Birth Before 39 Weeks’ Gestation Is Associated With
Worse Outcomes in Neonates With Heart Disease
WHAT’S KNOWN ON THIS SUBJECT: Premature birth is associated with adverse outcomes in neonates with critical congenital heart disease.
WHAT THIS STUDY ADDS: Compared to neonates with critical congenital heart disease delivered at 39 to 40 completed weeks’ gestation, those delivered at 37 to 38 weeks had increased adjusted mortality and morbidity rates. Patients delivered at or beyond 41 completed weeks also had greater adjusted mortality rates.
abstract
BACKGROUND:Recent studies have revealed increased morbidity and mortality rates in term neonates without birth defects who were deliv-ered before 39 weeks of completed gestation. We sought to determine if a similar association exists between gestational age at delivery and adverse outcomes in neonates with critical congenital heart disease, with particular interest in those born at 37 to 38 weeks’ gestation.
PATIENTS AND METHODS:We studied 971 consecutive neonates who had critical congenital heart disease and a known gestational age and were admitted to our cardiac ICU from 2002 through 2008. Gestational age was stratified into 5 groups:⬎41, 39 to 40, 37 to 38, 34 to 36, and
⬍34 completed weeks. Multivariate logistic regression analyses were used to evaluate mortality and a composite morbidity variable. Multi-variate Poisson regression was used to evaluate duration of ventila-tion, intensive care, and hospitalization.
RESULTS:Compared with the referent group of neonates who were delivered at 39 to 40 completed weeks’ gestation, neonates born at 37 to 38 weeks had increased mortality (6.9% vs 2.6%; adjustedP⫽.049) and morbidity (49.7% vs 39.7%; adjustedP⫽.02) rates and tended to require a longer duration of mechanical ventilation (adjustedP⫽.05). Patients born after 40 or before 37 weeks also had greater adjusted mortality rates, and those born before 37 weeks had increased mor-bidity rates and required more days of mechanical ventilation and intensive care.
CONCLUSIONS:For neonates with critical congenital heart disease, delivery before 39 weeks’ gestation is associated with greater mortal-ity and morbidmortal-ity rates and more resource use. With respect to neona-tal morneona-tality, the ideal gestational age for delivery of these patients
may be 39 to 40 completed weeks.Pediatrics2010;126:e277–e284
AUTHORS:John M. Costello, MD, MPH,aAngelo Polito, MD
MPH,a,bDavid W. Brown, MD,aThomas F. McElrath, MD,
PhD,cDionne A. Graham, PhD,dRavi R. Thiagarajan, MBBS,
MPH,aEmile A. Bacha, MD,eCatherine K. Allan, MD,a
Jennifer N. Cohen, MD,fand Peter C. Laussen, MBBSa
aDepartment of Cardiology,dClinical Research Program, eDepartment of Cardiac Surgery, andfDivision of Neonatology, Department of Medicine, Children’s Hospital Boston, Boston, Massachusetts;bDepartment of Cardiology and Cardiac Surgery, Bambino Gesu` Pediatric Hospital, Rome, Italy; and cDepartment of Obstetrics and Gynecology, Brigham and Woman’s Hospital, Harvard Medical School, Boston, Massachusetts
KEY WORDS
heart defects congenital, pregnancy, premature birth, gestational age, intensive care
ABBREVIATION
RACHS-1—Risk Adjustment in Congenital Heart Surgery-Version 1
This manuscript was presented in part at the American Heart Association’s Scientific Sessions; November 16, 2009; in Orlando, FL.
www.pediatrics.org/cgi/doi/10.1542/peds.2009-3640
doi:10.1542/peds.2009-3640
Accepted for publication Apr 12, 2010
Address correspondence to John M. Costello, MD, MPH, Division of Cardiovascular Critical Care, Department of Cardiology, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Ave, Bader 600, Boston, MA 02115-5737. E-mail: john.costello@cardio.chboston.org
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2010 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have no financial relationships relevant to this article to disclose.
1000 live births or 32 000 newborns each year in the United States.1Critical
congenital heart disease may be de-fined as a congenital heart defect that requires surgical or transcatheter cardiac intervention or results in death during the first 28 days of life.2
As with any other infants who are born at term or near term, those with criti-cal congenital heart disease may be delivered before 39 weeks of com-pleted gestation for a variety of ob-stetric indications including the onset of spontaneous labor, maternal co-morbidities, pregnancy complications, or nonreassuring results of fetal test-ing. However, in the absence of these indications, the ideal gestational age for the delivery of these infants is un-clear. Many fetuses with known critical congenital heart disease are electively delivered between 37 and 40 weeks’ gestation, when organ maturity is as-sumed to be complete. The practice of early delivery is advantageous be-cause it allows the efficient mobiliza-tion of expert personnel and critical-care resources and the transport in utero of the fetus to appropriate ter-tiary care facilities and possibly limits the risk of infant death during the late fetal period. However, results of recent
investigations of gestational-age–
specific neonatal morbidity, mortality, and resource use in the delivery of in-fants at term have raised questions about this practice.3–5 We therefore
sought to determine whether an asso-ciation exists between gestational age at delivery and adverse outcomes in neonates with critical congenital heart disease. In particular, we were inter-ested in those patients born at 37 to 38 completed weeks’ gestation. We hy-pothesized that in neonates with criti-cal congenital heart disease who were delivered at 37 to 38 weeks, infant mor-tality and morbidity rates and the con-sumption of health care resources
PATIENTS AND METHODS
This was a retrospective, single-center cohort study. We included all neonates (ⱕ28 days old) with critical congenital heart disease who were admitted to the cardiac ICU at Children’s Hospital Boston between January 1, 2002, and December 31, 2008. We excluded pa-tients whose gestational age could not be determined, medical records were incomplete, or initial cardiac surgical procedure was performed at another institution. We also excluded patients who had congenital heart disease and fatal genetic syndromes (eg, trisomy 13) or additional major malformations if life support was withdrawn before cardiac intervention.
The primary exposure variable was gestational age at delivery as assigned by providers of obstetric care and recorded from review of obstetric records, fetal echocardiogram re-ports, assessments by neonatologists, and ICU records. The estimated date of term delivery, and therefore the gesta-tional age at delivery, is usually deter-mined on the basis of either the results of a first-trimester ultrasound or the date of the first day of the mother’s last menstrual period with supporting in-formation from a second-trimester ul-trasound. If the available records indi-cated that delivery occurred at “40 weeks,” we recorded it as 400⁄7weeks.
Gestational age was stratified into 5 groups: 390⁄7to 406⁄7completed weeks
(referent group), ⱖ410⁄7 completed
weeks, 370⁄7to 386⁄7completed weeks,
340⁄7 to 366⁄7 completed weeks, and
⬍340⁄7completed weeks.
The primary outcome variable was hospital mortality. Secondary outcome variables included a composite mor-bidity variable, and durations of me-chanical ventilation, intensive care, and hospitalization were assessed as
bidity variable when at least 1 of the following 11 events occurred during the period from birth until first discharge from our institution: respi-ratory distress syndrome requiring surfactant, cardiopulmonary resusci-tation requiring chest compressions, necrotizing enterocolitis with pneuma-tosis observed on abdominal radio-graph, extracorporeal membrane oxy-genation, selected infections associated with health care (bloodstream, surgical-site, and urinary tract infection), cere-bral vascular injury (grade II or greater intraventricular hemorrhage, ischemic stroke, or intraparenchymal hemorrhage documented by neuroim-aging), unplanned reintubation, un-planned cardiac reoperation for re-sidual anatomic lesions, unplanned interventional cardiac catheterization for residual anatomic lesions,
un-planned cardiac ICU readmission,
transfer to an outside institution for additional convalescence, and un-planned readmission within 30 days of hospital discharge.
factors. We noted whether the neo-nate’s congenital heart disease was di-agnosed prenatally, the location of de-livery (at 1 of 2 tertiary care hospitals adjacent to our institution, Brigham and Woman’s Hospital or Beth Israel Deaconess Medical Center, versus other institution), vaginal or cesarean delivery, birth weight, birth weight that was low for gestational age (z score less than⫺2), gender, presence of ma-jor noncardiac birth defects, and pres-ence of chromosomal abnormalities. We recorded whether each neonate underwent primary cardiac surgical or transcatheter intervention and the neonate’s exposure to and duration of cardiopulmonary bypass. We assigned each patient a Risk Adjustment in Congenital Heart Surgery-Version 1 (RACHS-1) category.6RACHS-1 is a
vali-dated risk-adjustment method that groups cardiac surgical procedures with similar expected in-hospital mor-tality rates into 6 predefined risk cate-gories, in which category 1 has the lowest risk for death (eg, atrial-septal defect closure) and category 6 has the highest risk for death (eg, stage 1 Nor-wood operation).
This study was approved by the Chil-dren’s Hospital Boston committee on clinical investigation, and the re-quirement for informed consent was waived.
Statistics
SAS 9.1 of the SAS System for Windows (SAS Institute, Inc, Cary, NC), was used for statistical analysis. For each gesta-tional age group, we calculated sum-mary statistics for the outcomes of
in-terest. For the primary analysis,
multivariate logistic regression was used to calculate adjusted odds ratios for mortality. A similar approach was used for analysis of the composite morbidity outcome. Multivariate Pois-son regression was used to generate adjusted rate ratios for the duration of
mechanical ventilation, ICU stay, and hospitalization. Statistical significance was achieved when the 95% confi-dence intervals for adjusted odds ra-tios did not include 1 (P⬍.05). To ad-dress potential confounding variables, univariate associations were sought between each of the covariates listed above and the outcomes of interest. Covariates were considered for inclu-sion in each multivariate model that were associated (P⬍.1) by univariate analysis with the outcome.
RESULTS
Between January 1, 2002, and Decem-ber 31, 2008, 1071 neonates with criti-cal congenital heart disease were ad-mitted to our cardiac ICU. Of these patients, 100 (9%) were excluded from the study, and 971 (91%) were in-cluded (Fig 1). Demographic data and exposure variables for the study pa-tients are summarized in Table 1. Com-pared with patients delivered at 39 to 40 completed weeks’ gestation, pa-tients delivered at 37 to 38 completed weeks had lower birth weight and higher rates of prenatal diagnosis of congenital heart disease, delivery at an adjacent institution, cesarean deliv-ery, and major noncardiac structural anomalies.
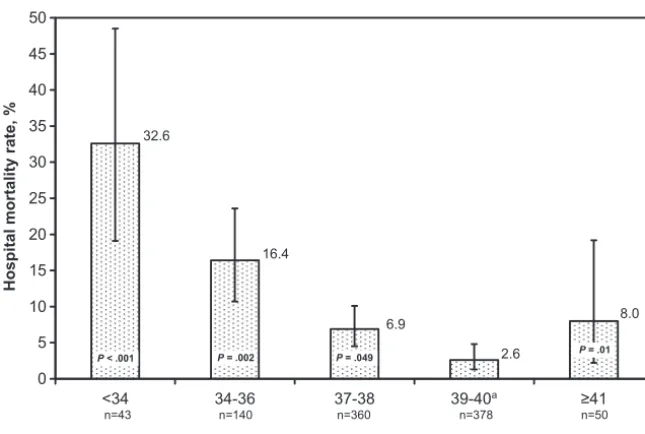
Of the 971 study patients, 76 (7.8%) died during their initial hospitalization, including 8 patients (0.8%) who suf-fered irreversible end-organ injury af-ter birth and, thus, did not undergo cardiac intervention. Compared with the referent group of patients born at 39 to 40 completed weeks’ gestation, patients born before 39 or after 40 weeks were more likely to die during their initial hospitalization (Table 2 and Fig 2). Delivery before 39 or after 40 completed weeks’ gestation remained significantly associated with greater odds of mortality after adjustment for prenatal diagnosis of congenital heart disease, nonreassuring antepartum fetal status, birth weight, the presence of major noncardiac structural anom-alies, RACHS-1 category, and exposure to and duration of cardiopulmonary bypass (Table 2).
One or more morbid events occurred in 480 of 971 study patients (49%). Compared with the referent group of patients born at 39 to 40 completed weeks’ gestation, those born before 39 completed weeks had significantly greater odds of reaching the compos-ite morbidity end point after adjust-ment for prenatal diagnosis of
congen-ital heart disease, nonreassuring
1071 neonates with critical congenital heart disease admitted to cardiac ICU
971 neonates included: 858- primary intervention = cardiac surgery 105- primary intervention = cardiac catheterization 8- no cardiac intervention due to postnatal end-organ injury
100 neonates excluded: 84- gestational age not available
7- initial primary cardiac intervention at outside institution 6- support withdrawn for multiple anomalies
3- incomplete medical records
FIGURE 1
Neonates with critical congenital heart disease admitted to the cardiac ICU between January 1, 2002, and December 31, 2008.
antepartum fetal status, birth weight, a birth weight that was low for gesta-tional age, the presence of major noncardiac structural anomaly or chromosomal syndrome, RACHS-1 cat-egory, and exposure to and duration of cardiopulmonary bypass (Table 2 and Fig 3). Individual morbid-event rates are summarized in Table 3. Eight of 11 individual morbid events occurred more commonly in patients in the group delivered at 37 to 38 completed weeks compared with the group deliv-ered at 39 to 40 completed weeks. There was no difference in the occur-rence of necrotizing enterocolitis be-tween these 2 groups, but infection and 30-day rehospitalization rates were slightly higher in patients deliv-ered at 39 to 40 completed weeks. In addition, after adjustment for these
same variables, our data indicated that patients delivered at 37 to 38 com-pleted weeks tended to have a greater adjusted risk for receiving more days
of mechanical ventilation (P ⫽ .05). Compared with patients in the referent group, patients born before 37 com-pleted weeks had greater adjusted risks for receiving ongoing
mechani-cal ventilation and intensive care (Ta-ble 2). It should be noted that univari-ate relationships between mode and location of delivery and the outcomes of interest were insignificant when considered along with a prenatal
diag-nosis of congenital heart disease; thus, they were not included in the final mul-tivariate models discussed above.
A secondary analysis was conducted that included only those neonates who
pared with the referent group deliv-ered at 39 to 40 completed weeks, elec-tively delivered patients in the group delivered at 37 to 38 completed weeks had similar rates of cesarean delivery and chromosomal anomalies and a similar distribution of RACHS-1 catego-ries and duration of cardiopulmonary bypass (data not shown). However, these neonates were more likely to have had a prenatal diagnosis of criti-cal congenital heart disease (95% vs 84%;P⫽.02), to have had been deliv-ered at an adjacent institution (89% vs
77%;P⫽.03), to have had a
noncar-diac structural anomaly (18% vs 2%;
P⬍.001), and to have had a lower birth weight (3.19 vs 3.30 kg;P⫽.001). Con-sidering the outcomes of interest for these electively delivered patients, we observed that compared with the ref-erent group delivered at 39 to 40 com-pleted weeks, those delivered at 37 to 38 weeks tended to have had a greater mortality rate, although this finding did not reach statistical significance (10 of 94 [10.6%] vs 4 of 82 [4.9%];P⫽
.16). Because of the small number of deaths that occurred in patients who were born via an elective delivery, we were unable to perform a multivariate analysis of mortality outcomes. Signif-icantly more patients in the group of infants who underwent elective deliv-ery at 37 to 38 completed weeks reached the composite morbidity
out-come (57% vs 41%;P⫽.03).
DISCUSSION
The results of this study helped us to determine the optimal time of delivery of patients with critical congenital heart disease. It is known that preterm delivery is associated with adverse outcomes in this patient population, but the timing of delivery at term has not been previously studied. The novel and most important finding of this study was that delivery at 37 to 38 com-(N⫽971) (n⫽43) (n⫽140) (n⫽360) (n⫽378) (n⫽50)
Multiple gestation, % 5 28 19 3 0 0
Prenatal diagnosis of CHD, % 51 51 59 60 43 22
Factors associated with timing of delivery, %
Spontaneous labor 44 42 37 37 46 66
Fetala 12 23 27 11 6 0
Maternalb 15 28 24 15 14 8
Elective 20 0 8 26 22 10
Unknown 10 7 4 11 11 16
Delivery at adjacent institution, %c 44 47 49 53 36 20
Cesarean delivery, % 40 57 54 40 33 22
Male gender, % 57 51 58 60 55 58
Birth weight, mean⫾SD, kg 3.1⫾0.7 1.6⫾0.5 2.5⫾0.5 3.1⫾0.5 3.4⫾0.4 3.6⫾0.5
Small for gestational age, % 3.2 9.3 5.0 3.6 0.8 8.0
Major noncardiac structural anomaly, %
10 14 17 12 5 16
Chromosomal anomaly, % 7 12 5 9 6 6
Cardiac intervention, %
Surgery 88 84 82 89 92 84
Catheterization 11 12 15 11 8 14
None: end-organ injury 1 5 3 0.3 0 2
RACHS-1 category, %
1–2 or cardiac catheterization 19 21 29 20 14 20
3–4 53 49 38 52 60 50
5–6 24 21 22 25 24 22
Unclassified or no intervention because of end-organ injury
4 9 11 3 2 8
CPB, median, min 121 121 112 120 127 115
CHD indicates congenital heart disease; CPB, cardiopulmonary bypass.
aIncludes intrauterine growth retardation, polyhydramnios/oligohydramnios, hydrops fetalis, failed stress test, and breech
presentation.
bIncludes placental abruption, preeclampsia, and repeat cesarean delivery.
pleted weeks of gestation was associ-ated with more than twofold greater adjusted odds of patient hospital mor-tality and significantly greater morbid-ity compared with delivery at 39 to 40 completed weeks. Not surprisingly, we previously identified significantly greater morbidity and mortality rates
and resource consumption for neo-nates who were born in the late-preterm period (34 –36 weeks), and this burden was even more pro-nounced for those patients delivered before 34 completed weeks.6,7Finally,
patients with critical congenital heart disease who were delivered beyond 40
weeks’ gestation had greater adjusted odds of death. Overall, the findings demonstrated a U-shaped relationship between gestational age and out-comes and indicated that the ideal ges-tational age for delivery may be 39 to 40 completed weeks.
The importance of the timing of deliv-ery of term neonates who do not have major structural anomalies has been investigated in recent studies. In a large study of data from birth and death certificates, Young et al found that the risk ratio for neonatal death was 1.9 for infants without birth de-fects who were born at 37 to 38 com-pleted weeks of gestation compared with a referent group born at 40 weeks.3Several additional recent
stud-ies also revealed associations be-tween delivery at 37 to 38 completed weeks’ gestation and adverse out-comes.4,5,8–10Using a multicenter
regis-try, Tita et al4studied 13 258 elective
term deliveries that were repeat ce-sarean deliveries for which there were no maternal conditions, major birth defects, or other factors that influ-enced the timing of delivery. These in-vestigators found that birth during
8.0
2.6 6.9
16.4 32.6
0 5 10 15 20 25 30 35 40 45 50
<34 34-36 37-38 39-40a ≥41
Completed weeks of gestation at birth
Hospital mortality rate, %
n=43 n=140 n=360 n=378 n=50
P = .049 P = .01
P = .002
P < .001
FIGURE 2
Crude hospital mortality rate for each gestational age group. Error bars represent 95% confidence intervals. ThePvalues were adjusted for prenatal diagnosis of congenital heart disease, nonreassur-ing antepartum fetal status, birth weight, and presence of major noncardiac structural anomalies, RACHS-1 category, and exposure to and duration of cardiopulmonary bypass.aReferent group.
TABLE 2 Relationships Between Gestational Age Groups and Outcome Variables
⬍34 wk (n⫽43)
34–366⁄7wk (n⫽140)
37–386⁄7wk (n⫽360)
39–406⁄7wk (n⫽378)
ⱖ41 wk (n⫽50)
Hospital mortality, % 32.6 16.4 6.9 2.6 8.0
Adjusted mortalitya 15.23 (4.04–57.45) 5.05 (1.86–13.74) 2.30 (1.00–5.30) 1.00 5.06 (1.38–18.60)
P ⬍.001 .002 .049 .01
Composite morbidity, % 95.4 62.9 49.7 39.7 44.0
Adjusted composite morbidityb 33.23 (7.19–156.65) 2.61 (1.58–4.31) 1.45 (1.06–1.99) 1.00 1.07 (0.57–2.01)
P ⬍.001 ⬍.001 .02 .83
Ventilator days 16 (6–54) 6.5 (1–43.5) 5 (1.5–23) 5 (1–13) 4 (1–13)
Adjusted ventilator daysb 2.39 (1.69–3.39) 1.59 (1.25–2.02) 1.19 (1.00–1.41) 1.00 0.70 (0.46–1.06)
P ⬍.001 ⬍.001 .05 .09
ICU days 21 (9–43) 12 (4–47) 9.5 (4–32.5) 9 (5–11) 8 (4–22.5)
Adjusted ICU daysb 1.98 (1.48–2.66) 1.30 (1.06–1.59) 1.10 (0.95–1.26) 1.00 0.79 (0.57–1.08)
P ⬍.001 .01 .20 .14
Hospital days 24 (9–61) 19 (7–58) 15 (8–46.5) 14 (8–35) 13 (7–32)
Adjusted hospital daysb 1.47 (1.13–1.93) 1.19 (0.99–1.41) 1.03 (0.91–1.16) 1.00 0.89 (0.69–1.14)
P .005 .06 .62 .35
Adjusted data are reported as odds ratios (95% confidence intervals). Ventilator, ICU, and hospital days are reported as median (10th–90th percentile), along with adjusted rate ratios (95% confidence intervals).
aAdjusted for prenatal diagnosis of congenital heart disease, nonreassuring antepartum fetal status, birth weight, presence of major noncardiac structural anomalies, RACHS-1 category
and exposure to and duration of cardiopulmonary bypass.
bAdjusted for prenatal diagnosis of congenital heart disease, nonreassuring antepartum fetal status, presence of major noncardiac structural anomalies and chromosomal syndrome, birth
weight, a birth weight that was low for gestational age, RACHS-1 category, and exposure to and duration of cardiopulmonary bypass.
week 37 or 38 of gestation was associ-ated with greater adjusted odds (2.1 and 1.5, respectively) of reaching a composite morbidity end point when compared with the referent group of patients delivered at 39 completed weeks. Similarly, Cheng et al10
evalu-ated 2.5 million low-risk term deliver-ies and found that compared with a referent group delivered at 39 com-pleted weeks, infants delivered at 37 weeks had lower Apgar scores, more incidents of respiratory distress syn-drome, and a greater need for
me-chanical ventilation, whereas those born at 41 completed weeks were more likely to have meconium-aspiration syndrome, macrosomia, and neonatal injury. The common finding in all of these studies was that neonatal mor-bidity and mortality rates were lowest when delivery occurred between 39 and 40 completed weeks’ gestation. Our data confirmed our hypothesis that an association exists between birth at 37 to 38 weeks’ completed ges-tation and increased morbidity and mortality rates in neonates with
criti-cal congenital heart disease in our center and others11are born before 39
completed weeks’ gestation, and that some of these deliveries are purely elective (26% in our group of infants born at 37–38 completed weeks of ges-tation) or on the basis of relative ma-ternal or fetal indications, we believe that we have identified a risk factor for death and adverse outcomes that is po-tentially modifiable in selected patients.
The precise etiology of adverse out-comes for patients born at 37 to 38 completed weeks’ gestation in this study is unknown. During the last few weeks of pregnancy physiologic events that involve nitric oxide and surfactant production and fetal lung-fluid clear-ance are important for postnatal lung function.12,13Incomplete maturation of
the lungs and other organ systems in term neonates with critical congenital heart disease may partially explain the association we found between delivery at 37 to 38 completed weeks’ gestation and increased morbidity. The “full-term” designation for infants deliv-ered at or beyond 37 weeks was con-trived by the World Health Organization in 1950 and has been perpetuated for decades despite a lack of data to indi-cate that such patients are free of the
hazards of premature birth.8 The
weight of evidence from our study and several recent investigations of neo-nates without heart disease clearly in-dicates that the 37-week threshold was arbitrarily established and sug-gests that morbidity and mortality rates are lowest when delivery occurs at 39 to 40 weeks.3–5,8–10
The fact that 58% of our patients with critical congenital heart disease were delivered before 39 weeks also must be considered in light of recent data regarding brain immaturity in neo-nates with hypoplastic left-heart syn-drome or transposition of the great
44.0 39.7
49.7 62.9
0 20 40 60 80
<34 34-36 37-38 39-40 ≥41
Composite morbidity rate, %
n=43 n=140 n=360 n=378 n=50
Completed weeks of gestation at birth
P < .001 P < .001 P = .02 referentgroup P = .83
FIGURE 3
Crude morbidity rates for each gestational age group. Error bars represent 95% confidence intervals. ThePvalues were adjusted for prenatal diagnosis of congenital heart disease, nonreassuring ante-partum fetal status, major noncardiac structural or chromosomal anomalies, birth weight, birth weight low for gestational age, RACHS-1 category, and cardiopulmonary bypass exposure and duration.
TABLE 3 Individual Morbid-Event Rates for Each Gestational Age Group
⬍34 wk (n⫽43)
34–366⁄7wk (n⫽140)
37–386⁄7wk (n⫽360)
39–406⁄7wk (n⫽378)
ⱖ41 wk (n⫽50)
Respiratory distress syndrome 62.8 9.3 1.7 0 0
Necrotizing enterocolitis 4.7 4.3 2.8 2.9 0
Nosocomial infection 37.2 17.9 13.6 14.0 12.0
Cardiopulmonary resuscitation 32.6 20.0 8.6 7.1 10.0
Cerebral vascular injury 27.9 8.6 2.5 1.1 6.0
Extracorporeal membrane oxygenation 11.6 16.4 9.2 6.1 10.0
Reoperation/interventional catheterization 23.4 19.3 17.5 11.1 18.0
Unplanned reintubation 34.9 16.4 15.6 10.3 14.0
Cardiac ICU readmission 4.7 8.6 11.4 7.8 6.0
Transfer to other hospital 44.2 19.3 11.4 6.6 2.0
vessels. In these patients, brain matu-ration, as assessed by the structural appearance of the cerebral cortex on MRI, is delayed by ⬃1 month.14,15 It is
plausible that such neonates, whose brains at the time of delivery at 37 to 38 weeks are structurally similar to those of premature infants delivered at 34 to 35 weeks, may be predisposed to neu-rologic injury before, during, and after major cardiac interventions during the first few days of life. Such a predispo-sition to injury may contribute to ad-verse late neurodevelopmental out-comes.16Additional work is needed to
elucidate the developmental changes of the central nervous system, lungs, and other organ systems in the fetus during the final weeks of gestation.
Our findings have important patient-management implications for perina-tologists and fetal cardiologists. In the absence of clear indications that deliv-ery is necessary, greater efforts seem warranted to extend the duration of pregnancy in a woman who presents in early labor before 39 completed weeks’ gestation and is carrying a fe-tus with critical congenital heart dis-ease. When the decision is made to electively deliver such fetuses, our data suggest that every additional week in utero is important, and waiting until 39 weeks may lead to better out-comes. We recognize that this change in practice may have practical implica-tions for pregnant women who live far from a center that treats congenital heart disease in neonates, because the
precipitous delivery of a fetus with crit-ical congenital heart disease in a re-mote hospital may lead to adverse out-comes. Clearly these data should not be used to guide management in all clinical scenarios. For example, fe-tuses with severe Ebstein malforma-tion of the tricuspid valve and evolving hydrops fetalis have a high rate of in utero demise and possibly may benefit from earlier delivery.17Our data also
have implications for outcomes re-search in neonates with critical con-genital heart disease. Investigators who conduct outcomes studies should consider exact gestational age of neo-nates rather than birth weight or the presence or absence of prematurity.
This study had several limitations. The associations we identified between gestational age and the outcomes of interest do not prove causality. How-ever, the patterns we found for mor-bidity and mortality were consistent with but amplified compared with those recently documented in larger samples of infants without birth de-fects.3–5,9,10There is inherent potential
for inaccuracy in the assignment of gestational age by obstetric providers. We do not have data from our referring perinatal centers regarding the inci-dence of in utero demise in fetuses with congenital heart disease, and such data would be difficult to obtain given the broad referral base of our program. However, the risk of in utero demise during late gestation must be considered by those who determine
the timing of delivery for any fetus. A more detailed understanding of the various pregnancy-related disorders, such as placental insufficiency, that in-fluenced the timing of nonelective de-liveries would have provided us with additional insight, but the gathering of such information would require a pro-spective study.18 The clinical
signifi-cance likely differs between the indi-vidual events that were included in our composite morbidity outcome vari-able. These findings may not be gener-alizable to all infants with major
non-cardiac birth defects, and such
patients warrant additional study.19
Fi-nally, our findings may not be general-izable to other institutions, and multi-center studies are needed.
CONCLUSIONS
Delivery before 39 or after 40 weeks’ completed gestation was associated with increased adjusted mortality rates in neonates with critical congen-ital heart disease. Perinatologists and fetal cardiologists must carefully con-sider these data when they determine the timing of delivery for these pa-tients. Birth before 39 weeks was also associated with greater morbidity and resource use. The ideal age for deliv-ery for these patients may be when they have reached 39 to 40 completed weeks’ gestation.
ACKNOWLEDGMENT
This study was supported in part by the Rochelle E. Rose Clinical Research Fund.
REFERENCES
1. Fyler DC, Buckley LP, Hellenbrand WE. Re-port of the New England Regional Infant Car-diac Program. Pediatrics. 1980;65(2): 377– 461
2. Schultz AH, Localio AR, Clark BJ, Ravishan-kar C, Videon N, Kimmel SE. Epidemiologic features of the presentation of critical con-genital heart disease: implications for screening.Pediatrics.2008;121(4):751–757
3. Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G. Mortality of late-preterm
(near-term) newborns in Utah.Pediatrics.2007; 119(3). Available at: www.pediatrics.org/ cgi/content/full/119/3/e659
4. Tita AT, Landon MB, Spong CY, et al. Timing
of elective repeat cesarean delivery at term and neonatal outcomes.N Engl J Med.2009; 360(2):111–120
5. Zhang X, Kramer MS. Variations in mortality and morbidity by gestational age among
in-fants born at term.J Pediatr.2009;154(3): 358 –362
6. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for sur-gery for congenital heart disease.J Thorac Cardiovasc Surg.2002;123(1):110 –118
7. Tanner K, Sabrine N, Wren C. Cardiovascular malformations among preterm infants. Pe-diatrics. 2005;116(6). Available at: www. pediatrics.org/cgi/content/full/116/6/e833
8. Madar J, Richmond S, Hey E. Surfactant-deficient respiratory distress after elective
9. De Luca R, Boulvain M, Irion O, Berner M, Pfister RE. Incidence of early neonatal mor-tality and morbidity after late-preterm and term cesarean delivery.Pediatrics.2009; 123(6). Available at: www.pediatrics.org/ cgi/content/full/123/6/e833
10. Cheng YW, Nicholson JM, Nakagawa S, Bruckner TA, Washington AE, Caughey AB. Perinatal outcomes in low-risk term preg-nancies: do they differ by week of gestation? Am J Obstet Gynecol.2008;199(4):370.e1– 370.e7
11. Dorfman AT, Marino BS, Wernovsky G, et al. Critical heart disease in the neonate: pre-sentation and outcome at a tertiary care center.Pediatr Crit Care Med.2008;9(2): 193–202
developmentally regulated in the fetus and
newborn.Am J Physiol. 1993;265(4 pt 2): H1056 –H1063
13. Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Se-min Perinatol.2006;30(1):34 – 43
14. Miller SP, McQuillen PS, Hamrick S, et al.
Abnormal brain development in newborns with congenital heart disease.N Engl J Med. 2007;357(19):1928 –1938
15. Licht DJ, Shera DM, Clancy RR, et al. Brain
maturation is delayed in infants with com-plex congenital heart defects.J Thorac Car-diovasc Surg.2009;137(3):529 –536
16. Wernovsky G. Improving neurologic and
quality-of-life outcomes in children with
135(2):240 –242, 242.e1–242.e2
17. McElhinney DB, Salvin JW, Colan SD, et al. Improving outcomes in fetuses and neo-nates with congenital displacement (Eb-stein’s malformation) or dysplasia of the tricuspid valve.Am J Cardiol.2005;96(4): 582–586
18. McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epide-miologic approach to classification.Am J Epidemiol.2008;168(9):980 –989
DOI: 10.1542/peds.2009-3640 originally published online July 5, 2010;
2010;126;277
Pediatrics
and Peter C. Laussen
Cohen
Graham, Ravi R. Thiagarajan, Emile A. Bacha, Catherine K. Allan, Jennifer N.
John M. Costello, Angelo Polito, David W. Brown, Thomas F. McElrath, Dionne A.
Neonates With Heart Disease
Birth Before 39 Weeks' Gestation Is Associated With Worse Outcomes in
Services
Updated Information &
http://pediatrics.aappublications.org/content/126/2/277
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/126/2/277#BIBL
This article cites 16 articles, 3 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/neonatology_sub
Neonatology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2009-3640 originally published online July 5, 2010;
2010;126;277
Pediatrics
and Peter C. Laussen
Cohen
Graham, Ravi R. Thiagarajan, Emile A. Bacha, Catherine K. Allan, Jennifer N.
John M. Costello, Angelo Polito, David W. Brown, Thomas F. McElrath, Dionne A.
http://pediatrics.aappublications.org/content/126/2/277
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.