International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 4, Issue 3, March 2014)
11
Use of Glass Particles for Development of Low Cost
Aluminium Matrix Material
Dr. Vandana J. Rao
1, Anil Parmar
2 1Associate Professor, Department of Metallurgical and Materials Engineering, Faculty of Technology & Engineering, The M. S. University of Baroda, Vadodara Gujarat, India
2Assistant Professor, Dept. of Mechanical Engg., ITM Baroda Abstract-Aluminum matrix composites (AMCs) are
lightweight high performance material. In the present work attempt made to prepare Al-Glass powder base composites for automobile field where high hardness and strength are important. Prepared composites have composition ranging from 3-7 wt % glass reinforcement which produced by low cost, stir cast route by using commercially pure Aluminum as matrix material. Two types of attempts made to prepare the composite one is the direct addition of glass particles & other is by using aluminum foil for addition of glass particles. The prepared composite shows increases in mechanical properties like hardness and tensile strength. Hardness increases from 24 to 72 BHN and Tensile value increased from 80 to 136 N/mm2, due to change in microstructure of samples. Along with increasing mechanical properties, the ductility values observed are in the range of 25 to 30 % by varying the wt% of glass particles.
Keywords--Aluminum, Glass particles, Stir cast method, Microstructure, Tensile, Hardness and Ductility.
I. INTRODUCTION
Composites are attractive with their special properties and low cost and high chemical resistance. [ 1, 2] The use of glass particles presents some important advantages like low cost, in comparison with fibers , ease of dispersion into the matrix and possibility to obtain isotropic properties etc. [3] There is an increasing interest in the development of metal matrix composites (MMCs) having low density and low cost of reinforcements. Although these MMCs have better properties including high strength, high stiffness and better wear resistance but their usage is limited due to their high manufacturing cost. Among the various discontinuous reinforcements used, glass particulate is one of the most inexpensive and low-density reinforcement. Incorporation of glass particles reduces the cost and density of aluminum and its alloys. Aluminum and its alloys are used in various industries like aerospace, engineering fields as internal combustion engines, machines, thermal control, electronic padding, automobile as breaks, drive shafts, flywheels, tank pressure vessels etc.[4,5] Some innovative changes can wider the useful range of aluminum materials.
In this article idea is to prepare the glass reinforced commercially pure aluminum by stir cast route than characterize it by the study of microstructure, hardness and tensile.
II. EXPERIMENTAL WORK
The preparation of Aluminium matrix composite in the present study carried out by stir cast method. Commercially pure aluminum melted at 710 0C in graphite crucible. Care taken to produce sound liquid metal like to avoid oxidization, used the flux and hexachloro-ethane tablet for degassing, before addition of preheated glass particles. After glass particles addition, melt stirred for 5 min. The stirred melt poured into the metallic die in the forms of rods (20mm diameter and 160mm height). The melt solidified in the metallic die. Microstructural characterizations include optical (Neophote 2) & SEM (Jeol JSM 5610LV model scanning electron microscope) study. Mechanical test conducted by using Brinell hardness and Monsento tensile testing machine. Hardness values calculated by using Brinell hardness tester as per ASTM E-10:2008. We performed test with Indenter Dia. 2.5mm in size and with 31.250 kg load. To avoid the segregation of particles eight readings taken for each sample and average value reported. Tensile test conducted on round sample 42mm long and 5mm dia with 25mm gauge length. Tensile speed maintained 0.05 mm/min until the failure. Elongation properties evaluated at same sample by using standard gauge. Following figure 1 indicate tensile test specimen.
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 4, Issue 3, March 2014)
12
III. RESULT AND DISCUSSION
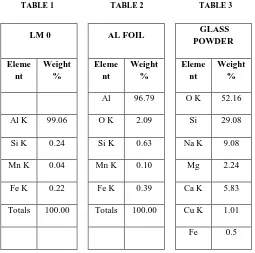
[image:2.612.340.546.135.308.2]Following table 1 indicates the chemical composition of commercial pure aluminum (LM 0). Commercially pure aluminium used to prepare composite because it is easy to understand the effect of addition of glass particles in it. Table 2 indicates composition of aluminum foil, which used for addition of glass particles as a holder and table 3 indicates chemical composition of glass powder.
TABLE 1 TABLE 2 TABLE 3
LM 0 AL FOIL GLASS
POWDER
Eleme nt
Weight %
Eleme nt
Weight %
Eleme nt
Weight %
Al 96.79 O K 52.16
Al K 99.06 O K 2.09 Si 29.08
Si K 0.24 Si K 0.63 Na K 9.08
Mn K 0.04 Mn K 0.10 Mg 2.24
Fe K 0.22 Fe K 0.39 Ca K 5.83
Totals 100.00 Totals 100.00 Cu K 1.01
Fe 0.5
The commercially pure aluminium contains impurities like Si, Mn, Fe and Mg etc in minor quantities. Aluminium foils also contains impurities like Si, Mn and Fe in different amount. The glass particles are oxides mixture of Si, Ca, Mg and Cu. Figure 2 shows the size and shape of glass particles. The average size of glass particles are around 25 micrometers with more or less oval in shape.
Figure 2 SEM image represent the shape and size of glass particles By using above materials, composites prepare with and without using foil. Foil reflects different chemical composition than commercially pure aluminium. The amount of Si in foil is around 0.63 Wt % which is higher than commercially pure aluminium. However, the use of foil is only 5 gram that is quite low compare to raw matrix material. Thus, the change of variation in chemistry of final composite due to use of foil is very low. Glass particles have Si around 29.08, Na is 9 wt%, Ca 6wt%, Mg is 2 wt%, Cu is around 1wt% and Fe is around 0.5wt% in form of their oxides. Chances of reaction of oxides of all say Na, Ca, Mg and Cu metal with liquid commercially aluminium is quite less compare to Fe and Si in its oxides forms. The thermodynamic data’s at 7270
C for possible reactions are like,[6,7,8]
3SiO2 + 4Al → 2Al2O3 + 3Si;
ΔG0 = -532.2KJ……… (1)
Fe2O3 + 2Al → Al2O3 + 2Fe;
[image:2.612.42.297.236.489.2]International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 4, Issue 3, March 2014)
13
The reaction (2) has more chance to release free Fe than reaction (1) because of more negative ΔG0 value (free energy value). The release Fe and Si change the chemistry of glass powder added system. The release oxygen by reduction of SiO2 and Fe2O3 as per equation (1) and (2) immediately react with aluminium and produce the product of small size Al2O3 particles. In-situ Al2O3, distributed uniformly into the matrix and increases the properties of various glass-reinforced systems. The x-ray analysis may confirm the same. The release ‘Si’ and ‘Fe’ depends on the way of addition of glass powder. In case of foil enveloped glass powder, the time for reaction and controlled over reaction is more and hence the wt % release of Si and Fe increases with increase in wt % glass powder.
The released Si and Fe finally control the tensile property of all systems. To check the alternation of chemistry of prepared composites system after addition of 3 Wt%, 5 Wt% and 7 Wt% with and without using foil is represented in table 4. Following table 4 indicates the various chemical compositions of Al-Glass composites with 3-5-7 wt % of glass powder. The % recovery of Si and Fe increases by using foil because it increases the reaction time and protect the glass particles flotation on molten metal. The % Si recovery increases more than Fe because the % Si oxides are more than Fe oxides in the glass powder. The other alloying elements are in the range of same as that in commercially pure aluminium. The x-ray analysis of solid samples confirms the results. The un-reduced other oxides of glass particles and in-situ Al2O3 confirms by the presence of oxygen along with other elements.
TABLE 4
CHEMICAL ANALYSIS OF DIFFERENT SYSTEMS
Systems Al Si Mg Fe Mn O
Commercially
pure Al 99.06 0.24 0.26 0.22 0.04
Al-3% Glass
Powder 91.39 0.30 0.35 0.40 0.038 7.40
Al-3% glass powder with
foil
93.35 0.48 0.43 0.29 0.023 5.40
Al-5% Glass
Powder 95.30 0.38 0.27 0.40 0.044 3.64
Al-5% Glass Powder with
foil
93.21 1.74 0.42 0.35 0.032 4.59
Al-7% Glass
Powder 90.23 0.36 0.45 0.32 0.028 8.65
Al-7% Glass Powder with
foil
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 4, Issue 3, March 2014)
14
[image:4.612.325.560.135.335.2]The hardness value varies from 24 to 73 BHN. Tensile value varies from 80 N/mm2 to 136 N/mm2, and % elongation varies from 10 % to 30% generally. By increasing hardness and tensile strength value there should be a decreasing in % elongation (ductility) but here the reinforcement behavior of glass particles are quite different then direct addition of alloying elements. Following table 5 shows the hardness and tensile strength of various glass-reinforced composites.
TABLE 5
HARDNESS (BHN) AND TENSILE STRENGTH (MPA)
[image:4.612.43.295.259.545.2]Following figure, 3 indicate the optical microstructure of various glass particle added system with and without using foil.
Fig 3 Optical microstructure of glass added system with and without using foil at 100X without using etchant
It is observed from the figure that the released Si, Fe and in-situ Al2O3 were dispersed uniformly in the commercially pure aluminum matrix. The size of the phase appears to be uniform throughout the matrix in the forms of black small dots at grain boundary and in the grain. The uniform distribution attributed due to the effective stirring action and the use of appropriate process parameters. Homogeneous distribution enhances the mechanical properties of the matrix alloy. It may notice that a good adhesion between the matrix and the generated phase observed. Generally when the solidification front meets on insoluble particles, two situations can appear. (a)The solidified material retains the particles, which remain inside the crystalline grains. (b)The solidified material does not include the particle from the beginning, they bring pushed into the liquid phase. [9,10] Both the phenomena observed here like some black particle like phase is present at centre of the grains and some are at the end of grains as a grain boundary. The same phenomena represent in the SEM (BSE mode) analysis of various glass particle added system at 270X magnification. Following figure no. 4 shows the SEM (BSE) micrograph.
Pro
p
er
ties
Amount of Glass Powder
Co m m er cia l p u re a lu m in iu m (LM 0 ) G la ss p o wder (3 %) G la ss p o wder (with fo il ), (3 %) G la ss p o wder (5 %) G la ss p o wder (with fo il ), (5 %) G la ss p o wder (7 %) G la ss p o wder (with f o il ), (7 %) Hardness (BHN)
24 47 56 60 73 62 78
Tensile Strength
(Mpa)
70 93 109 99 136 109 125
Elongation
(%)
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 4, Issue 3, March 2014)
[image:5.612.56.284.139.341.2]15
Fig 4 SEM microstructure of Aluminum-glass compositemicrostructures at 270X
The microphotograph indicates the presence of some phase as network like grain boundary and some are at the centre of the grains. Three different colors indicate the three different phases like major gray is of aluminium base metal, white lines for segregations of heavy elements generated due to reaction between glass particles and liquid aluminium metal. The last is of dark black particles are of generates oxides of Al2O3 and may be of other oxide phase. In presence of foil, the quantification can easily indentify in the SEM micrograph, which is not possible to observe in optical micrograph.
IV. CONCLUSIONS
(1) By addition of glass particles hardness value increases in both the conditions with and without using, foil. (2) Hardness value increases more from 24 to 78 BHN by
using different amount of glass powders with using foil.
(3) Tensile strength value increases from 81 to 110N/mm2 with using foil.
(4) % of elongation decreases from 42 to 30% by adding the glass particle but the values are quite higher than the other cast aluminium alloys.
(5) % of silicon recovery increases maximum 1.74% by using foil.
Acknowledgment
Authors like to thanks the AICTE RPS Scheme of India for their financial support for this research activity.
REFERENCES
[1] Paul A. Ihom, G.B. Nyior, O.O. Alabi, E.E. Anbua, J. Ogbodo, S. Segun. ‘Solid waste management The use of broken waste bottles as reinforcement agent for aluminum matrix composite.’ International Journal of Science and Technology Vol.1, No.10, pp 546-551 (Oct.2012.).
[2] K. Annamalai. Influence of E-glass and Graphite particle on Al-356
alloy composite produced by vortex method’. International Journal of Engineering science and Technology Vol.3 No.2 pp1606-1609 (Feb 2011).
[3] Madhu Kumar, Uma Shankar ‘Evaluation of mechanical properties
of Aluminum Alloy 6061- glass particulates reinforced metal matrix
composites’. International Journal of Modern Engineering
Research Vol.2, Issue.5, pp-3207-3209 ( Sep. –Oct. 2012)
[4] Noboru Yoshikawa, Yuuya Nakano, Kentarou Sato and Shoji
Taniguchi Fabrication of composite materials using Al Scrap and Wasted Glass. Materials Transactions, Vol.46, No.12 (2005) pp. 2582-2585. Special Issue on Growth of Ecomaterials as a Key to Eco-Society II 2005 The Japan Institute of Metals.
[5] Edited by Karl U. Kainer. Metal Matrix Composites. Custom-made
Materials for Automotive and Aerospace Engineering. Copyright © 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-31360-5.
[6] V J Rao, H.N.Panchal and Sonam Patel Fabrication and
characterization of cast aluminum fly ash composite vol 6 2013 ISSN2249-6157
[7] R.C. Weast et al CRC handbook of chemistry and physics 70th ed
(CRC press, Boca Raton. Florida, USA 1990) D-33
[8] N Yoshikawa, Y Nakano, K Sato and S Taniguchi, ‘Fabrication of
composite material using Al scrape and Wasted Glass’ Material Transactions, Vol 46,No.12 pp.2582 to 2585 (2005)
[9] Isil Kerti &Faith Toptan Microstructural Variations in cast B4C-reinforced aluminium matrix composites (AMCS) material Letters vol. 62, pp. 1215-1218, (2008)
[10] Thoguluva Raghavan Vijiayaram ‘Metallography of silicon dioxide