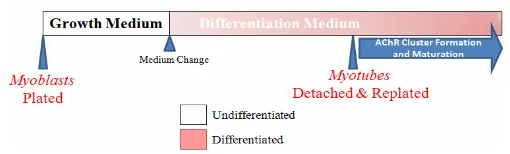
Cell culture-based analysis of postsynaptic membrane assembly in muscle cells
Full text
Figure



Related documents
Field experiments were conducted at Ebonyi State University Research Farm during 2009 and 2010 farming seasons to evaluate the effect of intercropping maize with
The scattergram represents the distribution with age of 69 determinations of concentration of potassium in serum of 39 premature infants with respiratory distress syndrome (Table
19% serve a county. Fourteen per cent of the centers provide service for adjoining states in addition to the states in which they are located; usually these adjoining states have
Many operations have been described for the correc- tion of claw hands following involvement in leprosy, from Sir Harold Stiles (1922) of Edinburgh, through Bunnell, Brand and
Since section 3004 has been amended to extend corrective action re- quirements to all solid waste management units at facilities seeking a RCRA permit, the
ON THE APPEARANCE OF PRIMES IN LINEAR RECURSIVE SEQUENCES JOHN H JAROMA Received 16 August 2004 and in revised form 5 December 2004 We present an application of difference equations
In summary, it is clear that horizontal and vertical position disparities, orientation disparity and spatial frequency disparity are used by the visual system in the
There are different media systems in the world as there are countries. What primarily determines the media system a country adopts and practices is largely dependent