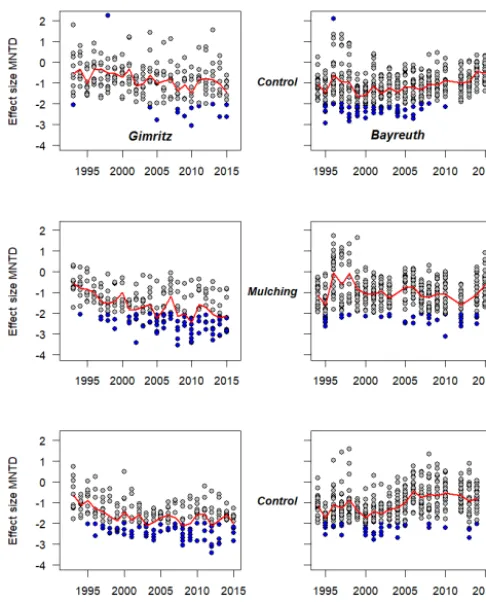
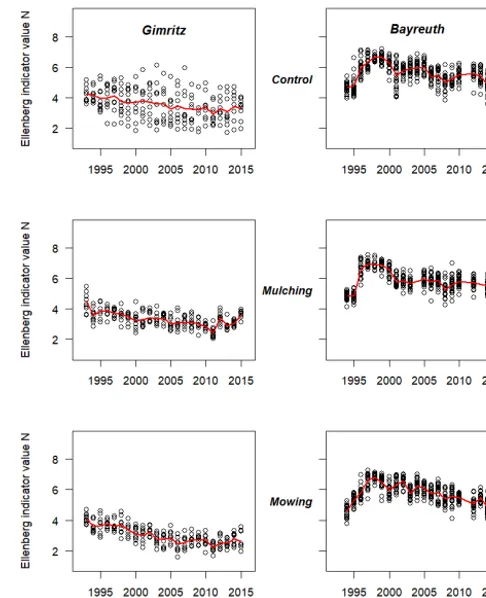
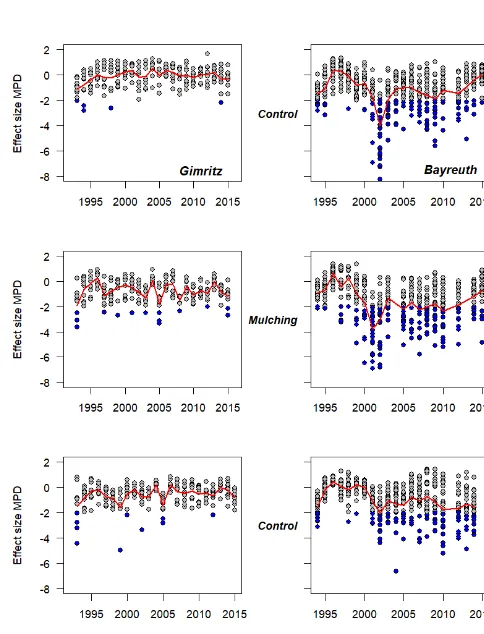
Species richness and phylogenetic structure in plant communities: 20 years of succession
Full text
Figure


Related documents
Although ontology is a kind of data model, the UML metamodel, which is a rich in the purpose of representing data, widely used, and well supported with software tools, was not
Here we show that each codon as well as each nucleo- tide in cellular genomes follows a very similar trend when GC content varies (Figures 2, 3, 4 and Additional files 2, 3, 4),
Se informa sobre este caso para recordar el peligro potencial de este pro- cedimiento y Ia necesidad de proceder con cuidado en su ejecuci#{243}n. Este m#{233}todono debera emplearse
A system of major characteristics indicators for rural household energy consumption includes effective heat consumption/or livelihood per capita per day (EHC), the share
After successfully supporting the development of the wind power technology, an approach is needed to include the owners of wind turbines in the task of realizing other ways, other
En fait, il suffit qu'il yait initialement (avant une nouvelle integration verticale) moins de firmes integrees que de firmes independantes sur Ie marche amont pour que Ie
In conclusion, the finding of this cross-sectional study of pre and post menopausal women show no evidence of a significant difference in BMD between Ocs users and never user
The aim of this study is to evaluate the incidence and severity of postoperative swelling and pain following mandibular third molar surgery using PRF as a healing material in