Copyright © 2000, American Society for Microbiology. All Rights Reserved.
Development of a PCR-Based Line Probe Assay for
Identification of Fungal Pathogens
CARA MARTIN,
1DAVID ROBERTS,
1MARJO
VAN DERWEIDE,
2RUDI ROSSAU,
2GEERT JANNES,
2TERRY SMITH,
1ANDMAJELLA MAHER
1*
National Diagnostics Centre, BioResearch Ireland, National University of Ireland,
Galway, Ireland,
1and Innogenetics NV, Ghent, Belgium
2Received 22 February 2000/Returned for modification 27 March 2000/Accepted 2 June 2000
We report on a reverse-hybridization line probe assay (LiPA) which when combined with PCR amplification
detects and identifies clinically significant fungal pathogens including
Candida
,
Aspergillus
, and
Cryptococcus
species. DNA probes have been designed from the internal transcribed-spacer (ITS) regions of
Candida
albicans
,
Candida parapsilosis
,
Candida glabrata
,
Candida tropicalis
,
Candida krusei
,
Candida dubliniensis
,
Cryp-tococcus neoformans
,
Aspergillus fumigatus
,
Aspergillus versicolor
,
Aspergillus nidulans
and
Aspergillus flavus
. The
probes were incorporated into a LiPA for detection of biotinylated ITS PCR products, and the specificity of the
probes was evaluated. We established LiPA detection limits for ITS 1 and for full ITS amplicons for genomic
DNA from
C. albicans
,
A. fumigatus
, and
C. neoformans
. Further evaluation of the LiPA was carried out on
clinical fungal isolates. One hundred twenty-seven isolates consisting of dimorphic yeasts and dermatophytic
and filamentous fungi were tested by the LiPA, which correctly identified 77 dimorphic yeasts and 23 of the
filamentous isolates; the remaining 27 isolates represented species of fungi for which probes were not included
in the LiPA. The fungal-PCR-LiPA technology was applied to blood samples inoculated with
Candida
cells
which were pretreated by minibead beating to mechanically disrupt the cells, with the DNA extracted by either
a previously described guanidium thiocyanate-silica method or the commercially available QIAmp tissue kit.
PCR amplification of the extracted DNA and subsequent DNA probe hybridization in the LiPA assay yielded
detection limits of 2 to 10 cells/ml. An internal standard control was included in the PCR amplification to
monitor for PCR inhibition. This fungal PCR-LiPA assay is robust and sensitive and can easily be integrated
into a clinical-testing laboratory with the potential for same-day diagnosis of fungal infection.
Improvements in the management and treatment of
debili-tated medical and surgical patients have led to an unwelcome
increase in the number of life-threatening infections due to
true pathogenic and opportunistic fungi (2, 17, 30). Candidosis
and aspergillosis are the most common mycoses in
immuno-compromised patients (6). The advent of new antifungal drugs
has improved the prospects for management of these
infec-tions; however, diagnosis remains difficult, and early initiation
of antifungal therapy is critical in reducing the high mortality
rate in immunocompromised patients (8, 11).
Conventional methods for diagnosis of fungal infection rely
primarily on the identification of species- or genus-specific
morphological characteristics that often require histological
examination or in vitro culture (6, 19). Recently several
au-thors have described PCR assays targeting different regions of
the fungal genome for detection of
Candida
(9, 38) and
As-pergillus
(10, 18, 27, 32) species. DNA-based assays for the
detection and identification of fungal species provide a
poten-tially more specific and sensitive alternative to conventional
culture and detection methods.
We describe a reverse hybridization line probe assay (LiPA)
which when combined with PCR amplification allows the
spe-cific detection and identification of fungal species. The
previ-ously described fungus-specific PCR primers (40) were used to
amplify the internal transcribed-spacer (ITS) region of the
ribosomal RNA complex from all fungi. The ITS region has
sufficient sequence variation to allow for the design of
species-specific probes to discriminate between different species (33,
41). In this study, species-specific oligonucleotide probes were
designed from within the ITS region for the following species:
Candida albicans
,
Candida parapsilosis
,
Candida tropicalis
,
Candida krusei
,
Candida glabrata
,
Candida dubliniensis
,
Cryp-tococcus neoformans
,
Aspergillus fumigatus
,
Aspergillus
nidu-lans
,
Aspergillus flavus
, and
Aspergillus versicolor
. These probes
were incorporated into a LiPA, combined with PCR
amplifi-cation of the ITS region, and evaluated on a panel of typed
fungi and clinical isolates.
Several researchers have described DNA-based tests for
Candida
infections with detection limits as low as 2 cells/ml of
blood sample (14–16, 39). In this report we describe the
ap-plication of the fungal PCR-LiPA technology to the detection
of
Candida
spp. inoculated into blood samples. As part of the
study we evaluated a number of sample preparation methods
and concluded that pretreatment of inoculated blood samples
(10
5to 10
1Candida
cells) with a minibead-beating step
fol-lowed by DNA extraction using a previously described method
(4) or by using the QIAmp tissue extraction kit from Qiagen
enabled detection limits of 2 to 10 cells/ml of blood tested. The
fungal PCR-LiPA assay can easily be integrated into clinical
laboratories for the identification of fungal species following
isolation, with the potential to identify the pathogen directly
from clinical samples within a single working day.
MATERIALS AND METHODS
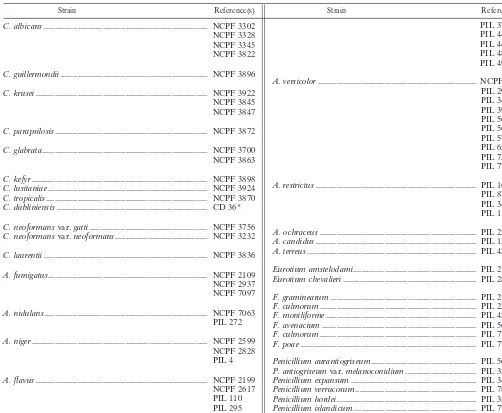
Microorganisms.All typed fungal isolates (Table 1) used in this study were
obtained from the National Collection of Pathogenic Fungi, Mycology Reference Laboratory, PHLS Central Public Laboratory, London, and from John Banks, Central Sciences Laboratory, Sand Hutton, York, United Kingdom. The typed culture ofC. dubliniensisCD36 was supplied by Derek Sullivan, Moyne Institute of Preventative Medicine, Trinity College, Dublin, Ireland. Clinical isolates were
* Corresponding author. Mailing address: National Diagnostics
Centre, BioResearch Ireland, National University of Ireland, Galway,
Ireland. Phone: 353-91-586559. Fax: 353-91-586570. E-mail: majella
.maher@nuigalway.ie.
3735
on May 15, 2020 by guest
http://jcm.asm.org/
obtained on agar plates from the Department of Medical Microbiology, Univer-sity College Hospital Galway, Galway, Ireland. For the purpose of generating serial dilutions ofCandidacells for sensitivity studies in inoculated blood sam-ples, 1 ml of a 10-ml overnight culture ofCandidacells was centrifuged at 13,000 rpm (14,926⫻g; Heraeus Sepatech Biofuge A) for 5 min, the cell pellet was resuspended in 1 ml of dH2O, and the blastoconidia were microscopically
enu-merated on a hemocytometer.
Culture conditions.All fungi were grown from lyophilized pellets on
Sab-ouraud glucose agar (Oxoid Ltd.), and a single yeast colony or a 50-l aliquot of a spore suspension in 0.2% Tween 20 was transferred to 25 ml of Sabouraud glucose (Oxoid Ltd.) broth for DNA extraction. All yeast isolates were grown at 37°C for 48 h, with shaking at 160 rpm for liquid cultures, with the exception of Cryptococcus albidus, which was grown at 30°C for 72 h. AllAspergillusisolates were grown at 30 to 37°C for 48 to 72 h, while all other filamentous isolates were grown at 22 to 30°C for 5 through 10 days. Clinical isolates were subcultured onto Sabouraud agar and grown at 37°C for 72 h.C. albicansclinical isolates were verified using either the germ tube test in serum or the MurexC. albicansCA50 kit (Murex Diagnostics Ltd., Bereluk, B.V. Straatweg, Dreubelen).
DNA extraction. (i) Preparation of template DNA from filamentous fungi.The
method used for extraction of DNA from filamentous fungi was a modification of methods previously described (3, 10). Briefly, filamentous fungal mycelia grown in 25 ml of Sabouraud broth as described above were harvested by filtration and washed once with sterile distilled water. The harvested mycelia were transferred to a microcentrifuge tube containing 0.5-mm-diameter zirco-nium glass beads (Biospec Products, Bartlesville, Okla.) stored in 0.2% sodium dodecyl sulfate (SDS) (BDH). Cell destruction was achieved by shaking the
microcentrifuge tube at maximum speed for 190 s in a Mini Beadbeater (Biospec Products, Bartlesville, Okla.). Nucleic acids were purified with 900l of L6 buffer (10 M guanidium thiocyanate, 0.1 M Tris-HCl [pH 6.4], 0.2 M EDTA, 2.6% [vol/vol] Triton X-100) (Sigma-Aldrich Ltd.) and 40l of silica dioxide suspen-sion (Sigma-Aldrich Ltd.) at room temperature for 10 min. The sample was then centrifuged at 13,000 rpm (14,926⫻g) for 1 min, and the silica pellet was washed twice with 1 ml of L2 buffer (10 M guanidium thiocyanate, 0.1 M Tris-HCl [pH 6.4]), followed by two washes in 1 ml of 70% ethanol (BDH; Merck Ltd.) and one wash in 1 ml of 100% acetone (BDH; Merck Ltd.). The pellet was air dried, and the DNA was eluted in 100l of sterile 0.1⫻TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) (Sigma-Aldrich Ltd.).
(ii) Preparation of template DNA from yeast isolates.DNA was extracted
[image:2.612.52.554.84.497.2]from yeast isolates using a modification of a previously described method (21). Briefly, 25 ml of yeast culture grown as described above was harvested by centrifugation at 6,000 rpm (5,000⫻g; Beckman J2-21 centrifuge) for 15 min and washed once in distilled water. The cell pellet was resuspended in 7 ml of lysis buffer (10 mM Tris-HCl [pH 8.0], 250 mM EDTA [pH 8.0], 0.5% Triton X-100 [vol/vol]) supplemented with 3 mg of lyticase enzyme (Sigma-Aldrich Ltd.) per ml and incubated at 37°C overnight. Spheroplasts were subsequently lysed by incubating the samples with 3 mg of proteinase K per ml (Roche-Boehringer Mannheim Diagnostics, Mannheim, Germany) at 55°C for 2 h. The proteinase K was inactivated at 95°C for 10 min. An equal volume of phenol-chloroform-isoamyl alcohol (25:24:1; Sigma-Aldrich, Steinheim, Germany) was added, the tube was mixed by inversion and centrifuged at 12,000 rpm (19,800⫻g; Beckman J2-21 centrifuge) for 1 h. The aqueous layer was transferred to a fresh tube, and an equal volume of ice-cold isopropanol was added. The DNA was precipitated
TABLE 1. List of typed fungal isolates used in this study
Strain Reference(s) Strain Reference(s)
C. albicans
... NCPF 3302
NCPF 3328
NCPF 3345
NCPF 3822
C. guillermondii
... NCPF 3896
C. krusei
... NCPF 3922
NCPF 3845
NCPF 3847
C. parapsilosis
... NCPF 3872
C. glabrata
... NCPF 3700
NCPF 3863
C. kefyr
... NCPF 3898
C. lusitaniae
... NCPF 3924
C. tropicalis
... NCPF 3870
C. dubliniensis
... CD 36*
C. neoformans
var.
gatti
... NCPF 3756
C. neoformans
var.
neoformans
... NCPF 3232
C. laurentii
... NCPF 3836
A. fumigatus
... NCPF 2109
NCPF 2937
NCPF 7097
A. nidulans
... NCPF 7063
PIL 272
A. niger
... NCPF 2599
NCPF 2828
PIL 4
A. flavus
... NCPF 2199
NCPF 2617
PIL 110
PIL 295
PIL 345
PIL 377
PIL 378
PIL 444
PIL 447
PIL 480
PIL 499
A. versicolor
... NCPF 7088
PIL 293
PIL 347
PIL 399
PIL 564
PIL 565
PIL 576
PIL 656
PIL 725
PIL 770
A. restrictus
... PIL 167
PIL 87
PIL 34
PIL 116
A. ochraceus
... PIL 253
A. candidus
... PIL 129
A. terreus
... PIL 422
Eurotium amstelodami
... PIL 218
Eurotium chevalieri
... PIL 280
F. graminearum
... PIL 210
F. culmorum
... PIL 234
F. moniliforme
... PIL 450
F. avenacium
... PIL 569
F. culmorum
... PIL 772
F. poae
... PIL 773
Penicillium aurantiogriseum
... PIL 563
P. antiogriseum
var.
melanoconidium
... PIL 333
Penicillium expansum
... PIL 346
Penicillium verrucosum
... PIL 781
Penicillium hordei
... PIL 351
Penicillium islandicum
... PIL 778
Penicillium martensii
... PIL 9
Penicillium ruber
... PIL 162
on May 15, 2020 by guest
http://jcm.asm.org/
by centrifugation at 12,000 rpm (19,800⫻g; Beckman J2-21 centrifuge) for 15 min. The pellet was washed twice with 70% ethanol. Air-dried pellets were resuspended in 200l of 0.1⫻TE buffer (Sigma-Aldrich Ltd.) and stored at
⫺20°C. DNA concentrations were estimated against known concentrations of lambda DNA (New England Biolabs) using a densitometer with Bio-ID V.96 software (Vilber Lourmat, Marne La Valle´e, France) and by spectrophotometric analysis of absorbances at 260 and 280 nm using a Heios␣spectrophotometer. CandidaandCryptococcusDNA was diluted to a working concentration of 10 ng/l.AspergillusDNA was diluted to a 1-ng/l working stock.
(iii) Rapid preparation of template DNA from yeast cells.To facilitate PCR
analysis of a large number of clinical isolates, a method was developed for the rapid preparation of template DNA from yeast isolates. A single colony was removed from the plate using a micropipette tip and resuspended by grinding in 100l of lysis buffer (0.1 M EDTA, 0.1 M NaOH). The sample was vortexed, and a 5-l aliquot was used per 100-l PCR mixture.
(iv) Preparation ofC. albicansDNA template from inoculated blood samples.
Blood samples (200l) inoculated with yeast cells (1⫻105to 1⫻101cells) were
lysed in 800l of lysis buffer (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 50 mM NaCl) at room temperature for 10 min and centrifuged at 13,000 rpm (14,926⫻
g; Heraeus Sepatech Biofuge A) for 5 min, the supernatant was discarded, and the pellet was resuspended in 100l of sterile H2O. Blood samples (1 ml and 5
ml) inoculated with yeast cells (105to 101cells) were lysed in 3 ml of lysis buffer
and centrifuged at 2,000 rpm (500⫻g; Beckman J2-21 Centrifuge) for 10 min, the supernatant was discarded, and the pellet was resuspended in 100l of sterile H2O. Glass beads (0.5-mm-diameter zirconium glass beads stored in 0.2% SDS)
were added to the resuspended pellet, the sample was vortexed in a minibead beater at top speed for 190 s, and the DNA was extracted as previously described (4). Briefly, the suspension was removed, following bead beating, to a fresh microcentrifuge tube containing 900l of L7 buffer (10 M GuSCN, 100 mM Tris-HCl [pH 6.4], 200 mM EDTA, 2.6% [wt/vol] Triton X-100, and alpha-casein [Sigma-Aldrich Ltd.]) (added to a final concentration of 1 mg/ml), and 40l of silica suspension for 200-l and 1-ml samples and was scaled up to 80l for 5-ml samples. The sample was vortexed at maximum speed for 30 s followed by incubation at room temperature for 10 min. The sample was vortexed again for 30 s and spun at 12,000 rpm (12,700⫻g; Heraeus Sepatech Biofuge A) for 1 min. The supernatant was removed, and the pellet was washed twice in 1 ml of L2 buffer (10 M GuSCN, 100 mM Tris-HCl [pH 6.4]) which was vortexed for 30 s and spun at 12,000 rpm (12,700⫻g; Heraeus Sepatech Biofuge A) for 1 min. The pellet was then washed twice in 1 ml of 70% ethanol followed by a final wash in 1 ml of 100% acetone. The pellet was dried in a heating block at 60°C for 10 min and resuspended in 100l of 0.1⫻TE buffer for 30 min. Similarly, blood samples (200l, 1 ml, and 5 ml) inoculated with yeast cells (105to 101cells/ml) were lysed
and minibead beaten, and DNA was extracted from the suspensions by using a QIAmp tissue kit (Qiagen, Los Angeles, Calif.).
Sequence information, oligonucleotide probes, and primer design.
Oligonu-cleotide primers were obtained from Genosys Biotechnologies (Europe) Ltd., Cambridgeshire, United Kingdom. The oligonucleotide primer pairs used in this study were previously shown to amplify the 5.8S rDNA and the adjacent ITS regions (40). To facilitate the detection of ITS PCR products in the LiPA, PCR primers were modified at the 5⬘end with a biotin moiety. The oligonucleotide primers UP1 and RP1 (UP1, 5⬘-GCCTAATGTAATCCATGGCG-3⬘; RP1, 5⬘ -CTCCATTGGATTATCCCAGCA-3⬘) for amplification of a 306-bp internal standard control (ISC) were designed by Innogenetics (Ghent, Belgium) and were supplied modified with 5⬘biotin moieties for this study.
Species-specific oligonucleotide probes were designed from ITS sequences
available in the GenBank database. Oligonucleotide probes CA1, CA2, CA3, CP, CG1, CT, CK, CD1, CD2, AN1, AN2, AFL1, AFL4, and AV1 were designed from GenBank entries (Table 2).C. neoformansNCPF 3756 and NCPF 3232,C. dubliniensisCD36, and A. fumigatusNCPF 2109 and NCPF 7097 sequence information was obtained following amplification of the full ITS region using the ITS 5-ITS 4 primer pair and direct sequencing of the PCR products (MWG Biotech, Milton Keynes, United Kingdom). Oligonucleotide probes CD3, CN2, CN4, AF1, and AF2 were designed from the sequences generated in this study. All sequence analysis was carried out using the University of Wisconsin Ge-netics Computer Group (UWGCG) package (Version 9.1, September 1997; GCG, Madison, Wis.). Database searches were run using Blast and Fasta pro-grams against the EMBL and GenBank DNA databases. Pairwise comparisons were made using the Bestfit programs. Multiple sequence alignments were car-ried out using the Clustal W program (37). Sequence data obtained from the ABI DNA sequencer were imported into the Acer Network at the Irish National Centre for BioInformatics, Trinity College, Dublin, Ireland. Sequence data were assembled using the Fragment Assembly program.
PCR.PCRs were performed in a final volume of 100l. PCR conditions for the amplification of the ITS region from yeast isolates were as follows; each reaction mixture contained 0.25 mM deoxynucleotide triphosphates (DU:dNTPs [2:1]), 1⫻reaction buffer (Promega), 3 mM MgCl2, 1 U of uracil DNA
glycosy-lase (25) (Roche-Boehringer Mannheim), 40 pmol each of the forward and reverse primers (ITS 5-ITS 4 pair for full ITS amplification, and ITS 5-ITS 2 pair for ITS-1 amplification), 2.5 U of Taqpolymerase (Promega), and 5 l of template DNA made to a final volume of 100 l with nuclease-free water (Sigma-Aldrich, Ltd.). PCR amplification was performed in a Touchdown ther-mocycler (Hybaid, Middlesex, United Kingdom), with the following cycling con-ditions: 37°C for 10 min for one cycle followed by 94°C for 2 min for one cycle followed by 30 cycles of DNA denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, and DNA extension at 72°C for 2 min, with a final extension cycle at 72°C for 10 min.
PCR amplification ofCandidaDNA extracted from inoculated blood samples was performed in a final volume of 100l with 20l of DNA extracted from the blood samples (for DNA extracted from 5-ml blood samples, 20l of a 1/10 dilution was included in the PCR mixture) added to the PCR mixture containing a final concentration of 0.25 mM DU/dNTPs (2:1), 1⫻reaction buffer (Pro-mega), 3 mM MgCl2, 1 U of uracil DNA glycosylase (25) (Roche-Boehringer
Mannheim), 40 pmol each of ITS 5 and ITS 4 primer, and 2.5 U of Taq polymerase (Promega) made to a final volume of 100l in nuclease-free water (Sigma-Aldrich Ltd.). An ISC plasmid supplied by Innogenetics was included when appropriate at a concentration of 105molecules and PCR amplified with 5
pmol each of the forward and reverse primers UP1 and RP1. PCR amplification was performed in a Touchdown thermocycler (Hybaid), with the following cy-cling conditions: 37°C for 10 min for one cycle followed by 94°C for two min for 1 cycle followed by 40 cycles of DNA denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, and DNA extension at 72°C for 2 min, with a final extension cycle at 72°C for 10 min.C. albicansDNA (50 ng) extracted was included in a PCR as a positive control, along with a no-template negative control in each PCR run.
PCR conditions for amplification of the ITS region from filamentous fungi were performed as above with the following modifications; each reaction mixture contained 1⫻reaction buffer (Perkin Elmer), 3 mM MgCl2(Perkin Elmer), 2.5
U of Amplitaq Gold (Perkin Elmer), and 15% glycerol (Sigma-Aldrich Ltd.). Cycling conditions for amplification of the ITS from filamentous fungi were as follows; 37°C for 10 min for one cycle followed by 95°C for 10 min for one cycle followed by 30 cycles of DNA denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, and DNA extension at 72°C for 2 min, with a final extension cycle at 72°C for 10 min. A no-template negative control was included in each PCR run. Ten-microliter aliquots of PCR products were analyzed by gel electrophore-sis on 1 to 2% agarose gels. Agarose gels composed of 1 to 2% (wt/vol) agarose (Roche-Boehringer Mannheim) were run in TBE buffer (0.045 M Tris, 0.089 M boric acid, 0.002 M EDTA [pH 8.4]) at 100 to 120 V for 1 to 2 h.
LiPA.The INNO-LiPA fungal assay (Innogenetics) was performed essentially
as described previously (35) and is based on the reverse-hybridization principle. Oligonucleotide probes for the LiPA were enzymatically provided with a polydeoxythreonine tail. Subsequently, probes were immobilized as parallel lines onto a nitrocellulose membrane, with the top line containing a positive-control biotinylated DNA. Briefly, 10l of biotinylated PCR product was denatured in a LiPA tray by adding an equal volume of denaturing solution (NaOH-EDTA) and incubating at room temperature for 5 min. A 1-ml aliquot of preheated (50°C) hybridization buffer (2⫻SSC [1⫻SSC is 0.15 M NaCl plus 0.015 M sodium citrate] and 0.1% SDS) together with a LiPA strip was added and incubated at 50°C for 30 min in a shaking water bath. The strips were stringently washed three times in 1 ml of hybridization buffer—twice for 1 min at room temperature and once at 50°C for 15 min. These washes were followed by one wash in 1-ml of rinse solution for 1 min followed by incubation in 1 ml of an alkaline phosphatase-linked streptavidin conjugate at room temperature for 30 min. The strips were washed twice in 1 ml of rinse solution and once in 1 ml of substrate buffer for 1 min each at room temperature. Finally the strips were incubated in 1 ml of substrate solution (5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium diluted in substrate buffer) for 30 min at room
tem-TABLE 2. Oligonucleotide probe design and corresponding
GenBank accession numbers
Probe Organism regionITS Accession no.
CA 1 C. albicans ITS 1 AB032172, AB01803, X 71088 CA 2 C. albicans ITS 1 AB032172, AB01803, X 71088 CA 3 C. albicans ITS 1 AB032172, AB01803, X 71088 CP 2 C. parapsilosis ITS 1 U10987
CG 1 C. glabrata ITS 1 L47108, L11351 CT 1 C. tropicalis ITS 1 L47112 CK 1 C. krusei ITS 1 L477133 CD 1 C. dubliniensis ITS 2 U96719 CD 2 C. dubliniensis ITS 2 U96719 CN 2 C. neoformans ITS 1 L14068 CN 4 C. neoformans ITS 2 L14068
AF 1 A. fumigatus ITS 1 U18355, AF078889, U93683 AF 2 A. fumigatus ITS 2 U18355, AF078889, U93683
AN 1 A. nidulans ITS 1 L76747, AF138289, AJ000933, U03520 AFL 1 A. flavus ITS 1 AB008414, AB008415, AF027863 AV 1 A. versicolor ITS 1 L76745
on May 15, 2020 by guest
http://jcm.asm.org/
[image:3.612.53.294.90.265.2]perature. The color reaction was stopped by adding distilled water to the strips. After drying, the strip results were interpreted by eye.
RESULTS
PCR amplification of the ITS with universal fungal primers.
Four PCR primers designed from the conserved nucleotide
sequences of 18S, 5.8S, and 28S rRNA genes were used to
amplify the ITS region. These primers were used in the
fol-lowing combinations: ITS 5-ITS 4, ITS 5-ITS 2, and ITS 3-ITS
4 to amplify the full ITS region, the ITS 1 region, and the ITS
2 regions, respectively. Using these primer combinations and
the optimized PCR conditions described in Materials and
Methods, PCR products were generated from all of the typed
fungi listed in Table 1. Figure 1 illustrates the different
ampli-con sizes obtained for the full ITS region amplified from a
selection of different fungus species. For consistent
amplifica-tion of the ITS region from filamentous fungi, PCR condiamplifica-tions
were modified to contain 15% glycerol and a hotstart at 95°C
for 10 min. No PCR products were amplified with the ITS PCR
primers for genomic DNA isolated from
Clostridium
perfrin-gens
,
Mycobacterium bovis
,
Listeria monocytogenes
,
Escherichia
coli
, or human DNA.
Sequence analysis and probe design.
Oligonucleotide
probes were designed from ITS sequences available in the
GenBank database. For
C. dubliniensis
,
C. neoformans
, and
A.
fumigatus
strains for which insufficient sequence information
was available in the GenBank database, the full ITS region was
sequenced from two isolates of each species. One sequencing
reaction was performed on each strand, and a consensus
se-quence was obtained using the UWGCG Fragment Assembly
program.
Analysis of the ITS regions of the rRNA complex from a
range of fungi including
Candida
,
Cryptococcus
,
Aspergillus
,
and
Penicillium
species was carried out following alignment of
these sequences using the UWGCG Clustal W bioinformatics
program. Based on these sequence comparisons, potential
spe-cies-specific probes were designed for the following species:
C.
albicans
,
C. parapsilosis
,
C. tropicalis
,
C. glabrata
,
C. krusei
,
C.
dubliniensis
,
C. neoformans
,
A. fumigatus
,
A. flavus
,
A.
versi-color
, and
A. nidulans.
This panel of species-specific probes,
which ranged in size from 17 to 21 bp, was designed to
hybrid-ize specifically to the appropriate complimentary biotinylated
PCR product at a temperature of 50°C to enable
multiparam-eter detection with the LiPA technology. Confidence in the
specificity of the probes was strengthened by a sequence
data-base search using the Fasta or Blast programs. When possible,
species-specific probes were designed from the ITS 1 region
with probes for the ITS 2 region also designed for the following
species:
C. dubliniensis
,
C. neoformans
, and
A. fumigatus
. All
oligonucleotide probes were incorporated into the LiPA for
evaluation, and relevant accession numbers are listed in Table
2.
[image:4.612.92.516.70.280.2]Specificity of LiPA.
The specificities of the individual probes
in the LiPA were evaluated against the panel of fungi listed in
Table 1. Biotinylated full-ITS amplicons generated from these
species were reverse hybridized to the LiPA strips at 50°C.
Figure 2 illustrates the specificity of a selection of these probes
in the LiPA. All of the probes hybridized specifically with the
ITS PCR products from the appropriate species with the
fol-lowing exceptions. The CA1 probe reacted with the full-ITS
PCR products from
C. dubliniensis
. The CN1 probe reacted
weakly with
C. laurentii
PCR products (data not shown).
A.
versicolor
full-ITS amplicons generated from 11 individual
iso-lates varied in size from approximately 650 to 700 bp and were
found to cross-react with the
A. nidulans
AN1 probe and the
A.
flavus
AFL1 probe (data not shown). Additionally, during this
study, ITS sequences for
A. parasiticus
and
A. nominus
were
submitted to the GenBank database (accession numbers
AF027862, AF027860, AF027864, and AF027861). The AFL1
probe designed for the specific detection of
A. flavus
shared
100% homology with both of these ITS sequences, resulting in
the design of a new
A. flavus
probe (AFL4) for the specific
identification of
A. flavus
ITS PCR products. The AFL4 probe
was found to hybridize with full-ITS and ITS1 PCR products
from
A. flavus
, and although it showed weak
cross-hybridiza-tion with full-ITS PCR products from some
A. versicolor
iso-lates, it did not show cross-reaction with the ITS1 PCR
prod-ucts from these isolates. The full-ITS regions from three
A.
FIG. 1. Full-ITS PCR products amplified from different fungal species. Lanes 1 through 12 show full-ITS amplicons fromC. albicansNCPF 3302,C. guillermondii NCPF 3896,C. glabrataNCPF 3700,C. tropicalisNCPF 3870,C. dubliniensisCD36,C. kruseiNCPF 3847,C. krusei3922,C. kruseiNCPF 3845,C. kefyrNCPF 3898, A. fumigatusNCPF 2109,A. nidulansNCPF 7063, andA. nigerNCPF 2828. Lane 13 represents a no-template PCR negative control. M, 100-bp molecular size marker.
on May 15, 2020 by guest
http://jcm.asm.org/
versicolor
isolates were sequenced to investigate whether the
cross-reaction of some
A. versicolor
full-ITS PCR products with
the AFL4 probe was a result of sequence homology within the
full-ITS sequence of these
A. versicolor
isolates and the AFL4
probe sequence. The full-ITS sequences from these
A.
versi-color
isolates showed 100% homology with the GenBank
se-quence entry (L76745) for
A. versicolor
and showed no
signif-icant homology with the AFL1 or AFL4 probe sequences in
similar Blast searches. As a result of cross-reaction of
A.
ver-sicolor
full-ITS PCR products with the
A. nidulans
AN1 probe,
a second DNA probe, AN2, was designed for the specific
detection of
A. nidulans
which has been shown to have no
cross-reaction with the full-ITS PCR products from
A.
versi-color
and will replace AN1 for the detection of
A. nidulans
in
future LiPA strips. The two DNA probes CD1 and CD2 for the
detection of
C. dubliniensis
were designed from ITS 2 sequence
information. DNA sequencing of the full-ITS region from
C.
dubliniensis
allowed the design of an ITS 1 probe CD3, specific
for the detection of
C. dubliniensis
.
Analysis of clinical isolates.
Further evaluation of the LiPA
was carried out on a collection of clinical isolates and quality
control samples from the Quality Assurance Laboratory,
PHLS Central Public Laboratory, and isolates from diverse
specimen types obtained from the Bacteriology Department,
University College Hospital, Galway. PCR template DNA was
prepared from the clinical isolates using DNA extraction
meth-ods A and C as described in Materials and Methmeth-ods. Full-ITS
PCR amplicons generated from the isolates were hybridized to
the LiPA strips. A total of 127 clinical isolates were analyzed,
and of these, 100 isolates were identified, including 77
dimor-phic yeast species (66
C. albicans
isolates, 4
C. glabrata
isolates,
2
C. parapsilosis
isolates, 2
C. krusei
isolates, and 1 isolate each
from
C. tropicalis
,
C. dubliniensis
, and
C. neoformans
) and 23
members of the
Aspergillus
genus (20 isolates of
A. fumigatus
,
2 isolates of
A. flavus
, and 1 isolate of
A. nidulans
). The identity
of the
C. albicans
isolates was confirmed using the Murex
C.
albicans
CA50 kit. The LiPA did not identify the 27 other
fungal isolates for which DNA probes were not designed and
included in the LiPA assay. These included 3 dimorphic yeast
isolates (1
Saccharomyces
sp., 1
C. laurentii
isolate, and 1
C.
guilliermondii
isolate), 10 dermatophytes (isolates of
Microspo-rum canis
,
Trichophyton rubrum
, and
Trichophyton
verruco-sum
), and 14 filamentous fungal isolates which did not
hybrid-ize to DNA probes in the LiPA assay (3
A. niger
isolates, 2
A.
terreus
isolates, 1
A. glaucus
isolate, 4
Penicillium
sp. isolates, 2
Fusarium
sp. isolates, and 2 unknown isolates). With the
ex-ception of
C. albicans
, the number of isolates of each species
tested was low, and while these isolates demonstrated the
po-tential of the PCR-LiPA for species identification, ideally the
study should be expanded to a larger number of isolates to
confirm the reliability of the PCR-LiPA for species
identifica-tion.
Sensitivity of the LiPA.
The sensitivity of the PCR-LiPA was
evaluated by performing PCR amplification of serial dilutions
of purified genomic DNA (100 ng to 1 fg) isolated from
C.
albicans
,
C. neoformans
, and
A. fumigatus
isolates using both
the ITS 5-ITS 4 and the ITS 5-ITS 2 primer pairs. PCR
prod-ucts were hybridized to the LiPA strips, and detection limits of
50 pg for full-ITS and 50 fg for ITS 1 PCR amplicons
gener-ated from
A. fumigatus
genomic DNA were obtained by the
LiPA with the
A. fumigatus
probes. Detection limits of 100 fg
for full-ITS and ITS 1 amplicons generated from
C. albicans
DNA were obtained by the LiPA, with all three
C. albicans
probes, and 10 pg for full ITS and 1 pg for ITS 1 amplicons
were obtained for the
C. neoformans
probes. Comparable
de-FIG. 2. LiPAs of full-ITS products amplified from typed fungal isolates. The positions of the control line and the 16 species-specific probes for fungal pathogens are shown on the left hand side of the photograph. LiPAs 1 through 12 represent full-ITS PCR amplicons from the following species: 1,C. albicansNCPF 3345; 2,C. parapsilosisNCPF 3872; 3,C. glabrataNCPF 3700; 4,C. tropicalisNCPF 3870; 5,C. kruseiNCPF 3922; 6,C. kruseiNCPF 3847; 7,C. dubliniensisCD36; 8,C. neoformans var.gattiNCPF 3756; 9,C. neoformansvar.neoformansNCPF 3232; 10,A. fumigatusNCPF 2109; 11,A. nidulansNCPF 7063; 12,A. flavusNCPF 2199.on May 15, 2020 by guest
http://jcm.asm.org/
tection limits were achieved for each species by Southern blot
hybridization with the appropriate digoxigenin-labeled probes.
Application of the PCR-LiPA to blood samples.
In this study,
200-
l, 1-ml, and 5-ml blood samples were inoculated with
decreasing concentrations of
C. albicans
cells (10
5to 10
1cells).
The inoculated blood samples were pretreated to lyse and
remove the erythrocytes, and the pellet of lymphocytes and
yeast cells was resuspended, glass beads were added, and the
suspension was minibead beaten for 190 s to mechanically
disrupt the yeast cell walls in preparation for DNA extraction.
DNA was extracted from the suspensions as previously
de-scribed (4) and scaling up the silica suspension from 40 to 80
l
to facilitate DNA extraction from the 5-ml inoculated samples.
A 20-
l aliquot of extracted DNA from the 200-
l and 1-ml
blood samples and a 20-
l aliquot of a 1/10 dilution of DNA
extracted from 5-ml blood samples containing the decreasing
[image:6.612.71.544.63.442.2]concentrations of yeast cells were PCR amplified with the ITS
5-ITS 4 primer pair, and 10
l of each of the PCR products was
analyzed by agarose gel electrophoresis with a second 10-
l
aliquot hybridized to the LiPA strips. The detection limits
achieved following agarose gel electrophoresis and
hybridiza-tion in the LiPA assay were comparable at 50 cells/ml for
200-
l and 10 cells/ml for 1-ml blood samples, with a detection
limit of 2 cells/ml for 5-ml blood samples (Fig. 3a and b). The
200-
l, 1-ml, and 5-ml blood samples inoculated with yeast
cells (10
5to 10
1cells/ml) were pretreated as previously
de-scribed, and the DNA was extracted from the suspensions by
using the QIAmp tissue kit from Qiagen by following the kit
manual. PCR amplification of 20
l of the extracted DNA with
the ITS 5-ITS 4 primer pair followed by hybridization of 10
l
of the PCR mixtures to the LiPA strips yielded detection limits
of 50 cells/ml for 200-
l blood samples, 10 cells/ml for 1-ml
FIG. 3. PCR amplification and LiPA detection of ITS region fromC. albicansinoculated into 1-ml blood samples and extracted as previously described (4). (A) PCR amplification of ITS region fromC. albicansinoculated into 1-ml blood samples and extracted as previously described (4). Lane 1, 100-bp ladder; lane 2, 1⫻105cells; lane 3, 104cells; lane 4, 103cells; lane 5, 102cells; lane 6, 101cells; lane 7, PCR positive control; lane 8, PCR positive control; lane 9, negative PCR control; lane
10, 100-bp ladder. (B) LiPA hybridization of 10l of full-ITS PCR products fromC. albicansinoculated into 1-ml blood samples and extracted as previously described (4). LiPA 1, 105cells; LiPA 2, 104cells; LiPA 3, 103cells; LiPA 4, 102cells; LiPA 5, 101cells, LiPA 6, positive control; LiPA 7, positive control; LiPA 8, negative control.
(C) PCR amplification of ITS region in the presence of an ISC fromC. albicansinoculated into 1-ml blood samples and extracted as previously described (4). Lane 1, 100-bp ladder; lane 2, 104cells; lane 3, 103cells; lane 4, 102cells; lane 5, 101cells; lane 6, sample preparation negative control; lane 7, positive control; lane 8, positive
control; lane 9, PCR-negative control; lane 10, PCR-negative control. (D) LiPA hybridization of 10l of full-ITS PCR products fromC. albicansand ISC PCR products fromC. albicansinoculated into 1-ml blood samples and extracted as previously described (4). LiPA 1, 104cells; LiPA 2, 103cells; LiPA 3, 102cells; LiPA 4, 101cells;
LiPA 5, sample preparation negative control; LiPA 6, PCR-negative control; LiPA 7, PCR-positive control. Positive, positive control probe; CA1,C. albicansITS probe; CA2,C. albicansITS probe; CA3,C. albicansITS probe; ISC, internal standard control probe.
on May 15, 2020 by guest
http://jcm.asm.org/
blood samples, and 2 cells/ml for the 5-ml blood samples,
which were comparable to the detection limits achieved with
the guanidium thiocyanate-silica DNA extraction method. An
ISC in the form of an “artificial plasmid” was included in the
PCR to monitor for PCR inhibition in the extracted DNA
samples. This ISC was designed to be PCR amplified with a
UP1-RP1 primer pair to yield a PCR product of 306 bp, which
was smaller than the full-ITS PCR amplicons from
Candida
spp. (
⬎
600 bp). When the ISC was included at a concentration
of 10
5copies of the PCR ISC plasmid/100
l of mixtures of
decreasing dilutions of
C. albicans
cells extracted from 1 ml of
blood, a detection limit of 10 cells/ml was achieved (Fig. 3c and
d).
DISCUSSION
Invasive mycotic infections are contributing to increased
morbidity and mortality among immunocompromised patients.
While the availability of new antifungal drugs has improved the
management of infections, in many cases diagnosis of the
fun-gal agent remains difficult. In this study we report on the
development of a reverse-hybridization LiPA which, when
combined with PCR amplification, detects and identifies
clin-ically significant fungal pathogens including
Candida
,
Aspergil-lus
and
Cryptococcus
species.
Previous reports describe PCR assays for the detection of
fungal pathogens that target highly conserved regions such as
the 18S (8, 13, 27) and the 28S (27, 34) regions or single copy
genes such as actin (20) or heat shock protein 90 (7). In this
study the ITS region of the rRNA complex was chosen as a
target for species-specific-probe design. This region is an ideal
target for species identification because it has sufficient
varia-tion to allow for discriminavaria-tion between species (1, 12), and
this target is present in multiple copies in the fungal genome.
In this study we describe universal amplification of the ITS
region from fungi combined with the hybridization of the ITS
PCR products to species-specific oligonucleotide probes on the
LiPA strip for the specific identification of fungal pathogens.
The new species,
C. dubliniensis
, first described by Sullivan et
al. (36), is typically identified as
C. albicans
by routine
pheno-typic methods although a PCR assay based on amplification of
the pH-regulated
PHR1
and
PHR2
genes has been recently
described for distinguishing these species (22). Sequence
anal-ysis of the ITS regions from both
C. albicans
and
C. dubliniensis
in this study revealed a high degree of homology between both
species, and we observed cross-reaction between the ITS PCR
products from these two species and their respective
oligonu-cleotide probes. The CD2 probe, which was specific for
C.
dubliniensis
, was designed from the ITS 2 region, and a second
probe, CD3, designed from ITS 1 with a single-base-pair
mis-match to
C. albicans
in the ITS1 probe region, was also specific
for detection of
C. dubliniensis
. In this study we also observed
cross-reaction of ITS PCR products from
A. versicolor
isolates
with the
A. flavus
probe (AFL1) and the
A. nidulans
probe
(AN1). In addition we observed different full-ITS amplicon
sizes (650 to 700 bp) among 11 different isolates of
A. versicolor
that were PCR amplified as part of this study. Other authors
have reported on genetic variation within the ITS region
be-tween isolates of the same species (28, 31, 32), in particular for
Pneumocystis carinii
(23, 26), which has been found to have up
to 15 different ITS subtypes. However, cloning and sequencing
the full-ITS region from three
A. versicolor
isolates showed
100% homology with
A. versicolor
sequences in the GenBank
database.
The detection limit of the LiPA was 10-fold lower than that
of agarose gel electrophoresis for DNA that was PCR
ampli-fied for
C. albicans
,
A. fumigatus
, and
C. neoformans
. Detection
of
C. albicans
inoculated into blood was as low as 2 to 10
cells/ml and compared well to sensitivities reported by other
researchers. A microtiter enzyme immunoassay (EIA) for the
detection of
Candida
species in blood with a detection
sensi-tivity of 2 cells/200
l of sample and a real-time fluorescent
PCR-based assay for the detection of three
Candida
species in
a single reaction tube with a detection sensitivity of 100 cells/
200
l have previously been described (12, 15). A nested PCR
assay for the detection of
C. neoformans
in cerebrospinal fluid
is reported to have a detection limit of 10 cells (29). Other
researchers determined a detection level of 5 pg for
A.
fumiga-tus
rDNA 18S gene amplicons in a microtiter plate EIA (10).
A nested specific PCR-EIA for
A. fumigatus
(13) based on
sequences derived from the 18S rRNA gene had a detection
limit of 1.7 ng/
l of genomic DNA, while a similar PCR-EIA
for an
Aspergillus
mitochondrial gene had a sensitivity of 0.6
fg/ml (18). We report a detection limit for ITS 1 PCR
ampli-cons from
A. fumigatus
of 50 fg or one fungal genome
equiv-alent (5) in the PCR-LiPA assay.
During the course of this study we evaluated a number of
different DNA extraction methods with the aim of developing
a universal approach for the preparation of fungal DNA from
Candida
,
Cryptococcus
, and
Aspergillus
spp. in blood and/or
respiratory specimens. Evaluation of different methods for the
extraction of fungal DNA from blood has previously been
reported in the literature (24). Most of the methods evaluated
involved enzymatic approaches previously described (16, 24),
with some modifications. In our laboratory these methods
failed to consistently yield high-quality DNA, resulting in
vari-ation in the detection limits achieved between experiments
(data not shown). We also noted that DNA extracted from
A.
fumigatus
by these methods required a “hot start” PCR
ap-proach for sensitive PCR amplification of the extracted DNA.
The modified guanidium thiocyanate-silica method recently
described (4) proved to be an efficient DNA extraction method
and enabled detection limits of 2 to 10 cells/ml to be
consis-tently achieved from fungal DNA extracted from blood
sam-ples. The QIAmp tissue kit from Qiagen also proved to be an
equally reliable and user-friendly approach for extracting DNA
from blood samples.
We have developed a PCR-LiPA for detection and
identifi-cation of clinically important species of
Candida
,
Aspergillus
,
and
Cryptococcus
, and we have demonstrated the potential of
the technology for species confirmation of a small number of
clinical fungal isolates and the direct detection of
C. albicans
in
inoculated blood samples. Application of the PCR-LiPA is
being expanded to a range of clinical specimen types, including
tissue specimens in many cases of which fungal morphology is
not sufficiently distinct to be diagnostic, and a rapid
noncul-ture-based identification method would have direct
applica-tion.
ACKNOWLEDGMENTS
We thank Geraldine Corbett-Feeney and John Kelehan from the
Department of Medical Microbiology, University College Hospital,
Galway, for the clinical isolates used in this study. We also thank John
Banks, Central Sciences Laboratory, Sand Hutton, York, and Derek
Sullivan, Moyne Institute of Preventative Medicine, Trinity College,
Dublin, for supplying other fungal strains used in the study.
REFERENCES
1.Bainbridge, B. W.1994. Modern approaches to the taxonomy ofAspergillus,
p. 291–301.InK. A. Powell, A. Renovich, and J. F. Peberdy (ed.), The genus Aspergillus. Plenum Press, New York, N.Y.
2.Bodey, G. P.1997. New fungal pathogens. Curr. Clin. Top. Infect. Dis.
17:205–235.
on May 15, 2020 by guest
http://jcm.asm.org/
3.Boom, R., C. J. A. Sol, M. M. M. Salimens, C. L. Jansen, P. M. E. Wertheim
van Dillen, and J. van der Noordae.1990. A rapid and simple method for
purification of nucleic acids. J. Clin. Microbiol.28:495–503.
4.Boom, R., C. Sol, M. Beld, J. Weel, J. Goudsmit, and P. W. van Dillen.1999.
Improved silica-guanidiniumthiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J. Clin. Microbiol.
37:615–619.
5.Brody, H., and J. Carbon.1989. Electrophoretic karyotype ofAspergillus
nidulans. Proc. Natl. Acad. Sci. USA86:6260–6263.
6.Cohen, J.1991. Clinical manifestations and management of aspergillosis in
the compromised patient, p. 117–152.InD. W. Warnock and M. D. Rich-ardson (ed.), Fungal infections in the compromised patient, 2nd ed. Wiley & Sons, New York, N.Y.
7.Crampin, A. C., and R. C. Matthews.1993. Application of the polymerase
chain reaction to the diagnosis of candidiasis by amplification of an HSP 90 gene fragment. J. Med. Microbiol.39:233–238.
8.Einsele, H., H. Hebart, I. Roller, J. Lo¨ffler, I. Rothenho¨fer, C. A. Mu¨ller, R.
A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher.1997.
Detection and identification of fungal pathogens in blood using molecular probes. J. Clin. Microbiol.35:1353–1360.
9.Elie, C. M., T. J. Lott, E. Reiss, and C. J. Morrison.1998. Rapid
identifi-cation ofCandidaspecies with species specific DNA probes. J. Clin. Micro-biol.36:3260–3265.
10. Fletcher, H. A., R. C. Barton, and P. E. Verweij.1998. Detection of
Aspergil-lus fumigatusPCR products by a microtiter plate based DNA hybridisation assay. J. Clin. Pathol.51:617–620.
11. Fraser, V., M. Jones, J. Dunkel, et al.1992. Candidemia in a tertiary care
hospital: epidemiology, risk factors and predictors of mortality. Clin. Infect. Dis.15:414–421.
12. Fujita, S., B. A. Lasker, T. J. Lott, E. Reiss, and C. J. Morrison.1995.
Microtitration plate enzyme immunoassay to detect PCR-amplified DNA fromCandidaspecies in blood. J. Clin. Microbiol.33:962–967.
13. Golbang, N. J., P. Burnie, and R. C. Matthews.1999. A polymerase chain
reaction enzyme immunoassay for diagnosing infection caused byAspergillus fumigatus. J. Clin. Pathol.52:419–423.
14. Hee Shin, J., F. S. Nolte, and C. J. Morrison.1997. Rapid identification of
Candidaspecies on blood cultures by a clinically useful PCR method. J. Clin. Microbiol.35:1454–1459.
15. Hee Shin, J., F. S. Nolte, B. P. Holloway, and C. J. Morrison.1999. Rapid
identification of up to threeCandidaspecies in a single reaction tube by a 5⬘
exonuclease assay using fluorescent DNA probes. J. Clin. Microbiol.37:165– 170.
16. Holmes, A. R., R. D. Cannon, M. G. Shepherd, and H. F. Jenkinson.1994.
Detection ofCandida albicansand other yeasts in blood by PCR. J. Clin. Microbiol.32:228–231.
17. Jantunen, E., P. Rueitu, L. Niskanen, et al.1997. Incidence and risk factors
for invasive fungal infections in allogenic bone marrow transplant recipients. Bone Marrow Transplant.19:801–808.
18. Jones, M. E., A. J. Fox, A. J. Barnes, B. A. Oppenheim, P. Balagopal, G. R.
Morgenstern, and J. H. Scraffe.1998. PCR-ELISA for the early diagnosis of
invasive pulmonary Aspergillus infection in neutropenic patients. J. Clin. Pathol.51:652–656.
19. Kahn, F. W., J. M. Jones, and D. M. England.1986. The role of
bronchoal-veolar lavage in the diagnosis of invasive pulmonary Aspergillus infection. Am. J. Clin. Pathol.86:518–523.
20. Kan, V. L.1993. Polymerase chain reaction for the diagnosis of candidiasis.
J. Infect. Dis.168:779–782.
21. Kitamura, K., T. Kaneko, and Y. Yamamoto.1971. Lysis of yeast cells by
enzymes of Arthrobacter luteus. Arch. Biochem. Biophys.145:402–404.
22. Kurzai, O., W. J. Heinz, D. J. Sullivan, D. C. Coleman, M. Frosch, and F. A.
Muhlschlegel.1999. Rapid PCR test for discriminating betweenCandida
albicansandCandida dubliniensisisolates using primers derived from pH-regulated PHR1 and PHR2 genes ofC. albicans. J. Clin. Microbiol.37:1587– 1590.
23. Lee, C. H., J. Helweg-Larsen, X. Tang, S. Jin, B. Li, M. S. Bartlett, J. J. Lu,
B. Lundgren, J. D. Lundgren, M. Olsonn, S. B. Lucas, P. Roux, A. Cargnel,
C. Atzori, O. Matos, and J. W. Smith.1998. Update onPneumocystis carinii
f. sp.hoministyping based on nucleotide sequence variations in internal transcribed spacer regions of rRNA genes. J. Clin. Microbiol.36:734–741.
24. Loffler, J., H. Hebart, U. Schumacher, H. Reitze, and H. Einsele.1997.
Comparison of different methods for extraction of fungal pathogens from cultures and blood. J. Clin. Microbiol.35:3311–3312.
25. Longo, M. C., M. S. Beringer, and J. L. Hartley.1990. Use of uracil DNA
glycosylase to control carry-over contamination in polymerase chain reac-tions. Gene93:125–128.
26. Lu, J. J., M. S. Bartlett, M. M. Shaw, S. F. Queener, J. W. Smith, M.
Ortiz-Rivera, M. J. Leibowitz, and C. H. Lee.1994. Typing ofPneumocystis
cariniistrains that infect humans based on nucleotide sequence variations of the internal transcribed spacers of the rRNA genes. J. Clin. Microbiol.
32:2904–2912.
27. Melchers, W. J. G., R. E. Verweij, P. van den Hurk, A. van Belkum, B. E. De
Pauw, J. A. A. Hoogkamp-Korstanje, and J. F. G. M. Meis.1994. General
primer mediated PCR for detection ofAspergillusspecies. J. Clin. Microbiol.
32:1710–1717.
28. O’Donnell, K.1992. Ribosomal DNA internal transcribed spacers are highly
divergent in the phytopathogenic ascomyteFusarium sambucinum. Curr. Genet.22:213–220.
29. Rappelli, P., R. Are, G. Casu, P. L. Fiori, P. Cappuccinelli, and A. Aceti.
1998. Development of a nested PCR for detection ofCryptococcus neofor-mansin cerbrospinal fluid. J. Clin. Microbiol.36:3438–3440.
30. Richardson, M. D., and M. H. Kokki.1998. Diagnosis and prevention of
fungal infection in the immunocompromised patient. Blood Rev.12:241– 254.
31. Sanders, I. R., M. Alt, K. Groppe, T. Boller, and A. Weiken.1995.
Identifi-cation of ribosomal DNA polymorphisms among and within spores of the Glomaes: application to studies on genetic diversity for arbuscular mycor-rhizal fungal communities. New Phytol.130:419–427.
32. Sandhu, G. S., B. C. Cline, L. Stockman, and G. D. Roberts.1995. Molecular
probes for diagnosis of fungal infection. J. Clin. Microbiol.33:2913–2919.
33. Shin-Ichi, F., B. A. Lasker, T. J. Lott, E. Reiss, and C. J. Morrison.1995.
Microtitration plate enzyme immunoassay to detect PCR-amplified DNA fromCandidaspecies in blood. J. Clin. Microbiol.33:962–967.
34. Spreadbury, C., D. Holde, A. Aufauvre-Brown, B. Bainbridge, and J. Cohen.
1993. Detection ofA. fumigatusby polymerase chain reaction. J. Clin. Mi-crobiol.31:615–621.
35. Stuyver, L., A. Wyseur, W. van Arnhem, F. Hernandez, and G. Maertens.
1996. Second generation line probe assay for hepatitis C virus genotyping. J. Clin. Microbiol.34:2259–2266.
36. Sullivan, D. J., T. J. Westerneng, K. A. Hayes, D. E. Bennet, and D. C.
Coleman.1995.Candida dubliniensissp. nov.: phenotypic and molecular
characterisation of a novel species associated with oral candidosis in HIV infected individuals. Microbiology141:1507–1521.
37. Thompson, J., D. Higgins, and T. Gibson.1994. Clustal W: improving the
sensitivity of progressive multiple sequence alignment through sequence weighting, position specific weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res.22:4673–4680.
38. Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M.
Kabani.1999. Rapid identification of fungi using the ITS2 genetic region and
automated fluorescent capillary electrophoresis system. J. Clin. Microbiol.
37:1846–1851.
39. Van Burik, J.-A., D. Myerson, R. W. Schreckhise, and R. A. Bowden.1998.
Panfungal PCR assay for detection of fungal infection in human blood specimens. J. Clin. Microbiol.36:1169–1175.
40. White, T., T. Bruns, S. Lee, and J. Taylor.1990. Amplification and direct
sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315–322.In PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, N.Y.
41. Williams, D. W., M. J. Wilson, M. A. G. Lewis, and A. J. C. Potts.1995.
Identification ofCandidaspecies by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J. Clin. Microbiol.33:2476–2479.