Intra-arterial infusion of chemotherapy agents for cancer of the head and neck has been used for several decades. This article provides the historical background, rationale, and current state of our research on the use of this treatment regimen for patients with advanced nasal and paranasal sinus carcinoma.1
Historical background
Malignancies of the nasal cavity and paranasal sinuses are rare neoplasms that account for only 3% of head and neck carcinomas and about 0.5% of all malignant diseases.2 However, this condition is more frequently observed in Japan, with 7 % of all cancers arising in the upper aerodigestive tract,3 although the incidence has been on the decline of late.
based on the pioneer work of sullivan and colleagues4 (Figure 1), intra-arterial chemotherapy investigations were begun in Japan as early as the 1960s, with particular emphasis on its application to maxillary sinus cancer. this site is particularly suitable for intra-arterial chemotherapy, as it tends to be encompassed mostly within the territory
abstract
Intra-arterial infusion of chemotherapy agents for cancer of the head and neck has been used for several decades. Subsequent advances in vascular radiology techniques have enabled superselective arterial infusion to head and neck structures, and a specific concomitant chemotherapy protocol for head and neck cancer that employs the pharmacologic principles of IA cisplatin while capitalizing on the cisplatin-neutralizing agent sodium thiosulfate has shown promising results. This article provides the historical background, rationale, and current state of our research on the use of this treatment regimen for patients with advanced nasal and paranasal sinus carcinoma.
Superselective Arterial Cisplatin Infusion with
Concomitant Radiation Therapy for Advanced
Nasal Cavity and Paranasal Sinus Carcinoma
Akihiro Homma M.D. PhD
Department of Otolaryngology - Head & Neck Surgery, Hokkaido University Graduate School of Medicine, Japan.
of the internal maxillary artery.5
sato and colleagues6 at tokyo university developed a multidisciplinary approach known as “trimodality therapy”. their strategy was based on the continuous arterial infusion of fluorouracil (5-Fu), concomitant radiation, and cleaning of the antrum with a curette and forceps, followed by organ preservation surgery. the 5-year survival and preservation rate of the maxilla using this approach were both higher than had been reported up until then in Japan. Many investigators were encouraged by this success and applied this treatment method. Modifications of trimodality therapy developed by sato have been used in organ preservation therapy until recently.7-9
Mitani and colleagues10 at the cancer institute in tokyo reported the results for maxillary sinus cancer from their institute as follows. From 1970 to 1980, 78 maxillary sinus cancer patients were treated with radiotherapy together with intra-arterial chemotherapy with 5-Fu and reduction surgery. during that period, local control and overall survival rates at 5 years were 75.6% and 43.1%, respectively. From 1981 to 1990, 33 cases were
treated with preoperative radiotherapy, intra-arterial chemotherapy with 5-Fu and en bloc tumor resection. local control and overall survival rates were 93.9% and 69.7%, respectively, for that period. From 1991 to 1998, the extent of the tumor was precisely evaluated by ct and Mri, so radical surgery was the mainstay of the treatment. during that period, 40 cases were treated by preoperative radiotherapy and en bloc resection without intra-arterial chemotherapy. as a result, local control and overall survival rates were 82.5% and 61%, respectively. this was lower than the previous period, although there were no statistically significant differences. this rate shows that intra-arterial chemotherapy has some effect on local control. in fact, local recurrence was seen supero-posteriorly in all cases during the last period. tumors invading supero-posteriorly beyond the maxillary sinus are difficult to remove completely. thus, this shows that intra-arterial chemotherapy may have a role in controlling tumor invasion supero-posteriorly to the maxillary sinus.
in the 1980s, advances in vascular radiology techniques enabled superselective arterial infusion to head and neck structures,11 with tohnai,12 Fuwa,13 and other investigators all reporting on superselective infusion
therapy. under fluoroscopic guidance, an angiographic c a t h e t e r w a s i n s e r t e d retrogradely into the main feeding artery of the tumor via the superficial temporal artery. superselective intra-arterial infusion via the superficial temporal artery seems to be technically simple, and rarely leads to cerebrovascular accidents. However, catheter-related complications, such as obstruction of the vessel, infection and displacement of the catheter, sometimes occur. in addition, this method cannot be applied to tumors that are fed by multiple arteries. therefore, this technique is only technically simple and useful if the tumor is small and fed by one artery, such as a small tongue cancer or t3 maxillary sinus cancer.
i m a i ,1 4 a n d ko r o g i1 5
introduced superselective intra-arterial infusion using seldinger’s techniques for head and neck cancer. a microcatheter was placed in the target arteries, selected according to the location of the tumor, via the femoral artery. Hirai and colleagues16 described the theoretical advantages of their methods as follow: (1) catheterization is safe and due to improvements in catheters and angiography, (2) there is exact identification of feeding vessels, (3) even when the tumor has multiple feeding arteries, all feeders can be infused easily in one session and (4) patients were completely free from the need for treatments between sessions.
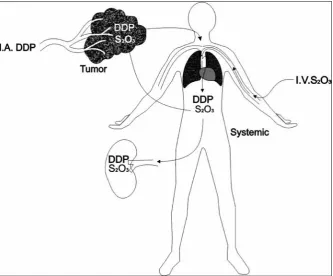
robbins and colleagues17 reported the results of a specific concomitant chemotherapy protocol for head and neck cancer that employed the pharmacologic principles of ia cisplatin while capitalizing on the cisplatin-neutralizing agent sodium thiosulfate (Figure 2). in a phase i study, it was determined that cisplatin could be safely administered to patients with advanced and recurrent head and neck cancer at a dose intensity of 150 mg/m2 per week. this dose intensity is at least five times greater than conventional cisplatin chemotherapy regimens. in the subsequent phase, a second therapeutic modality, concomitant radiotherapy, was added to the supradose
cisplatin infusion strategy. the regimen, referred to as radPlat, consists of ia cisplatin (150mg/m2) and concurrent iV bolus administration of sodium thiosulfate (9 g) followed by 12 g over 12 hours, four times per week; and concomitant irradiation 180-200 cgy/fraction 35 times over 7 weeks.18 between 1993 and 1997, 213 patients with stage iii and iV disease were treated with radPlat at the university of tennessee, Memphis.19 With a median follow-up of 30 months (range 16-69 months), the kaplan-Meier estimates for disease-specific and overall survival at 5 years were 53.6% (sd 3.9%), and 38.8% (sd 3.7%), respectively. the kaplan-Meier plot for the rate of disease control above the clavicle for all patients was 74.3%. ninety-four patients (44%) had t4 disease; 102 (47%) had t3 disease; 15 (7%) had t2 disease, and 2 (1%) had t1 disease (both of these patients had n3 nodal disease); 28.6% of patients had stage iii disease while the remaining 71.4% had stage iV disease. of the 213 patients entered into the treatment program, complete response in the primary site was obtained in 171 (80%).
Yokoyama and colleagues20 first reported radPlat in 1998 in Japan. they reported that large tumors could be eradicated using this therapy and that high-dose
Figure 2. diagrammatic illustration of the drug delivery technique used in the radPlat
program.1 i.a. ddP: intra-arterial cisplatin, i.V. s
weekly cisplatin infusion did not cause any serious side effects, which surprised Japanese head and neck surgeons and radiation oncologists. since then, intra-arterial chemotherapy has gained recognition and popularity again in Japan as the long history of its use has made it easy to accept.
rationale For intra-arterial
cisPlatin cHeMotHeraPY and
PHarMacodYnaMics
in cancer chemotherapeutics, there is evidence that drug resistance can be overcome by increasing drug dosage. However, a practical limitation to this strategy is toxicity to normal cells. With adequate hydration to protect the kidneys, conventional doses of cisplatin can be as high as 125mg/m2. clinically, it is possible to deliver higher concentrations of cisplatin through pharmacologic and technical manipulations. one strategy is through intra-arterial (ia) delivery. the relative advantage (rt) of ia infusion (relative to iV infusion of the same dose and schedule) is defined by the equation: rt = 1 + (plasma clearance/tumor plasma flow). the greater the plasma clearance of the drug and the smaller the tumor plasma flow, the greater the advantage of injecting the drug via the ia route. all of the advantages associated with ia infusion occur with the first pass of the drug through the tumor bed as once the drug has entered venous circulation, subsequent tumor exposure—because of recirculation—will be equivalent whether the drug entered the systemic circulation via an ia or an iV injection.21
to increase the therapeutic advantages associated with cisplatin, one must either decrease tumor plasma flow or increase plasma clearance.22 the former can be accomplished by giving the ia injection into as small an artery as possible. in the case of cisplatin, the latter can be accomplished by using the neutralizing agent thiosulfate.23 thiosulfate reacts covalently with cisplatin to produce a complex that is still soluble but is totally devoid of either toxicity or antitumor activity. When this neutralization occurs in the plasma it effectively increases the “clearance”. the extent of the reaction is a function of the concentration of both agents.24 thiosulfate is not a very potent neutralizing agent, and molar thiosulfate/cisplatin ratios in excess of ten are required before the reaction is fast enough to contribute significantly to the clearance of cisplatin.25 thiosulfate itself is very non-toxic, and doses in excess of 72 g can be given acutely, which is well above that needed to
provide effective cisplatin neutralization. thiosulfate has been used extensively at the university of california, san diego in conjunction with iP chemotherapy for ovarian carcinoma,23,26, and pharmacokinetic studies have demonstrated an additional important feature of its use.27 thiosulfate is extensively (>25-fold) concentrated in the urine, and this provides excellent protection against cisplatin-induced nephrotoxicity.28
the raPlat program uses a 2- to 4-minute ia infusion of cisplatin, and concurrent iV bolus administration of thiosulfate. during the brief interval of rapid ia infusion, the tumor is exposed to an extraordinarily high cisplatin concentration (approximately 250 times higher than the peak plasma concentration following standard iV dosing). because of the very high cisplatin/thiosulfate concentration ratio in the tumor bed, and the slow rate of reaction between cisplatin and thiosulfate, little neutralization is expected in the tumor.29 However, once the cisplatin passes through the tumor and reaches the plasma, it is diluted into the blood volume, and its concentration falls relative to the high concentration of thiosulfate in the plasma. thus, the tumor will receive a very brief exposure to an extremely high concentration of cisplatin, and the exposure of the systemic circulation to active cisplatin will be reduced.22
the nasal cavity and paranasal sinus region are particularly well suited for regional chemotherapy. Most patients who present with advanced carcinomas of the nasal cavity and paranasal sinuses do not have demonstrable distant metastases, although most patients have large, bulky lesions. the blood supply to these tumors is primarily derived from branches of the external carotid artery. significant technical advances in angiography now permit repeated safe superselective micro-catheterization of the dominant nutrient artery using a coaxial approach, which serves to decrease blood flow and further increase rt.30
because of infection and thrombosis. significant technical advances in vascular radiology techniques now permit safe repetitive superselective catheterization of the smaller nutrient arteries of the tumor.30, 33, 34
current state oF our researcH
We have been using radPlat for the definitive treatment of patients with cancer of the nasal cavity and paranasal sinuses since 1999 in Hokkaido university Hospital (sapporo, Hokkaido, Japan).35 Here, i present our methodology and latest outcomes.
Materials and Methods
Patients: Patients with advanced untreated nasal cavity
and paranasal sinus carcinoma were eligible for the study, but were excluded if they had distant metastases (M1) or had received prior treatment of any kind for their cancer. Further, eligible patients had to be no older than 75 years and had to have a World Health organization performance status of 0 to 2, adequate bone marrow reserve, and adequate liver and renal function.
Chemotherapy: all patients received concurrent ia
cisplatin and iV sodium thiosulfate infusions in the following manner: cisplatin (100–120 mg/m2 per week for four weeks) was infused through a microcatheter placed angiographically to selectively encompass only the dominant blood supply of the targeted tumor. tumors of the nasal cavity or paranasal sinuses are usually covered by the internal maxillary artery, but in cases when the facial artery, transverse facial artery, or ascending pharyngeal artery covered the tumor, part of the dose was administered through these alternative arteries.
at first, the catheter was positioned in the region of expected blood supply. a contrast agent was then injected as rapidly as possible until it refluxed slightly into the more proximal vessels during peak systole. next, selective intra-arterial computed tomographic arteriography (ia-cta) was performed to accurately and carefully identify the feeding arteries and their perfusion, and cisplatin was infused at the determined rate (Figures 3). simultaneously, sodium thiosulfate (20–24 g) was given intravenously, as described by robbins et al, to neutralize the cisplatin.19 all arterial catheterizations were accomplished transcutaneously through the femoral artery, and the catheters were removed immediately after infusion. so that patients excreted the cisplatin rapidly,
(a)
(b)
(c)
(d)
Figure 3. Magnetic resonance imaging (Mri) findings from
a 63-year-old man with a left maxillary sinus cancer that was classified as t4an0M0 (a). intra-arterial computed tomographic arteriography (ia-cta) was performed after a microcatheter was placed in 2ndportion of the internal maxillary artery (B and C, left).the ia-cta demonstrated the enhancement of the majority of the tumor. However, the anterior and lateral portions of the tumor were not enhanced
8 l of lactated ringer’s solution were given over a 24 h period. a 5Ht3-receptor antagonist was given to all patients before arterial infusion to minimize nausea and vomiting. chemotherapy was completed during the first four weeks, provided that patients responded well in the early treatment period and had received three arterial infusions.
radiotherapy: all patients received external radiotherapy using a 4-megavolt or 6-megavolt x-ray linear accelerator. the irradiation treatment volume included the entire maxilla, ethmoid sinus, ipsilateral nasal cavity, and pterygopalatine fossa. For patients with tumor extensions to the orbita, this area was also treated, but efforts were made to spare the lacrimal gland. the irradiation schedule was 65 gy in 26 fractions over 6.5 weeks until May 2006. it has since been changed to 70 gy in 35 fractions over seven weeks for all patients with advanced head and neck cancer. the treatment volume was reduced to 40 gy for cases with a low possibility of tumor extension to adjacent structures, such as the ethmoid sinus or orbita.
a modified 45-wedged pair technique was used, in which the lateral beams were tilted approximately 10 degrees anteriorly with a hope of reducing the risk of temporal lobe necrosis. Multileaf collimators were also used for this purpose and to reduce the dose to other critical structures, such as the optic chiasma and contralateral eye. For patients with lymph node metastases, the ipsilateral neck was irradiated (40 gy) using an anterior-posterior field and a 25-30 gy boost was given to the positive nodes. a thermo-plastic mask was used for immobilization for all patients. ct and Mri were undertaken in the same position using the mask so that accurate diagnosis of the extent of the tumor could be made. the treatment was planned with a ct simulator and a three-dimensional dose calculation computer. the dose to the spinal cord was kept below 40 gy in all instances.
evaluation of response and toxicity: responses were evaluated by clinical examination and/or ct or Mri studies 6–8 weeks after the completion of therapy. standard criteria were used to assess patient response. a complete response was defined as total resolution of the grossly visible tumor, and a partial response was defined as a 50% or greater reduction in the grossly visible tumor. as it is difficult to differentiate between radiographic changes related to the treatment and scar tissue from persisting tumors, we categorized patient outcomes to reflect this uncertainty. over time, scar tissue remains stable, but persistent tumor tissue will progress, so a patient with radiologic changes that
remained stable and with no signs or symptoms of disease was considered to be “progression-free”. a biopsy was performed only to document recurrence, if indicated. responses were evaluated by clinical examination and/or ct or Mri studies 6–8 weeks after the completion of therapy.
statistical analysis: the major endpoint of the study was overall survival. additional endpoints included local control rate (local progression-free rate) and toxicity. all patients were closely observed during the follow-up period, the mean of which was 5.7 years (range 1.2–11.1 years). cases of persistent or recurrent primary disease after completion of radPlat were considered to be local or regional failures, regardless of whether salvage was successful. Probabilities of overall survival, which included death from any cause, and local control rates (local progression-free rates computed from the beginning of treatment to the time of local relapse) were calculated using the kaplan-Meier method.
results
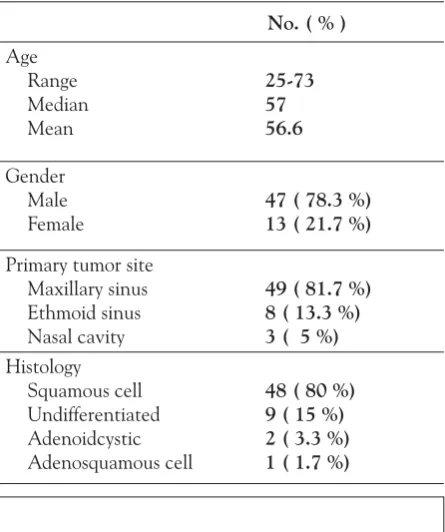
Patient characteristics: sixty patients were enrolled in this study from october 1999 to december 2009 and were treated by radPlat. subjects consisted of 47 males and 13 females, with a median age of 57 years (range 25–73 years). detailed patient characteristics are shown in table 1. Forty-seven patients (78.3 %) had tumors arising in
No. ( % )
age range Median Mean
25-73 57 56.6
gender Male
Female 47 ( 78.3 %)13 ( 21.7 %)
Primary tumor site Maxillary sinus ethmoid sinus nasal cavity
49 ( 81.7 %) 8 ( 13.3 %) 3 ( 5 %)
Histology squamous cell undifferentiated adenoidcystic adenosquamous cell
No. patients by toxicity grade
toxicity i ii iii iV
allergic reaction 1
Hearing 16 6
anemia 22 26 5 1
leucopenia 7 24 19 3
thrombocytopenia 13 10 3 1
arrhythmia 1
Fever 18 11 6
alopecia 28 13
dermatitis 13 29 1 2
nausea/vomiting 17 17 13
Mucositis 9 24 16 3
diarrhea 1
liver dysfunction 21 5 1
neuropathy 1 1
renal 5 2
Table 3. Acute Toxicity (n=50)
the maxillary sinus, eight (13.3 %) in the ethmoid sinus, and three (5 %) in the nasal cavity. Forty-eight patients (80 %) had squamous cell carcinomas, nine (15 %) undifferentiated carcinomas, two (3.3 %) adenoidcystic carcinomas, and the remaining one (1.7 %) had an adeno-squamous cell carcinoma.
t and n classifications are shown in table 2. twelve patients (20 %) were diagnosed with t3, 29 (48.3 %) with t4a, and 19 (31.7 %) with t4b disease. lymph node involvement was present in twelve patients (20
No. patients by N classification
T classification 0 1 2a 2b 2c Total
3 4a 4b total
9 25 14 48
2 4 1 7
1
1 2 2
2 2
12 29 19 60
Table 2. T and N Classification (n=50)
19), nausea/vomiting (n = 13), and neurologic signs (n =2). no patient experienced a cerebrovascular accident. Hematologic toxicity consisted of leukopenia (n = 22), anemia (n = 6), and thrombocytopenia (n =4). arterial infusion had to be stopped after only one infusion in one patient who developed sepsis because of toxicity. no surviving patients required feeding-tube support.
osteonecrosis, brain necrosis and ocular/visual problems occurred as late adverse reactions. seven patients experienced osteonecrosis, including five cases involving the maxilla, one the mandible, and one the frontal bone. Patients with grade 3 mandible necrosis required reconstruction of the mandible with free flap transfer. the remaining six patients suffered grade 2 osteonecrosis, which was manageable with minor sequestrectomy. Four patients suffered from brain necrosis. of these, one developed seizures that were well controlled by a convulsant drug. another suffered mild dementia. the remaining 2 had no symptoms.
severe ocular/visual problems (grade iii to iV) occurred in 19 of the 40 patients who were followed up over two years. one of these required enucleation of a painful eye ball. severe ocular/visual problems occurred in 17 (68 %) of 25 patients with tumors invading the orbit and/or invading inferior wall of the orbit. these patients were %). two patients with large tumors received induction
chemotherapy prior to radiotherapy to avoid exposing the eyeball and/or the optic nerve of the unaffected side to radiation. the induction chemotherapy protocol consisted of a combination of cisplatin, 5-fluorouracil, and docetaxel. one patient received one course and the other received two courses of treatment.
compliance: radPlat was feasible (three or four infusions of ia cisplatin and a full dose of radiation therapy within seven days of treatment interruptions) in 57 patients (95 %). one patient received only one cycle of ia chemotherapy and had his radiotherapy interrupted for 30 days, because of sepsis and poor general condition. a second patient experienced severe drug eruption after each ia chemotherapy, so radPlat was stopped after two courses of ia chemotherapy and 50 gy of radiotherapy. the patient then underwent a total maxillectomy. in the remaining patient, radiotherapy was stopped after 4 courses of ia chemotherapy and 48 gy of radiotherapy because of patient refusal. the remaining tumor was removed by endonasal resection.
considered for orbital exenteration if the need for radical surgery was indicated. severe ocular/visual problems also occurred in 4 (26.7 %) of 15 patients without tumors invading the orbit and/or invading inferior wall of the orbit.
local control and overall survival: the patient who experienced a severe drug eruption and underwent a total
(n=41, Figure 4). the 5-year overall survival was 72.6% for all patients, 56.9% for patients with t4b, and 79.6% for patients with t3-4a disease (Figure 5).
response of the Primary disease: of the 60 patients enrolled in the treatment program, complete responses in the primary site were obtained in 18 and partial responses in 39. However, the primary disease has been well controlled by radPlat in 46 patients (76.7%) so far. the remaining fourteen patients (23.3%) have experienced persistent or recurrent primary disease after the completion of radPlat. of these, eight patients received a total maxillectomy. as a result, seven of eight patients were successfully salvaged. in total, primary disease was controlled in 53 of 60 patients.
Pattern of relapse: the site of first uncontrolled recurrence was identified wherever possible. recurrence first occurred at the primary site in eight patients. of these, one patient is alive with disease. neck disease and distant metastasis were found at the same time in two cases, and distant metastasis was found in four patients without primary or neck recurrence. three patients died of other causes without disease.
discussion
a combination of radical surgery with radiation therapy constitutes the standard treatment for cancer of the nasal cavity and paranasal sinus as well as most epithelial malignancies.36, 37 However, the overall treatment of sinonasal malignancies has resulted in 5-year survival rates of only 30 to 50 percent, despite refinements in imaging tools such as ct scans and Mr imaging, surgical techniques and radiation therapy.37-41 Moreover, survival is further reduced for t4 tumors.
Functional and cosmetic outcomes after surgical treatment for advanced tumors, particularly those classed as t4, are also far from satisfactory from the standpoint of the patient. some patients, therefore, refuse surgical
Figure 4. local progression-free survival rate according to t classification in 50
patients with cancer of the nasal cavity and paranasal sinuses.
Figure: 5 overall survival according to t classification in 50 patients with cancer
of the nasal cavity and paranasal sinuses.
treatment, while others have unresectable disease. For these patients, radiation therapy is suggested, but is not expected to eliminate the tumor.42
Paranasal sinus carcinomas tend to be encompassed mostly within the region bordered by the terminal branches of the internal maxillary artery, which can be catheterized consistently and repeatedly, so such malignancies are considered good candidates for ia chemotherapy. the survival rate of maxillary sinus cancer in Japan has been reported to be 61-82% ,10, 43, 44, whereas those in other countries have been reported to be 29-62%.36, 37, 41, 46, 47, 48 this discrepancy might be due to the larger number of patients in Japan than in other countries,3 affording greater experience in treating such patients. in addition, the use of ia chemotherapy might play some role in providing the comparatively better results.
a phase ii protocol designed by the university of tennessee Health sciences center in Memphis in 1993 takes advantage of the benefits of multimodality therapy.49 Patients receive up to four-weekly infusions of high-dose cisplatin via superselective transfemoral catheterization of the internal maxillary artery. concurrently, they also receive 50 gy of external beam radiation over five weeks. definite surgical resection is performed 6–8 weeks following the completion of the therapy. results from the first 19 patients were recently reported within a median follow-up of 53 months. of these, 16 patients (84%) had t4 disease. surgery was conservative with a high rate of preservation of orbital contents, visual function, midface structures and palate, as well as a lack of any facial incisions resulting in an improved overall cosmetic and functional outcome. overall survival at 2 and 5 years was 67% and 51%, respectively. in the 13 patients in whom the disease was controlled after surgery, only two (15%) developed local failure. they reported no visual loss except for cataracts in 2 patients. However, 50 gy of radiation is not considered enough to eradicate advanced cancer, even in combination with surgery. conversely, delivering a high curative dose of radiation has been considered to result in damage to the optic nerve, chiasma, or brain.50 We are concerned that reducing the radiation dose results in poor local control, because to our knowledge wide resection and postoperative radiation therapy have not previously achieved satisfactory survival rates.37-41
in our institution, radPlat is the definitive treatment of choice for advanced nasal cavity and nasal paranasal sinus cancer so as to achieve improved survival rates
and avoid major surgery. We consider that patients with tumors invading orbital fat, orbital musculature, or involving the orbital apex usually require orbital content extirpation if surgery is indicated.51 therefore, we believe that eye-related complications may occur in such patients, although efforts should be made to spare vision and to avoid complications through the use of treatments such as intensity modulated radiation therapy and heavy particle radiation therapy. although some complications occurred in the present study, careful planning of radiation and ia infusion limited these to an acceptable level.
a previous multicenter, randomized phase 3 trial covering 239 patients with advanced head and neck cancer in the netherlands concluded that ia chemoradiation was not superior to intravenous chemoradiation.52 However, in an unplanned subgroup analysis, they found that there were significantly higher local and locoregional control rates and dFs for ia treatment for large (>30 ml) lateralized tumors. this result is consistent with my experience. nasal cavity and paranasal sinus cancer is generally large and lateralized. thus it appears suitable for treatment with radPlat therapy.
However, what concerns me in the dutch trial is that the paper made no mention of the angiographic technique. We did not know where or how cisplatin was administered intra-arterially, although it must influence the treatment outcome. therefore, we suppose that there were some issues regarding the angiographic technique.
although ia chemotherapy is sometimes regarded as dangerous because of the risk of catheter related problems, cerebrovascular accidents, and severe systemic complications,53, 54 no treatment-related death was encountered and no cerebrovascular accidents occurred in the present study; indeed we have experienced these complications in only one out of 240 cases in our institution to date, and that with full recovery. We consider the key to the successful application of radPlat is careful patient selection, superselective catheterization, and the management of side effects and toxicities during radPlat therapy. thus, the present study findings were of a better outcome than previous reports from many centers, and excellent cosmetic outcomes were achieved because surgery was not performed. as patient numbers were small and this was a single institution study, a multi-institutional trial is needed to confirm that this strategy is both feasible and effective.
cancer. cancer 1959;12:1248-62.
5. goepfert H, Jesse rH, lindberg, rd. arterial infusion and radiation therapy in the treatment of advanced cancer of the nasal cavity and paranasal sinuses. am J surg 1973;126:464-8.
6. sato Y, Morita M, takahashi Ho, Watanabe n, kirikae i. combined surgery, radiotherapy, and regional chemotherapy in carcinoma of the paranasal sinusitis. cancer 1970;25:571-9.
7. inuyama Y, Fujii M, tanaka J, takaoka t, Hosoda H, kohno n, saito s. neoadjuvant chemotherapy in maxillary sinus carcinoma with cisplatinum and peplomycin intraarterial infusion. auris nasus larynx. 1985;12 suppl 2:249-54.
8. nibu k, sugasawa M, asai M, ichimura k, Mochiki M, terahara a, et al. results of multimodality therapy for squamous cell carcinoma of maxillary sinus. cancer 2002;94:1476-82.
9. nishino H, Miyata M, Morita M, ishikawa k, kanazawa t, ichimura k. combined therapy with conservative surgery, radiotherapy, and regional chemotherapy for maxillary sinus carcinoma. cancer 2000;89:1925-32.
10. Mitani H, kamata s, nigauri t, Hoki t, Yonekawa H. results of treatment of patients with maxillary sinus carcinoma. Jibiinkokatenbo 2001,44:180-9. (Japanese)
11. Hattori t, Hirano t, toyoda s, nakagawa t, Yamaguchi n, sakakura Y. superselective continuous intra-arterial infusion therapy via superficial temporal artery for head and neck tumors. nippon igaku Hoshasen gakkai Zasshi 1985;45:1056-8. (Japanese)
12. tohnai i, Fuwa n, Hayashi Y, kaneko r, tomaru Y, Hibino Y, ueda M. new superselective intra-arterial infusion via superficial temporal artery for cancer of the tongue and tumour tissue platinum concentration after carboplatin (cbdca) infusion. oral oncol 1998;34:387-90.
13. Fuwa n, ito Y, Matsumoto a, kamata M, kodaira t, Furutani k, et al. a combination therapy of continuous superselective intraarterial carboplatin infusion and radiation therapy for locally advanced head and neck carcinoma. Phase i study. cancer 2000;89:2099-2105 14. imai s, kajihara Y, Munemori o, kamei t, Mori t, Handa t, et al.
superselective cisplatin (cddP)-carboplatin (cbdca) combined infusion for head and neck cancers. eur J radiol 1995;21:94-9. 15. korogi Y, Hirai t, nishimura r, Hamatake s, sakamoto Y,
Murakami r, et al. superselective intraarterial infusion of cisplatin for squamous cell carcinoma of the mouth: preliminary clinical experience. am J roentgenol 1995;165:1269-72.
16. Hirai t, korogi Y, Hamatake s, nishimura r, baba Y, takahashi M,et al. stages iii and iV squamous cell carcinoma of the mouth: three-year experience with superselective intraarterial chemotherapy using cisplatin prior to definitive treatment. cardiovasc intervent radiol 1999;22:201-5.
17. robbins kt, storniolo aMs, kerber c, Vicario d, seagren s, shea M, et al. Phase i study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J clin oncol 1994;12:2113-2120.
18. robbins kt, Vicario d, seagren s, Weisman r; Pellitteri P; kerber c, et al. a targeted supradose cisplatin chemoradiation protocol for advanced head and neck cancer. am J surg 1994;168:419-422. 19. robbins kt, kumar P, Wong Fs, Hartsell WF, Flick P, Palmer r, et
al. targeted chemoradiation for advanced head and neck cancer: analysis of 213 patients.Head neck 2000;22:687-93.
20. Yokoyama J, shiga k, saijo s, Matumoto k, ogawa Y. superselective intra-arterial infusion chemotherapy of high-dose cisplatin for advanced paranasal sinus carcinomas. gan to kagaku ryoho 1999;26:967-73. (Japanese)
21. dedrick rl. arterial drug infusion: pharmacokinetic problems and pitfalls. J natl cancer inst 1988;80:84-89.
22. Howell sb. Pharmacokinetic principles of regional chemotherapy. contr oncol 1988;29:1-8.
23. Howell sb, Pfeifle ce, Wung We, oshen ra, lucas We, Yon Jl, green M. intraperitoneal cisplatin with systemic thiosulfate
infusion with concomitant radiotherapy can result in organ preservation and cure in the majority of patients with advanced cancer of the nasal cavity and paranasal sinuses. toxicity was manageable and no patient died as a result of treatment toxicity. However, late adverse reactions, such as osteonecrosis, brain necrosis and ocular/ visual problems, should be monitored in future trials. nonetheless, this result does suggest that significant progress has been made in the management of these diseases.
Finally, due to the theoretical advantage of ia chemotherapy, it remains an attractive alternative method for delivering high dose chemotherapy to advanced head and neck cancer, particularly in cases of nasal cavity and paranasal sinus cancer. the promising results from trials conducted provide a strong impetus for continued investigations into this approach. We are now planning a multi-institutional trial of radPlat for very advanced cases of maxillary sinus cancer.
acknoWledgMents
I thank Satoshi Fukuda MD PhD for his advice. The efforts and collaboration of the neuroradiologists (Daisuke Yoshida MD, Satoshi Ushikoshi MD, and Tsuyoshi Asano MD), radiation oncologists (Rikiya Onimaru MD PhD, Kouichi Yasuda MD, Hiroki Shirato MD PhD, and so on), and my colleagues, head and neck surgeons (Yasushi Furuta MD PhD, Nobuhiko Oridate MD PhD, Fumiyuki Suzuki MD PhD, Hiromitsu Hatakeyama MD PhD, Jun Furusawa MD PhD, Satoshi Kano MD PhD, Takatsugu Mizumachi MD PhD, Shigenari Taki MD, Naoya Inamura MD, Tomohiro Sakashita MD, and so on) were also paramount and much appreciated. Finally, this work was supported in part by a Grant-in-Aid for Science Research from the Ministry of Education, Science, Sports and Culture, Japan.
reFerences
1. a. robbins kt, Homma a. intra-arterial chemotherapy for head and neck cancer: experiences from three continents. surg oncol clin n am. 2008;17:919-33, xi.
2. Muir cs, nectoux J. descriptive epidemiology of malignant neoplasms of nose, nasal cavities, middle ear and accessory sinuses. clin otolaryngol. 1980;5:195–211.
3. report of Head and neck cancer of Japan clinical statistics of registered Patients, 2001. Japan society for Head and neck cancer registry committee. Japanese Journal of Head and neck cancer. 2005; 31: supplement.
protection. ann int Med 1982;97:845-851.
24. Howell sb, taetle r. the effect of sodium thiosulfate on cis dichlorodaimminedplatin (ii) nephrotoxicity and antitumor activity in the l1210 leukemia. cancer treat rep 1980;64:611-615. 25. elferink WJF, van der Vijah ik, Pinedo HM. interaction of cisplatin
and carboplatin with sodium thiosulfate: reaction rates and protein binding. clin chem 1986;32:642-645.
26. kirmani s, lucas We, kim s, goel r, McVey l, Morris J, Howell sb. a phase ii trial of intraperitoneal cisplatin and etoposide as salvage treatment of ovarian carcinoma. J clin oncol 1991;9:649-657.
27. goel r, cleary sM, Horton c, kirmani s, abramson i, kelly c, Howell sb. effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J natl cancer inst 1989;81:1552-1560. 28. shea M, koziol Ja, Howell sb. kinetics of sodium thiosulfate, a
cisplatin neutralizer. clin Pharmacol ther 1984;35:419-425. 29. Howell, sb. editorial: improving the therapeutic index of
intra-arterial cisplatin chemotherapy. eur J of cancer clin oncol 1989;25:775-776.
30. Wolpert sM, kwan es, Heros d, et al. selective delivery of chemotherapeutic agents with a new catheter system. radiology 1988;166:547-549.
31. Forastiere aa, baker sr, Wheeler r, Medvec br. intra-arterial cisplatin and Fudr in advanced malignancies confined to the head and neck. J clin oncol 1987;5:1601-1606.
32. cheung dk, regan J, savin M, gibberman V, Woessner W. a pilot study of intraarterial chemotherapy with cisplatin in locally advanced head and neck cancers. cancer 1988;61:903-908. 33. lee Y-Y, dimery iW, Van tassel P, de Pena c, blacklock b, goepfert
H. superselective intra-arterial chemotherapy of advanced paranasal sinus tumors. arch otolaryngol Head neck surg 1989;115:503-511.
34. robbins kt, storniolo aM, kerber c, seagren s, berson a, Howell sb. rapid superselective high dose cisplatin infusion for advanced head and neck malignancies. Head and neck 1992;14:364-371. 35. Homma a, oridate n, suzuki F, taki s, asano t, Yoshida d,
onimaru r, nishioka t, shirato H, Fukuda s. superselective high-dose cisplatin infusion with concomitant radiotherapy in patients with advanced cancer of the nasal cavity and paranasal sinuses: a single institution experience. cancer. 2009;115:4705-14. 36. katz ts, Mendenhall WM, Morris cg, amdur rJ, Hinerman rW,
Villaret db. Malignant tumors of the nasal cavity and paranasal sinuses. Head neck. 2002;24:821–829.
37. dulguerov P, Jacobsen Ms, allal as, lehmann W, calcaterra t. nasal and paranasal sinus carcinoma: are we making progress? a series of 220 patients and a systematic review. cancer. 2001;92:3012–3029.
38. lavertu P, roberts Jk, kraus dH, et al. squamous cell carcinoma of the paranasal sinuses: the cleveland clinic experience 1977-1986. laryngoscope. 1989; 99:1130–1136.
39. spiro Jd, soo kc, spiro rH. squamous carcinoma of the nasal cavity and paranasal sinuses. am J surg. 1989;158:328–332. 40. Paulino ac, Marks Je, bricker P, Melian e, reddy sP, emami b.
results of treatment of patients with maxillary sinus carcinoma. cancer. 1998;83:457–465.
41. Myers ll, nussenbaum b, bradford cr, teknos tn, esclamado rM, Wolf gt. Paranasal sinus malignancies: an 18-year single institution experience. laryngoscope. 2002;112:1964–1969. 42. Hoppe bs, nelson cJ, gomez dr, et al. unresectable carcinoma of
the paranasal sinuses: outcomes and toxicities. int J radiat oncol biol Phys. 2008;72:763–769.
43. konno a, ishikawa k, terada n, numata t, nagata H, okamoto Y. analysis of long-term results of our combination therapy for squamous cell cancer of the maxillary sinus. acta otolaryngol suppl 1998;537:57-66.
44. Yao k, takahashi H, inagi k, nakayama M, Makoshi t, nagai H, okamoto M. treatment of maxillary sinus carcinoma: clinical results using the kitasato modality. acta otolaryngol suppl. 2002;(547):15-9.
45. kanoto M, oda a, Hosoya t, nemoto k, ishida a, nasu t, koike s, aoyagi M. impact of superselective transarterial infusion therapy of High-dose cisplatin on Maxillary cancer with orbital invasion. am J neuroradiol. 2010;31:1390-4.
46. cantu g, bimbi g, Miceli r, Mariani l, colombo s, riccio s, squadrelli M, battisti a, Pompilio M, rossi M. lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. arch otolaryngol Head neck surg. 2008;134:170-7.
47. bhattacharyya n. Factors affecting survival in maxillary sinus cancer. J oral Maxillofac surg. 2003;61:1016-21.
48. Hoppe bs, stegman ld, Zelefsky MJ, rosenzweig ke, Wolden sl, Patel sg, shah JP, kraus dH, lee nY. treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting--the Mskcc experience. int J radiat oncol biol Phys. 2007 ;67:691-702.
49. samant s, robbins kt, Vang M, Wan J, robertson J. intra-arterial cisplatin and concomitant radiation therapy followed by surgery for advanced paranasal sinus cancer. arch otolaryngol Head neck surg. 2004;130:948–955.
50. Parsons Jt, Mendenhall WM, Mancuso aa, cassisi nJ, Million rr. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. int J radiat oncol biol Phys. 1988;14:11–22.
51. Wong rJ, kraus dH. cancer of the nasal cavity and paranasal sinuses. in: shah JP, editor. cancer of the head and neck. Hamilton: bc decker, inc., 2001, p. 204-224.
52. rasch cr, Hauptmann M, schornagel J, Wijers o, buter J, gregor t, Wiggenraad r, Paul de boer J, ackerstaff aH, kroger r, Hoebers FJ, balm aJ. intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: results of a randomized phase 3 trial. cancer. 2010;116:2159-2165.
53. Foote rl, kasperbauer Jl, okuno sH, et al a pilot study of high-dose intraarterial cisplatin chemotherapy with concomitant accelerated radiotherapy for patients with previously untreated t4 and selected patients with t3n0-n3M0 squamous cell carcinoma of the upper aerodigestive tract. cancer. 2005;103:559–568. 54. Papadimitrakopoulou Va, ginsberg le, garden as, et al.