Jayaprabha K.N. and Suresh K.K.
1Kizhakkeveettil (H), Thrikkalathoor P.O.,
Muvattupuzha, Kerala – 683541 2Assistant Professor, Department of Zoology,
NSS College, Pandalam, Kerala - 689501 sureshkk1@gmail.com
(Received on: February 25, 2016)
ABSTRACT
Endosulfan is one of the known highly toxic organochlorine pesticides. The global ban on the manufacture and use of endosulfan was recommended by The Stockholm Convention held in April 2011 because of its adverse effects on human health and the environment. This report discusses about the pollution caused by endosulfan in rural areas of Kasargod district in Kerala where Plantation Corporation of Kerala started aerial spraying of the pesticide in the 1970s. Until the year 2000, more than 50,000 villagers of this district have been exposed to endosulfan, and over 3000 were affected by rare diseases like mental retardation, cerebral palsy, cancer etc. The reports from various government authorities and non-government organizations confirm the presence of endosulfan in water and soil in the exposed areas. The exposure and use of endosulfan contaminated food and water is said to cause endocrine disruptions, reproductive system disorders, central nervous system disorders, liver and kidney dysfunctions in animals and human beings. There are literature reports on the removal of endosulfan from water and soil using suitable adsorbents which mainly uses carbon derived from various sources. Here we discuss about the use of different nanocomposite for endosulfan adsorption and removal.
Keywords: Endosulfan, Method for its Removal.
1. Introduction
Fig.1 Endosulfan: Chemical structure
Endosulfan has two stereoisomers, α and β, form in the ratio 7:3, being highly toxic to fish and aquatic systems due to its low water solubility.2 Endosulfan enters the air, water and soil during its manufacture and use. It has different environmental fate depending on the medium it gets exposed to. Both the isomers of endosulfan undergo photolysis upon exposure to light to give endosulfan diol with a half life of about 7 years. In water, the hydrolysis product is the same but the rate depends on pH. In soil, it undergoes biodegradation forming endosulfan sulfate which is equally toxic and more persistent in the environment than the parent compound. The half life varies from 60 days for alpha form to 800 days for beta form. In water, endosulfan has a half life of about 35 to 150 days. It does not easily dissolve in water and may accumulate in bodies of fish and other aquatic organisms.3 It is readily absorbed by stomach, by lungs and through skin.
Endosulfan is widely found in both surface water and groundwater throughout the world, including in India.4 World Health Organization drinking water quality standards permits a maximum 20 μg/L for endosulfan. According to the Bureau of Indian Standards, 2012, the permissible limit of endosulfan in drinking water is 0.4μg/l. According to the United States Environmental Protection Agency (EPA), the maximum permissible limit for endosulfan in lakes, rivers and streams is 74μg/L.5 EPA classifies endosulfan as Category Ib – Highly Hazardous. The European Union also rates it as Highly Hazardous. World Health Organisation (WHO) classifies endosulfan in Category II – Moderately Hazardous. The Industrial Toxicological Research Centre (ITRC) in India, the nodal centre for the Regional Based Assessment of Persistent Toxic Substances (PTS) for the Indian Ocean region by the United Nations Environment Programme-Global Environment Facility (UNEP-GEF), classifies endosulfan as Extremely Hazardous.6 India is the fourth largest producer of pesticides in the world and the world’s largest producer and user of endosulfan, the major companies being Excel industries, Hindustan Insecticides Limited (HIL) and EID Parry.7
II. A few examples of Endosulfan poisoning around the world
1990 to 1993. In Columbia, around 200 cases of poisoning due to endosulfan were reported. In 2004, 36 persons of all age groups in a rural area of Jabalpur, India were poisoned after consuming wheat-grains or flour contaminated with endosulfan.8
III. Endosulfan poisoning in Kasargod, Kerala (India)
Kasaragod, the northern most district in Kerala (India) has about 5600 acres of cashew plantations, owned by the Plantation Corporation of Kerala (PCK), spreading through about 11 village panchayats. These villages are Enmakaje, Belur, Kumbadaje, Badiadka, Muliyar, Karadukka, Periya, Pullur, Ajanur, Kallar, Panathady, Kayyur, Cheemeni. The plantations were aerially sprayed with the chemical pesticide endosulfan since 1976, till 2001, regularly three times a year. As a result of public outcry, intervention from some courts and the government, spraying was stopped from 2003.9 Figure 2 is a map showing the cashew plantation area under PCK.
Fig.2: Map showing the PCK plantation area (Kasargod district, Kerala) and the surrounding affected areas.10
Table 1: Confirmed cases of various diseases in Kasargod district due to endosulfan spraying.
Diseases Number of cases
Cancer 49
Mental retardation 23
Congenital anomalies 9
Psychiatric cases 43
Epilepsy 23 Suicide 9 Total 156
After the Bhopal gas tragedy, Endosulfan spraying in Kasargod was the biggest chemical tragedy. Government of Kerala appointed 11 commissions to study the issue. Apart from this, various Non-government Organizations (NGOs) have put their efforts in analyzing the role of endosulfan in causing the abnormalities.12 The results obtained by various committees are discussed below.
The concentration of endosulfan residues in water in the affected villages of Kasargod were analysed by Centre for Science and Environment (CSE), New Delhi, on 17th February 2001, one and half month after the last aerial spray carried out on 26th December 2000. The amount of endosulfan was found to be about 7 to 51 times higher than the Maximum Residual Limit (MRL). The amount in one of the soil sample studied was 391 times larger than the MRL.11 The CSE study also revealed that about 189 parts per million (ppm) of endosulfan is present in blood samples.
Well 0.0086 0.0088 0.0035 0.0209
Suranga 0.0065 0.0022 Not detected 0.0087
Stream 0.0081 0.0123 Not detected 0.0204
Pond 0.0138 0.0416 0.0113 0.0667
Table 3: Endosulfan residue found in blood samples of school children. (NIOH report)14
Blood samples of children from Vaninagar school
Total endosulfan residue (ppb)
Code1 78.74
Code 4 28.44
Code 6 48.09
Code 104 33.57
In 2001, as a result of the appeal from a victim, Smt. Leela Kumari Amma, the Munsif Court of Hosdurg temporarily stayed the aerial spraying of endosulfan. Later in 2003, the High Court of Kerala permanently stopped the spraying of endosulfan.15 Eventhough the aerial spraying of endosulfan is banned in Kerala, there are rural areas in India which suffer from the side effects of this pesticide. In January 2011, the Down to Earth magazine published by Centre for Science and Environment, reported the sufferings of rural people in Dakshina Kannada, in the state of Karnataka, a neighboring place of Kasargod, Kerala, due to the aerial spraying of endosulfan.
IV. Removal of Endosulfan from water
pollutants, especially aromatic ones, are reported by various research groups using carbon nanotubes, fullerenes and their modified forms.20 There are literature reports on the removal of organic pollutants like benzene, toluene, trihalomethanes, 1,2-dichlorobenzene using materials such as multi-walled carbon nanotubes (MWCNTs), single-walled carbon nanotubes (SWCNTs), graphitized carbon nanotubes, fullerenes etc.21 Several methods like chemical oxidation with ozone, photo degradation, combined ozone and UV irradiation, biological degradation, and adsorption have been used for the removal of pesticides from water.22,23
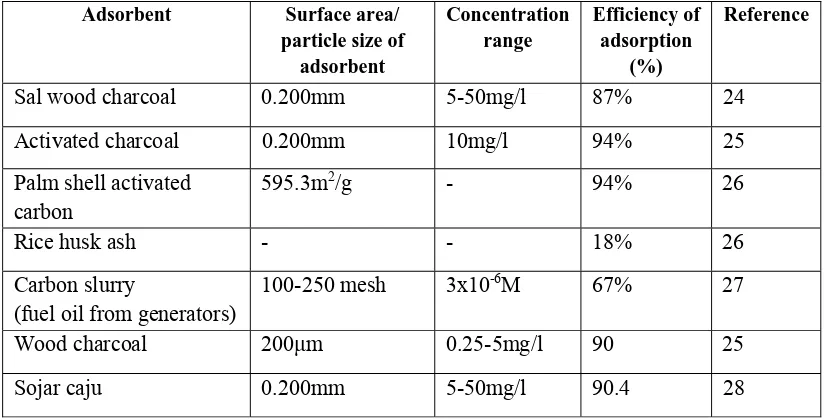
Table 4: A brief description of the adsorbents used so far for the removal of endosulfan from water
Adsorbent Surface area/
particle size of adsorbent
Concentration range
Efficiency of adsorption
(%)
Reference
Sal wood charcoal 0.200mm 5-50mg/l 87% 24
Activated charcoal 0.200mm 10mg/l 94% 25
Palm shell activated carbon
595.3m2/g - 94% 26
Rice husk ash - - 18% 26
Carbon slurry
(fuel oil from generators)
100-250 mesh 3x10-6M 67% 27
Wood charcoal 200μm 0.25-5mg/l 90 25
Sojar caju 0.200mm 5-50mg/l 90.4 28
Organic pollutants, especially pesticides, can be effectively detected using metal nanoparticles. The UV-visible absorption spectra of gold nanoparticles show considerable change when reacts with endosulfan.21 The biodegradation of endosulfan is another efficient pathway for the detoxification of this product.22 Even though gold nanoparticles can be used for the detection of endosulfan, the method is expensive as it deals with noble metals. In the present scenario, it is really difficult to extract endosulfan using such nanomaterials. Surfactants like Triton X100, tween, surfactin etc. has affinity towards endosulfan, which has been used for the separation of endosulfan from soil.1
byproduct of endosulfan and is more toxic and persistent than the parent compound.
Silica is chosen as an adsorbent because it adsorbs endosulfan with better efficiency than other adsorbents. As shown in table 2, only activated charcoal shows an adsorption capacity comparable with silica (90%).28 But source of charcoal is costly compared to the source of silica.
The rice husk ash is a source of silica is shown in Figure 3.
Fig.3 : Rice husk (left) and rice husk ash (right) - source of silica
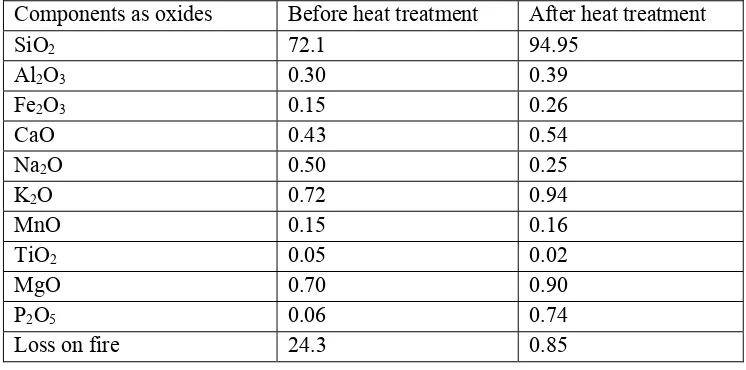
Rice husk ash (RHA) is mostly composed of silica. According to food and Agriculture organization of United Nations, the amount of rice produces per year is about 600 million tones and the rice husk is estimated to be 10 million tonnes. From the literature, it is clear that rice husk ash is an alternative source for silica. A 95% silica powder can be obtained by calcination at 700 oC for 6hours.29
Table 5: The composition of rice husk ash before and after calcinations
Components as oxides Before heat treatment After heat treatment
SiO2 72.1 94.95
Al2O3 0.30 0.39
Fe2O3 0.15 0.26
CaO 0.43 0.54
Na2O 0.50 0.25
K2O 0.72 0.94
MnO 0.15 0.16
TiO2 0.05 0.02
MgO 0.70 0.90
P2O5 0.06 0.74
The raw material can be obtained by burning rice husk obtained during the production of rice. The first step is to produce high specific surface area silica or active silica from rice husk ash by thermal treatment at different temperatures. Silica can be extracted from rice husk ash by the method developed by Combustion Gasification and Propulsion Laboratory, Indian Institute of Science, Bangalore.30 In this method of silica precipitation, the chemicals used can be regenerated making this method economical. The method involves three steps: digestion, precipitation and regeneration.
Digestion: In this step, rice husk ash is treated with sodium hydroxide, which results in the formation of sodium silicate. The resultant solution is filtered to remove undigested ash in the solution.
Precipitation: The sodium silicate solution is treated with carbon dioxide under continuous stirring to precipitate silica. The precipitated silica is filtered, washed and dried. The filtrate containing silica and sodium carbonate is further treated for regeneration.
Regeneration: The sodium carbonate present in the solution is converted to sodium hydroxide by treatment with calcium hydroxide. The solution on filtration gives calcium carbonate, and aqueous sodium hydroxide is used for digestion again.
The silica obtained is a white fluffy powder with purity of >98% and a surface area of 50-200m2/g. The size of the particles in silica will be in the nanoregime and can be varied by changing the initial heating temperature. The silica yield increases with increase in temperature and amount of caustic soda. The surface area, density etc. can be tuned by varying the precipitation conditions. The obtained silica can have quality comparable with that available in the market and the estimated cost for extracting silica is Rs. 30/kg, whereas reagent grade sodium silicate solution costs around Rs.3300 per litre.
V. Conclusion
From the above discussions it is clear that the villages in/near PCK area of Kasargod are polluted with endosulfan, which is highly toxic to the human community. The recent report which came in the journal Current Science clearly mentions the presence of endosulfan in soil and sediment samples collected from some of the affected panchayats of Kasargod district.5 Hence, there is a sure chance that the water in the affected areas also may be contaminated with this pesticide. Since the exposure to endosulfan-contaminated air, water and soil affects the immune, reproductive, endocrine and nervous systems of human beings, it is always better to purify water before use.
be an efficient method which is economically bearable by the rural people.
References
1. Jayashree R., Vasudevan N. and Chandrasekaran S., (2006). Surfactants enhanced recovery of endosulfan from contaminated soils, International Journal of Environmental Science and Technology, 3: 251.
2. ATSDR (1993) Toxicological profile for endosulfan. United States Agency for Toxic Substances and Disease Registry, Atlanta, USA.
3. Shivaramaiah H. M., Sanchez-Bayo F., Al-Rifai J. and Kennedy I. R., (2005) The Fate of Endosulfan in Water, Journal of Environmental Science and Health Part B, 40:711. 4. Quinete N., Castro J., Fernandez A., Zamora-Ley I. M., Rand G. M. and Gardinali P. R.,
(2013) Occurrence and distribution of endosulfan in water, sediment, and fish tissue: An ecological assessment of protected lands in South Florida, Journal of Agricultural and Food Chemistry, 61.
5. Harikumar P. S., Jesitha K., Megha T. and Kamalakshan Kokkal, (2014) Persistence of endosulfan in selected areas of Kasaragod district, Kerala, Current Science, 106:10. 6. Harikrishnan V. R. and Usha S., (2004) Endosulfan - A fact sheet and answers to common
questions, IPEN Pesticide Working Group Project.
7. Usha S., (2005) A will to kill - Role of pesticide regulators in the endosulfan tragedy in Kerala Newscape, 2:1.
8. End of the road for endosulfan-A call for an action against dangerous pesticide, A summary report by Environmental Justice Foundation.
9. Report on monitoring of endosulfan residues in the 11 panchayaths of Kasargod district, Kerala, Kerala State Council for Science, Technology and Environment, June 2011. 10. Embrandhiri A., Singh R. P., Ibrahim H. M. and Khan A. B., (2012)An epidemiological
study on the health effects of endosulfan spraying on cashew plantations in Kasargod District, Kerala, India, Asian Journal of Epidemiology, 5: 22.
11. A Centre for Science and Environment report on the contamination of endosulfan in the villagers, (February 28, 2001) Full report in Down to Earth, 9:19.
12. Preliminary findings of the survey on the impact of aerial spraying of endosulfan on the people and ecosystem in Kasaragod, Keralam, India, (February 2002) Report by Thanal Conservation Action & Information Network Thiruvananthapuram, Keralam, India. 13. Final report of the “Investigation of unusual illnesses allegedly produced by endosulfan
exposure in Padre Village of Kasargod District (N.Kerala)”, (July 2002) by National Institute of Occupational Health (Indian Council of Medical Research).
15. Report on health effects of endosulfan and progress of rehabilitation activities in Kerala, (April 2011) by Department of Health and Family Welfare, Government of Kerala. 16. Matlochova A., Placha D. and Rapantova N., (2013) The application of nanomaterials in
groundwater remediation, Polish journal of environmental studies, 22:5.
17. Hseih S. H. and Horng J. J., (2007)Adsorption behavior of heavy metal ions by carbon nanotubes grown on microsized Al2O3 particles, Journal of University of science and technology Beijing, 14:77.
18. Whang H., Zhou A., Peng F., Yu H. and Yang J., (2007) Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II), Journal of colloids and interfacial science, 316: 277.
19. Bystrzejewski M., Pyrzynska K., Huczko A. and Lange H., (2009) Carbon-encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions, Carbon, 47:1201.
20. Peng X., Li Y., Luan Z., Di Z., Wang H., Tian D. and Jia Z., (2003) Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes, Chemical physics letters, 376:154. 21. Nair A.S., Tom R.T. and Pradeep T., (2003) Detection and extraction of endosulfan by
metal nanoparticles”, Journal of Environmental Monitoring, 5:2.
22. Kong L., Zhu S., Zhu L., Xie H., Su K., Yan T., Wang J., Wang J., Wang F. and Sun F., (2013) Biodegradation of the organochlorine pesticide endosulfan by the bacterial strain
Alcaligenes faecalis JBW4, Journal of Environmental Sciences, 25:11.
23. Enjarlis and Ratnavati, (2011) Kinetics and identification of products degradation of endosulfan in a solution of carbofuran by hydrolysis, ozonation with and without activated carbon, International Journal of Chemical Engineering and Applications, 2:1.
24. Mishra P.C. and Patel R.K., (2008) Removal of endosulfan by sal wood charcoal, Journal of Hazardous Materials, 152:2.
25. Sudhakar Y. and Dikshit A. K., (2008) Removal of endosulfan from water using wood charcoal adsorption and desorption, Journal of Environmental Engineering, 134.
26. Lim Y. N., Shaaban M. G. and Yin C. Y., (2008) Removal of endosulfan from water using oil palm shell activated carbon and rice husk ash, Journal of Oil Palm Research, 20. 27. Gupta V. K. and Ali I., (2008) Removal of endosulfan and methoxychlor from water on
carbon slurry, Environmental Science and Technology, 42.
28. Sudhakar Y., Dikshit A. K., (1999) Adsorbent selection for endosulfan removal from water environment. Journal of Environmental Science and Health B., 34.
29. Della V.P., Kühn I. and Hotza D., (2002) Rice husk ash as an alternate source for active silica production, Materials Letters, 57.