplasia was based on the requirement for supplemental oxygen to maintain adequate oxygenation at 36 weeks’ postconceptional age.36Necrotizing enterocolitis was
diagnosed in the presence of abdominal distension, gastric resid-uals with or without bile-stained vomiting and bloody diarrhea or stools, hypotension, and suggestive abdominal radiographs (showing dilated and thickened bowel loops, parietal pneumatosis with or without perforation, or portal or hepatic venous air).37The
incidence of ROP, staged according to the international classifica-tion,38 was also recorded. All clinical and biological data were
reported on data sheets designed for this study.
Statistical Analyses
In our study, we considered as successful outcomes grade 1 IVH or no IVH at 7 days of life, whereas grade 2 to 4 IVH represented failure. We assumed a failure rate 20% higher in the placebo group than in the ibuprofen group. Therefore, at a power of 0.80 and␣⫽.05, the estimated sample size was 74 infants in each group.
mean values and SDs or rates and percentages. Statistical analyses were performed with Student’sttest for parametric continuous variables, the 2-sample Wilcoxon rank-sum test for nonparametric continuous variables, and Fisher’s exact test for categorical vari-ables such as frequencies. P ⬍ .05 was considered statistically significant.
Multiple stepwise logistic regressions were conducted to deter-mine which maternal, neonatal, or perinatal factors would influ-ence the risk for development of grade 2 to 4 IVH. Effect estimates are expressed as odds ratios (ORs) with profile, likelihood-based, 95% confidence intervals (CIs).
RESULTS
Two hundred fifty patients were eligible for the
study, but 80 were excluded because of the presence
of cardiac malformations (n
⫽
4), hydrops fetalis (n
⫽
3), PPHN (n
⫽
15), grade
ⱖ
2 IVH (n
⫽
20), platelet
counts of
⬍
50 000 platelets per mm
3or bleeding
tendency (n
⫽
25), or serum creatinine
concentra-tions of
⬎
1.5 mg/dL (n
⫽
13). Among the remaining
170 infants, 10 were excluded because of a lack of
parental consent and 5 were excluded after
random-ization because of incomplete data collection (4 in the
ibuprofen group and 1 in the placebo group).
There-fore, we studied 155 infants, 77 of whom were
as-signed to the ibuprofen group and 78 to the placebo
group. The initial dose of the drug was administered
at 5.2
⫾
0.6 hours and 5.4
⫾
0.5 hours in the
ibupro-fen and placebo groups, respectively. The groups
had comparable proportions of maternal and
obstet-ric factors (Table 1); they were also comparable in
clinical characteristics except for gestational age
(Ta-ble 2).
The proportions of infants with grade 1 IVH at
enrollment were similar in the ibuprofen (n
⫽
4, 5%)
and placebo (n
⫽
5, 6%) groups. Two of these infants
in each group experienced progression of
hemor-rhage; the 2 infants in the ibuprofen group
devel-oped grade 2 IVH, whereas 1 infant in the placebo
group developed grade 2 IVH and another
devel-oped grade 3 IVH. Grade 2 to 4 IVH develdevel-oped for
21% of the ibuprofen-treated infants and for 17% in
the placebo group (Table 3). The incidences of grade
1, grade 2, grade 3, and grade 4 IVH were similar in
the ibuprofen and placebo groups. We considered
the possibility that the occurrence of IVH could differ
between the groups at different times. For this
rea-son, we compared the IVH rates at baseline and at 24
hours, 48 hours, and 7 days of life, but we did not
find any difference between the ibuprofen group and
the placebo group (Table 4).
The infant survival rates were similar in the 2
groups (Table 5). The most frequent causes of death
were refractory respiratory failure and sepsis. There
was no significant difference between the groups in
the occurrence of iRDS or its severity, the
require-ment for or type of mechanical ventilation, or the
need for surfactant treatment (Table 2). The rates of
sepsis, bronchopulmonary dysplasia, necrotizing
en-terocolitis, ROP (all grades), and PVL (all grades)
and the lengths of stay in the hospital were similar in
the ibuprofen and placebo groups (Table 5). The
occurrence of PDA was less frequent on day 3 of life
in the ibuprofen group (Table 5). The incidences of
serum creatinine levels of
⬎
1.5 mg/dL, mean serum
creatinine levels, urine outputs, rates of oliguria,
bleeding tendency, and platelet counts of
⬍
50 000/
mm
3, and mean platelet counts did not differ
be-tween the ibuprofen and placebo groups (Table 5).
Logistic-regression analysis included all variables
in Tables 1 and 2 and also the occurrence of grade 1
IVH at enrollment, bleeding tendency,
thrombocyto-penia, and PDA in the day 3 of life. We found that
the following factors had significant predictive
val-ues for the development of grade 2 to 4 IVH: male
gender (OR: 2.3; 95% CI: 1.76 –2.84) and prenatal
steroid treatment (OR: 0.30; 95% CI:
⫺
1.66 to
⫺
0.71).
DISCUSSION
The anomalies of cerebral perfusion play an
im-portant role in the development of cerebral injury
among preterm infants. Prostaglandins, especially
prostaglandin E
2and F
2␣, are determinants in the
control of the upper range of CBF autoregulation;
they exert a minimal vasoconstrictor activity, which
could prevent an increase in CBF when systemic
blood pressure increases.
30Ibuprofen was reported
to enhance CBF autoregulation among newborn
pig-lets,
30and Li et al
39demonstrated that its effect was
secondary to the up-regulation of prostaglandin E
2and F
2␣receptors induced by inhibition of the
isoen-zyme cyclooxygenase-2. Therefore, with stabilization
of cerebral perfusion, a reduction in the incidence of
IVH among preterm infants might be expected.
Varvarigou et al
21reported a trend toward a
de-crease in the incidence of IVH among preterm
in-fants, although this was not statistically significant
and was not confirmed in other studies.
39Unfortunately, our study demonstrated that
ibu-profen was ineffective in preventing grade 2 to 4
IVH, confirming the results of a recent meta-analysis
study of the prevention of PDA with ibuprofen
pro-phylaxis.
40This result suggests that the action of
ibuprofen in improving CBF autoregulation among
preterm infants is insufficient to limit brain injuries.
We wondered why ibuprofen is ineffective in
reduc-TABLE 1. Comparison Between Ibuprofen and Placebo Groups in Obstetric and Maternal Factors
Ibuprofen (n⫽77)
Placebo (n⫽78)
Cesarean delivery, rate (%) 42/77 (55) 56/78 (72)
Prenatal steroids, rate (%) 56/77 (73) 58/78 (74)
No. of cycles, mean⫾SD 1.1⫾0.3 1.3⫾0.8
Preterm noninduced labor, rate (%) 46/77 (60) 58/78 (74)
Preeclampsia, rate (%) 24/77 (31) 16/78 (21)
Preterm premature rupture of membranes, rate (%) 6/77 (8) 6/78 (8)
Gestational diabetes mellitus, rate (%) 3/77 (4) 5/78 (6)
TABLE 2. Comparison Between Ibuprofen and Placebo Groups in Neonatal Factors
Ibuprofen (n⫽77)
Placebo (n⫽78)
Birth weight, g, mean⫾SD 832⫾215 812⫾209
Gestational age, wk, mean⫾SD 25.3⫾1.2 25.9⫾1.1*
Male/female,n 50/27 32/46
Apgar score, mean⫾SD
1 min 4.2⫾2.3 4.8⫾2.2
5 min 6.6⫾2.2 7.0⫾1.6
pH of umbilical artery blood, mean⫾SD 7.28⫾0.11 7.29⫾0.11
Respiratory diseases
Rate (%) 75/77 (97) 75/78 (96)
iRDS, rate (%) 70/75 (93) 71/75 (95)
Transient tachypnea of the newborn, rate (%) 5/75 (7) 4/75 (5)
Oxygen therapy
Rate (%) 74/77 (96) 75/78 (96)
Duration, d, mean⫾SD 40.9⫾33.1 42.6⫾35.2
Mechanical ventilation
SIMV/SIPPV, rate (%) 57/77 (74) 53/78 (68)
HFOV, rate (%) 26/77 (34) 17/78 (22)
Duration, h, mean⫾SD 397.6⫾426.7 339.0⫾347.1
Surfactant
Rate (%) 57/77 (74) 50/78 (64)
Age at treatment, min, mean⫾SD 63.3⫾47.7 79.5⫾75.8
No. of doses, mean⫾SD 1.8⫾0.9 2.1⫾1.3
Oxygenation index
Initial value, mean⫾SD 6.8⫾7.8 7.3⫾8.5
Highest value, mean⫾SD 10.9⫾12.3 12.3⫾14.5
Age at highest value, h, mean⫾SD 28.1⫾97.6 31.0⫾69.2
Ventilatory index
Initial value, mean⫾SD 257.0⫾246.1 232.9⫾167.0
Highest value, mean⫾SD 389.6⫾326.5 365.2⫾300.3
Age at highest value, h, mean⫾SD 29.6⫾99.1 35.1⫾68.6
Total or partial parenteral nutrition, rate (%) 67/77 (87) 70/78 (90)
Fluid intake, mL/kg, mean⫾SD
DOL 1 65.9⫾12.2 65.7⫾17.9
DOL 2 78.8⫾13.9 83.1⫾17.6
DOL 3 98.5⫾14.9 107⫾34.9
DOL 4 121.8⫾19.5 122.5⫾36.7
DOL 5 138.9⫾24.1 134.7⫾27.9
DOL 6 147.9⫾23.3 146.6⫾21.5
DOL 7 156.5⫾21.9 154.5⫾24.9
Vasoactive drugs
Rate (%) 38/77 (49) 37/77 (48)
Dopamine, rate (%) 37/38 (97) 37/37 (100)
Dopamine duration, d, mean⫾SD 10.3⫾9.9 8.0⫾7.9
Dobutamine, rate (%) 18/38 (47) 27/37 (73)
Dobutamine duration, d, mean⫾SD 6.4⫾1.8 7.0⫾6.5
PRC transfusions
PRCs transfused during week 1, rate (%) 49/77 (64) 48/78 (62)
No. of PRC transfusions, mean⫾SD 1.7⫾0.7 1.8⫾0.9
Plasma transfusions
Plasma transfused during week 1, rate (%) 25/77 (32) 23/78 (29)
No. of plasma transfusions, mean⫾SD 1.7⫾1.1 1.9⫾1.5
Postnatal steroid treatment
Rate (%) 28/77 (36) 27/78 (35)
Duration, d, mean⫾SD 12.8⫾14.7 10.3⫾9.0
PRC indicates packed red cell; DOL, day of life; SIMV, synchronized intermittent mandatory ventilation; SIPPV, synchronized intermit-tent positive pressure ventilation; HFOV, high-frequency oscillatory ventilation.
*P⫽.001, placebo group versus ibuprofen group.
TABLE 3. Comparison of Incidence of IVH at 7 Days of Life
Rate (%) P OR (95% CI)
Ibuprofen (n⫽77)
Placebo (n⫽78)
Successful outcome (no IVH or grade 1)
61 (79) 65 (83) .652 0.865 (0.387–1.932)
Grade 1 4 (5) 6 (8) .760 1.476 (0.761–5.390)
Grade 2 8 (10) 5 (6) .562 0.661 (0.206–2.117)
Grade 3 6 (8) 5 (6) .982 0.856 (0.253–2.898)
Grade 4 2 (3) 3 (4) .972 1.500 (0.227–9.225)
Grade 2–4 16 (21) 13 (17) .652 0.866 (0.246–3.050)
ing the occurrence of IVH whereas indomethacin,
which is also a cyclooxygenase inhibitor, is
effec-tive.
6–10We found that several authors observed
de-creased CBF after indomethacin administration, both
in newborn animals
12,41and among newborn
in-fants,
25,42but not after ibuprofen treatment.
12,25,42,43Therefore, we concluded that indomethacin can
af-fect cerebral circulation through mechanisms
differ-ent from cyclooxygenase blockade and
prostaglan-din synthesis,
43such as direct action on vascular
endothelium
44and the increase in the circulating
level of endothelins,
45which likely can explain also
the more frequent occurrence of adverse effects after
indomethacin treatment, rather than after ibuprofen
treatment. In other words, it is probable that the
ineffectiveness of ibuprofen in preventing grade 2 to
4 IVH and the lower occurrence of adverse effects
after its administration are secondary to
more-selec-tive inhibition of cyclooxygenase isoforms,
com-pared with indomethacin, which permits the closure
of PDA but is not sufficient to compensate for
inad-equate CBF autoregulation among preterm infants.
Another possible explanation is that we used an
inadequate ibuprofen dose. However, it is difficult to
suggest increasing this dose, because in a previous
study
21it was found to be associated with a plasma
ibuprofen level 2.5-fold higher than that generated
among adults with arthritis receiving similar doses
46and there are no studies of ibuprofen
pharmacoki-netics among newborn infants.
In the present study, we monitored the possible
adverse effects of ibuprofen treatment, in particular
on renal function and the clotting system, but we did
not find any difference between the ibuprofen and
placebo groups. This confirms previous studies,
40except that Van Overmeire et al
24found serum
cre-TABLE 4. Comparison of Rates of IVH Before Ibuprofen Ad-ministration, at 24 and 48 Hours, and at 7 Days of Life
IVH Rate (%)
Before Ibuprofen
24 h 48 h 7 d
Grade 1
Ibuprofen 4 (5) 4 (5) 4 (5) 4 (5)
Placebo 5 (6) 6 (8) 6 (8) 6 (8)
Grade 2
Ibuprofen 0 (0) 6 (8) 7 (9) 8 (10)
Placebo 0 (0) 4 (5) 5 (6) 5 (6)
Grade 3
Ibuprofen 0 (0) 4 (5) 6 (8) 6 (8)
Placebo 0 (0) 4 (5) 5 (6) 5 (6)
Grade 4
Ibuprofen 0 (0) 1 (1) 2 (3) 2 (3)
Placebo 0 (0) 2 (3) 2 (3) 3 (4)
Grade 2–4
Ibuprofen 0 (0) 11 (14) 15 (19) 16 (21)
Placebo 0 (0) 10 (13) 12 (15) 13 (17)
Total IVH
Ibuprofen 4 (5) 15 (19) 19 (25) 20 (26)
Placebo 5 (6) 16 (21) 18 (23) 19 (24)
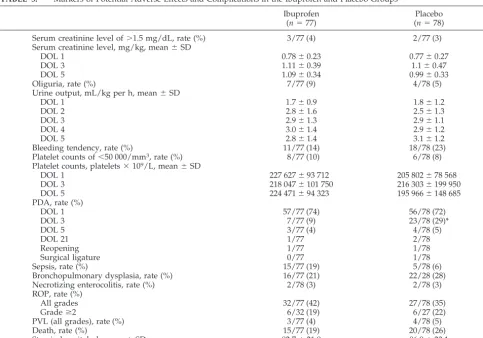
TABLE 5. Markers of Potential Adverse Effects and Complications in the Ibuprofen and Placebo Groups
Ibuprofen (n⫽77)
Placebo (n⫽78)
Serum creatinine level of⬎1.5 mg/dL, rate (%) 3/77 (4) 2/77 (3)
Serum creatinine level, mg/kg, mean⫾SD
DOL 1 0.78⫾0.23 0.77⫾0.27
DOL 3 1.11⫾0.39 1.1⫾0.47
DOL 5 1.09⫾0.34 0.99⫾0.33
Oliguria, rate (%) 7/77 (9) 4/78 (5)
Urine output, mL/kg per h, mean⫾SD
DOL 1 1.7⫾0.9 1.8⫾1.2
DOL 2 2.8⫾1.6 2.5⫾1.3
DOL 3 2.9⫾1.3 2.9⫾1.1
DOL 4 3.0⫾1.4 2.9⫾1.2
DOL 5 2.8⫾1.4 3.1⫾1.2
Bleeding tendency, rate (%) 11/77 (14) 18/78 (23)
Platelet counts of⬍50 000/mm3, rate (%) 8/77 (10) 6/78 (8)
Platelet counts, platelets⫻109/L, mean⫾SD
DOL 1 227 627⫾93 712 205 802⫾78 568
DOL 3 218 047⫾101 750 216 303⫾199 950
DOL 5 224 471⫾94 323 195 966⫾148 685
PDA, rate (%)
DOL 1 57/77 (74) 56/78 (72)
DOL 3 7/77 (9) 23/78 (29)*
DOL 5 3/77 (4) 4/78 (5)
DOL 21 1/77 2/78
Reopening 1/77 1/78
Surgical ligature 0/77 1/78
Sepsis, rate (%) 15/77 (19) 5/78 (6)
Bronchopulmonary dysplasia, rate (%) 16/77 (21) 22/28 (28)
Necrotizing enterocolitis, rate (%) 2/78 (3) 2/78 (3)
ROP, rate (%)
All grades 32/77 (42) 27/78 (35)
Gradeⱖ2 6/32 (19) 6/27 (22)
PVL (all grades), rate (%) 3/77 (4) 4/78 (5)
Death, rate (%) 15/77 (19) 20/78 (26)
Stay in hospital, d, mean⫾SD 82.7⫾21.9 86.9⫾23.1
DOL indicates day of life.
atinine levels to be increased on day 3 of life among
ibuprofen-treated infants. The recorded
complica-tions of prematurity also showed similar rates in the
2 groups, except for the incidences of PDA on day 3
of life (9% in the treated group and 29% in the
placebo group), which confirmed that prophylactic
treatment with ibuprofen reduced PDA occurrence
among preterm infants with iRDS at 3 days of life.
23However, among patients in the placebo group who
demonstrated PDA at 3 days of life, 83% (19 of 23
patients) experienced closure of the ductus arteriosus
after the first cycle of ibuprofen, as reported
previ-ously.
19,23,24A final issue in our study was the possible
occur-rence of PPHN among our patients after ibuprofen
administration, as reported by Gournay et al.
47None
of our patients demonstrated PPHN
47after
ibupro-fen treatment; we think this was because all infants
with PPHN were excluded from the study. In fact, it
is our opinion that the reported PPHN was
preexist-ing, not caused by ibuprofen, and that PDA closure
only worsened and manifested it.
CONCLUSIONS
Our study demonstrated that prophylactic
ibupro-fen was ineffective in preventing grade 2 to 4 IVH
and its use for this purpose cannot be recommended.
We confirmed that ibuprofen therapy for PDA
clo-sure is safe and effective. Because ibuprofen cannot
represent an alternative to indomethacin and
indo-methacin treatment might be followed by several
adverse effects, the question of pharmacologic IVH
prevention among preterm infants remains crucial
and additional efforts are necessary to identify other
potentially effective drugs.
ACKNOWLEDGMENTS
The IntraVentricular Ibuprofen Study Group includes G.L. Lista, MD (Milan, Italy); Hubert Messner, MD (Bolzano, Italy); Fabio Mosca, MD (Milan, Italy); and Paolo Tagliabue, MD (Monza, Italy).
REFERENCES
1. Rubaltelli FF, Dani C, Reali MF, et al. Acute neonatal respiratory dis-tress in Italy: a one year prospective study. Acta Paediatr. 1998;87: 1261–1268
2. Krishnamooorthy KS, Shannon DC, DeLong GR, Todres ID, Davis KR. Neurologic sequelae in the survivors of neonatal intraventricular hem-orrhage.Pediatrics.1979;64:233–237
3. Shinnar S, Molteni RA, Gammon K, D’Souza BJ, Altman J, Freeman JM. Intraventricular hemorrhage in the premature infant.N Engl J Med.
1982;306:1464 –1468
4. McMenamin JB, Shackelford GD, Volpe JJ. Outcome of neonatal intra-ventricular hemorrhage with periintra-ventricular echodense lesions.Ann Neurol.1984;15:285–290
5. Szymonowicz W, Yu VYH, Bajuk B, Astbury J. Neurodevelopmental outcome of periventricular hemorrhage and leukomalacia in infants 1250 g or less at birth.Early Hum Dev.1986;14:1–7
6. Banstra ES, Montalvo BM, Goldberg RN. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pedi-atrics.1984;74:1107–1112
7. Bada HS, Green RS, Pourcyrous M, et al. Indomethacin reduced the risks of severe intraventricular hemorrhage.J Pediatr.1989;115:631– 637 8. Ment LR, Duncan CC, Ehrenkrnaz RA. Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates.J Pediatr.1985;107:937–943
9. Ment LR, Oh W, Ehrenkrnaz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial.Pediatrics.1994;93:543–550
10. Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for pre-venting mortality and morbidity in preterm infants.Cochrane Database Syst Rev.2002;(3):CD000174
11. Leffler CW, Busija DW, Fletcher AM, Beasley DG, Hessler JR, Green RS. Effects of indomethacin upon cerebral hemodynamics of newborn pigs.
Pediatr Res.1985;19:1160 –1164
12. Chemtob S, Beharry K, Barna T, Varma DR, Aranda JV. Difference in the effects in the newborn piglet of various non-steroidal anti-inflammatory drugs on cerebral blood flow but not on cerebrovascular prostaglan-dins.Pediatr Res.1991;30:106 –111
13. Dahlgren N, Nilsson B, Sakabe T. The effect of indomethacin on cerebral blood flow and oxygen consumption in the rat at the normal and increased carbon dioxide tension.Acta Physiol Scand.1982;3:475– 485 14. Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA. Indomethacin
promotes germinal matrix microvessel maturation in the newborn bea-gle pup.Stroke.1992;23:1132–1137
15. Betkerur MV, Yeh TF, Miller K, Glasser RJ, Pildes RS. Indomethacin and its effects on renal function and urinary kallikrein excretion in prema-ture infants with patent ductus arteriosus.Pediatrics.1981;68:99 –102 16. Van Bel F, Guit GL, Schipper J, Van de Bor M, Baan J.
Indomethacin-induced changes in renal blood flow velocity waveform in premature infants investigated with color Doppler imaging.J Pediatr.1991;118: 621– 626
17. Grosfeld JL, Chaedt M, Molinari F. Increased risk of necrotizing entero-colitis in premature infants with patent ductus arteriosus treated with indomethacin.Ann Surg.1996;224:350 –357
18. Ojala R, Ikonen S, Tammela O. Perinatal indomethacin treatment and neonatal complications in preterm infants. Eur J Pediatr. 2000;159: 153–155
19. Lago P, Bettiol T, Salvadori S, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomized control trial.Eur J Pediatr.2002;161:202–207 20. Coceani F, White E, Bodach E, Olley PM. Age-dependent changes in the
response of the lamb ductus arteriosus to oxygen and ibuprofen.Can J Physiol Pharmacol.1979;57:825– 831
21. Varvarigou A, Bardin LC, Beharry K, Chemtob S, Papageorgiu A, Aranda JV. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants.JAMA.1996;275:539 –544 22. Van Overmeire B, Follens I, Hartmann S, Creten WL, van Acker KJ.
Treatment of patent ductus arteriosus with ibuprofen.Arch Dis Child.
1997;76:F179 –F184
23. Dani C, Bertini G, Reali MF, et al. Prophylaxis of patent ductus arteri-osus with ibuprofen in preterm infants.Acta Paediatr.2000;89:1369 –1374 24. Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibupro-fen and indomethacin for closure of patent ductus arteriosus.N Engl J Med.2000;343:674 – 681
25. Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effect of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arte-riosus.J Pediatr.1997;131:549 –554
26. Mosca F, Bray M, Colnaghi MR, Fumagalli M, Compagnoni G. Cerebral vasoreactivity to arterial carbon dioxide tension in premature infants: effect of ibuprofen.J Pediatr.1999;135:644 – 646
27. Grosfeld JL, Kamman K, Gross K, Cikrit D, Ross D, Wolfe M. Compar-ative effects of indomethacin, prostaglandin E1and ibuprofen on bowel
ischemia.Pediatr Surg.1983;18:738 –742
28. Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus.J Pediatr.1999;135:733–738 29. Malcom DD, Segar JL, Robillard E, Chemtob S. Indomethacin compro-mises hemodynamics during positive-pressure ventilation indepen-dently of prostanoids.J Appl Physiol.1993;74:1672–1678
30. Chemtob S, Beharry K, Barna T, Varma DR, Aranda JV. Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets.Stroke.1990;21:777–784
31. Chemtob S, Roy M-S, Abran D, Ferandez H, Varma DR. Prevention of post-asphyxial increase in lipid peroxides and retinal function deterio-ration in the newborn pig by inhibition of cyclooxygenase activity and free radical generation.Pediatr Res.1993;33:336 –340
32. Papile LA, Burstein J, Burstein R, Keffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweight less than 1500 grams.J Pediatr.1978;92:529 –534
33. De Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasounds.Behav Brain Res.1992;49:1– 6
35. American Academy of Pediatric, Committee on Drugs, Committee on Fetus and Newborn, and Committee on Infectious Diseases. Prophy-laxis and treatment of neonatal gonococcal infections.Pediatrics.1980; 65:1047–1048
36. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period.Pediatrics.1988;82:527–532 37. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L,
Brotherton T. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging.Ann Surg.1978;187:1–12
38. Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity.Arch Ophthal-mol.1984;102:1130 –1134
39. Li DY, Hardy P, Abran D, et al. Key role for cyclooxygenase-2 in PGE2
and PGF2␣receptor regulation and cerebral blood flow of the newborn.
Am J Physiol.1997;273:R1283–R1290
40. Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants.Cochrane Database Syst Rev.2003;(2):CD004213
41. Pappius HM, Wolfe LS. Effects of indomethacin and ibuprofen on cerebral metabolism and blood flow in traumatized brain.J Cereb Blood Flow Metab.1983;3:448 – 459
42. Patel J, Robert I, Azzopardi D, Hamilton P, Edwards AD. Randomized double-blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus.Pediatr Res.2000;47:36 – 42
43. Pellicer A, Aparicio M, Cabanas F, Valverde E, Quero J, Stiris TA. Effects of the cyclo-oxygenase blocker ibuprofen on cerebral blood flow during normocarbia and hypercarbia in newborn piglets.Acta Paediatr.
1999;88:82– 88
44. Siesjo BK. Cerebral circulation and metabolism.J Neurosurg.1984;60: 883–908
45. Therkelsen K, Jensen KA, Freundlich M, Thorshange H, Bunemann L, Bogeskov-Nielsen L. Endothelin-1 and cerebral blood flow: influ-ence of hypoxia, hypercapnia, and indomethacin on circulating en-dothelin levels in healthy volunteers.Scand J Clin Lab Invest.1994; 54:441– 451
46. Gallo JM, GalI IP, Gillespie WR, Albert KS, Perrier D. Ibuprofen kinetics in plasma and synovial fluid of arthritic patients. J Clin Pharmacol.
1986;26:65–70
47. Gournay V, Savagner C, Thiriez G, Kuster A, Roze´ JC. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lan-cet.2002;259:1486 –1488
WHILE BRAIN IMAGING OFFERS NEW KNOWLEDGE,
IT CAN BE AN ILLUSION
“There, right there, see that spot of blazing red in a cool blue sea of your cortex?
That’s your brain on drugs. Or, more specifically, that’s the brain of a recovering
cocaine addict, clean for months, who sees a mound of white powder. His brain
responds with the same craving and anxiety—marked by the red on the brain
scan—as when he was snorting every night, which is why addiction is so tenacious.
And here, see how a chess grandmaster activates the region of the brain that stores
memories, such as those of games he has played or studied? But in the brain
of a neophyte the activity is over here, in a region that analyzes positions from
scratch. . . . Neuroimaging such as PET and MRI are seducing laypeople and
scientists alike into believing we know more than we do about how and why we
think, feel and behave, some scientists say. The power of brain imaging, says
Frank Keil, a Yale University psychology professor, reflects ‘the illusion of
ex-planatory depth. If people see something, they are often deluded into thinking they
understand it better than they really do.’ . . . In a fit of physics envy, researchers
in economics, political science and even philosophy are deciding that
neuroimag-ing is just the thneuroimag-ing to make them more scientific. Yet the results are less than
groundbreaking. . . . For all its flaws, neuroimaging is here to stay. No
self-respect-ing psych department can afford to forgo it.”
Begley S.Wall Street Journal.2005