JOURNAL OFCLINICALMICROBIOLOGY,
0095-1137/00/$04.00⫹0 Feb. 2000, p. 669–676 Vol. 38, No. 2
Copyright © 2000, American Society for Microbiology. All Rights Reserved.
Characterization of
Mycobacterium tuberculosis
Isolates from
Patients in Houston, Texas, by Spoligotyping
HANNA SOINI, XI PAN, AMOL AMIN, EDWARD A. GRAVISS, ANEES SIDDIQUI,ANDJAMES M. MUSSER*
Institute for the Study of Human Bacterial Pathogenesis, Department of Pathology, Baylor College of Medicine, Houston, Texas 77030
Received 25 August 1999/Returned for modification 27 October 1999/Accepted 9 November 1999
Mycobacterium tuberculosisisolates (nⴝ1,429) from 1,283 patients collected as part of an ongoing popula-tion-based tuberculosis epidemiology study in Houston, Texas, were analyzed by spoligotyping and IS6110 pro-filing. The isolates were also assigned to one of three major genetic groups on the basis of nucleotide poly-morphisms located at codons 463 and 95 in the genes (katGandgyrA) encoding catalase-peroxidase and the A subunit of DNA gyrase, respectively. A total of 225 spoligotypes were identified in the 1,429 isolates. There were 54 spoligotypes identified among 713 isolates (n ⴝ 623 patients) assigned to 73 IS6110 clusters. In addition, among 716 isolates (nⴝ660 patients) with unique IS6110profiles, 200 spoligotypes were identified. No changes were observed either in the IS6110 profile or in the spoligotype for the 281 isolates collected sequentially from 133 patients. Five instances in which isolates with slightly different spoligotypes had the same IS6110profile were identified, suggesting that in rare cases isolates with different spoligotypes can be clonally related. Spoligotypes correlated extremely well with major genetic group designations. Only three very similar spoligotypes were shared by isolates from genetic groups 2 and 3, and none was shared by group 1 and group 2 organisms or by group 1 and group 3 organisms. All organisms belonging to genetic groups 2 and 3 failed to hybridize with spacer probes 33 to 36. Taken together, the results support the existence of three distinct genetic groups of M. tuberculosis organisms and provide new information about the relationship between IS6110profiles, spoligotypes, and major genetic groups ofM. tuberculosis.
Spacer oligonucleotide typing (spoligotyping) is a molecular method used to differentiateMycobacterium tuberculosis com-plex isolates. This method is based on the analysis of polymor-phisms in theM. tuberculosiscomplex direct repeat (DR) chro-mosomal region consisting of identical 36-bp DRs alternating with 35- to 41-bp unique spacer sequences. The method is PCR based and hence is more rapid and easier to perform than the standard typing technique based on IS6110profiling (10, 14). Spoligotyping can also be performed directly fromM. tubercu-losisorganisms, even those that are nonviable or that are found in tissues in paraffin-embedded blocks, or in archeological sam-ples (7, 19, 23).
Several studies have provided evidence that spoligotyping is less able to discriminate among isolates with high IS6110copy numbers, whereas spoligotyping is superior to IS6110profiling for isolates with fewer than five IS6110copies (3, 9, 14, 16, 27). Thus, a two-step protocol consisting of initial screening of isolates by spoligotyping, followed by IS6110 profiling of isolates with the same spoligotype, has been suggested (8). Spoligotyping also has been reported to be a useful method for the differentiation of Mycobacterium bovisisolates, because the majority of isolates of this species have only one IS6110element. In addition, the ab-sence of spacers 3, 9, 16, and 39 to 43 is characteristic ofM. bovis isolates (2, 5, 6, 11, 29). Similarly,Mycobacterium microtiand My-cobacterium canettiihave characteristic spoligotypes (15, 18, 26). For accurate interpretation of spoligotype data, it is neces-sary to obtain information about the evolution and relative stability of the DR region in large samples of isolates from
di-verse geographic sources. It is also necessary to gain insight into such issues as the relationship of spoligotypes to the three prin-cipal genetic groups ofM. tuberculosis(22) and the likelihood of evolutionary convergence to the same spoligotype. To address these and other issues, we studied the relationship between spoligotype, IS6110profile, and principal genetic group in 1,429 M. tuberculosisisolates causing disease in Houston, Texas.
MATERIALS AND METHODS
Bacterial isolates.The analysis is based on 1,429M. tuberculosis isolates
collected from 1,283 patients as part of an ongoing, population-based tubercu-losis epidemiology study in Houston, Texas. The strains were cultured from patients between September 1994 and February 1999. These organisms included 281 isolates recovered sequentially from 133 patients.
DNA methods.Chromosomal DNA extraction and IS6110profiling were
per-formed by an internationally standardized protocol (24). The IS6110profiles were analyzed with the BioImage (Ann Arbor, Mich.) Whole Band Analysis program, version 3.2. Spoligotyping was performed with a commercially available kit (Isogen Bioscience BV, Maarssen, The Netherlands) according to the instruc-tions supplied by the manufacturer. The isolates were assigned to one of three principal genetic groups on the basis of nucleotide polymorphism at codons 463 and 95 of the genes encoding catalase-peroxidase and the A subunit of DNA gyrase, respectively (22).
Statistical analysis.Statistical analysis was performed with Epi Info, version
6.04 (Centers for Disease Control and Prevention, Atlanta, Ga.). Chi-square analysis was used to test the association of clustered spoligotypes with IS6110 clustering and with the three major genetic groups. The sensitivity and specificity of spoligotyping were calculated by a method described by Hennekens and Buring (12), by using clustering by IS6110as the “gold standard.” The sensitivity was calculated as the number of patients clustered by both spoligotyping and IS6110profiling, divided by the total number of patients clustered by IS6110 profiling. The specificity was calculated as the number of patients clustered neither by spoligotyping nor by IS6110profiling, divided by the total number of patients not clustered by IS6110profiling.
RESULTS
Spoligotype and IS6110type.A sample of 1,429M. tubercu-losisisolates from 1,283 patients was analyzed by IS6110 pro-* Corresponding author. Present address: Laboratory of Human
Bacterial Pathogenesis, Rocky Mountain Laboratories, National Insti-tute of Allergy and Infectious Diseases, National InstiInsti-tutes of Health, 903 South 4th St., Hamilton, MT 59840. Phone: (406) 363-9315. Fax: (406) 363-9427. E-mail: jmusser@niaid.nih.gov.
669
on May 15, 2020 by guest
http://jcm.asm.org/
filing and spoligotyping. An isolate was assigned a print desig-nation if the same IS6110 pattern was found for isolates obtained from two or more patients. If no matching profiles were identified in the database, or if the IS6110pattern con-tained fewer than 5 copies, the isolate was defined as unique (print 999) (exception: print 006 has a copy number of 4). A total of 225 spoligotypes were identified in the 1,429 isolates
(Fig. 1). There were 54 spoligotypes identified among 713 iso-lates (n ⫽ 623 patients) assigned to 73 IS6110 clusters. In addition, among 716 isolates (n ⫽660 patients) with unique IS6110profiles, 200 spoligotypes were identified. Twenty-nine spoligotypes were shared by clustered and unique isolates.
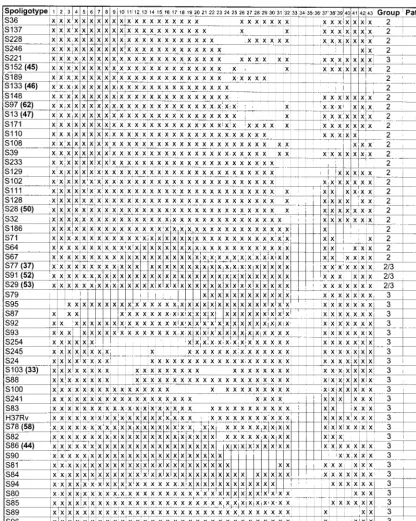
[image:2.612.92.513.72.599.2]By spoligotype alone, isolates from 1,146 patients were di-vided into 89 spoligotypes, whereas 137 patients were infected FIG. 1. Spoligotypes identified in HoustonM. tuberculosisisolates. Column heads: Spoligotype, arbitrary spoligotype designation; numbers 1 to 43, spoligotype probes (an “x” in the field below denotes hybridization, and an empty square indicates lack of hybridization); Group, major genetic group designation; Patients, number of patients infected by an isolate with this spoligotype. Boldfaced numbers in parentheses following arbitrary spoligotype designations correspond to the spoligotype designations provided by Sola et al. (21).
670 SOINI ET AL. J. CLIN. MICROBIOL.
on May 15, 2020 by guest
http://jcm.asm.org/
by organisms with unique spoligotypes. Patients with clustered spoligotypes (the same spoligotype identified in multiple pa-tients) were significantly associated with IS6110 clustering (2⫽59.16;P⬍0.001). Although the sensitivity of the
spoli-gotyping technique was fairly high (599 of 623; 96%), the specificity of spoligotyping in differentiating IS6110-clustered clones from nonclustered clones was relatively low (113 of 660; 17%).
Spoligotype and major genetic group.All isolates were also assigned to one of the three major genetic groups. In general,
[image:3.612.94.511.72.606.2]there was little sharing of major genetic groups and spoligo-types. Moreover, there was no association between the major genetic groups and spoligotype clustering (P⫽0.19). We iden-tified three very similar spoligotypes that were shared by group 2 and group 3 organisms (Table 1), but no sharing of spoligo-types among organisms belonging to major genetic groups 1 and 2, or among group 1 and group 3 organisms, was found. In addition, all isolates of major genetic groups 2 and 3 failed to hybridize with spoligotype probes 33 to 36. These results are consistent with the genetic affiliation of group 2 and group 3 FIG. 1—Continued.
VOL. 38, 2000 SPOLIGOTYPING OF M. TUBERCULOSIS ISOLATES 671
on May 15, 2020 by guest
http://jcm.asm.org/
organisms and the differentiation of group 1 and group 3 or-ganisms (22).
Analysis of large IS6110clusters.We next examined spoli-gotype variation among isolates classified on the basis of IS6110profile. To maximize the opportunity to identify spoli-gotype variation among isolates assigned to an IS6110profile, we studied seven large IS6110clusters with 40 to 123 isolates each. In general, isolates in each cluster had the same spoli-gotype. However, we identified two instances in which an
iso-late with a slightly different spoligotype had the same IS6110 profile. In the case of print 006, which has 4 IS6110copies, 10 different spoligotypes were obtained. In addition, an isolate with a different spoligotype was observed in three print groups with few isolates (005, 085, and 146) (Table 2).
[image:4.612.96.515.71.600.2]The more-abundant spoligotypes.Seventeen of the 23 IS6110 types in genetic group 1 had the same spoligotype, arbitrarily designated S1. This spoligotype was characterized by hybrid-ization with probes 35 to 43 and has been identified previously FIG. 1—Continued.
672 SOINI ET AL. J. CLIN. MICROBIOL.
on May 15, 2020 by guest
http://jcm.asm.org/
(25). Spoligotype S1 was identified in all 309 isolates from 264 patients infected with these 17 IS6110types and was also iden-tified in 67 isolates (62 patients) with unique IS6110profiles. The most common spoligotype found among isolates with low IS6110copy numbers was S12. This pattern was obtained from 147 isolates (from 134 patients) with copy numbers ranging from 2 to 5 and has been identified previously (3) (Table 3).
Serial isolates. Our study included 281 isolates collected sequentially from 133 patients. The interval between isolate collections varied from 1 to 1,043 days (average, 87 days).
Although 76% of the organisms were obtained within 60 days of one another, no changes were observed either in the IS6110 profile or in the spoligotype.
DISCUSSION
Correlation of spoligotypes with major genetic groups. Re-cently three genetic groups of M. tuberculosis isolates were identified on the basis of polymorphic nucleotides in katG codon 463 andgyrA codon 95 (22). According to the evolu-FIG. 1—Continued.
VOL. 38, 2000 SPOLIGOTYPING OF M. TUBERCULOSIS ISOLATES 673
on May 15, 2020 by guest
http://jcm.asm.org/
[image:5.612.98.514.70.591.2]tionary scenario proposed in that study, group 1 isolates are evolutionarily old and have further evolved into group 2 and group 3 organisms (22). Our results show that spoligotypes correlate extremely well with major genetic group designa-tions. Only three very similar spoligotypes were shared by group 2 and group 3 organisms, and, as anticipated, none was shared between group 1 and group 3 organisms. The three shared spoligotypes have been reported to be globally distrib-uted (21). It is probable that the group 3 isolates evolved from a group 2 precursor isolate. Consistent with this idea, we ob-served that all isolates belonging to major genetic groups 2 and 3 failed to hybridize with spacer probes 33 to 36, suggesting that these spacers and DRs have been deleted from the ge-nomes of all group 2 and group 3 organisms. Taken together, these results further support the division of M. tuberculosis isolates into three distinct genetic groups.
Stability of spoligotypes.Relatively little is known about the molecular evolution and stability of the DR region inM. tu-berculosiscomplex isolates. In a recent study, Niemann et al.
analyzed drug-resistantM. tuberculosisisolates recovered se-quentially from 56 patients (17). They found no change in the spoligotypes obtained from these isolates, whereas in five cases a change in the IS6110profile was observed (addition or de-letion of one band) (17). Similarly, in other studies that in-cluded duplicate or serial isolates from the same patient, the spoligotypes were identical (7–9, 13, 20). No change in spoli-gotypes or IS6110profiles was observed in the serial isolates included in our study. Although the interval between collec-tions of sequential isolates varied from 1 to 1,043 days, 76% of the organisms were obtained within 60 days of one another, which may be too short a period for variation to arise in this region.
[image:6.612.52.550.83.223.2]Our study also included IS6110clusters consisting of a large number ofM. tuberculosisisolates with identical or very similar IS6110profiles. We identified only five cases in which an ap-parent change in DR pattern occurred. These results suggest that in rare cases, isolates with different spoligotypes can be clonally related.
TABLE 1. Spoligotypes shared by major genetic groups Spoligotype
designationa Spoligotype pattern profileIS6110a copy no.IS6110 isolatesNo. of patientsNo. of Geneticgroupb
S29 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxxc 006 4 8 8 2
086 10 3 3 3
124 11 2 2 3
999 2–12 7 7 2
999 7–15 29 26 3
S91 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxx.xxxd 009 7 2 2 2
091 11 1 1 3
999 7–9 4 2 2
999 9–10 3 2 3
S77 xxxxxxxxxxxx.xxxxxxxxxxxxxxxxxxx....xxxxxxxe 999 7 1 1 2
999 11 1 1 3
aArbitrary designation.
bDefined by Sreevatsan et al. (22). cSpoligotype 53 defined by Sola et al. (21). dSpoligotype 52 defined by Sola et al. (21). eSpoligotype 37 defined by Sola et al. (21).
TABLE 2. IS6110profiles characterized by different spoligotypes IS6110
profilea copy no.IS6110 Geneticgroupb isolatesNo. of patientsNo. of designationSpoligotypea Spoligotype pattern
001 12 3 107 72 S24 xxxxxxxx...xxxxxxxxxxxxxxxxxxx....xxxxxxx
001 12 3 1 1 S245 xxxxxxxx...x....xxxxxxxxxxxxxx....xxxxxxx
004 6 2 73 59 S3 xxxxxxxxxxxxxxxxx.xxxxxxxxxxxxxx....xxxxxxx
004 6 2 1 1 S5 xxxxxxxxxxxx.xxx...xxxxxxxxxxxxx....xxxxxxx
005 14 3 1 1 H37Rv xxxxxxxxxxxxxxxxxxx..xxxxxxxxxxx....xxxxxxx
005 14 3 2 2 S83 xxxxxxxxxxxxxxxxxxx..xxxxxxxxxxx....xx..xxx
006 4 2 33 25 S25 xxx...x...xxxxxxxxxxxxxx....xxxxxxx
006 4 2 2 1 S33 xxx...xxxxx.xxxxxxxxxxxxxx....xxx....
006 4 2 1 1 S256 xxx...xxxxx.xxxxxxxxxxxxxx....xxxxxx.
006 4 2 8 8 S27 xxx...xxxxx.xxxxxxxxxxxxxx....xxxxxxx
006 4 2 3 3 S26 xxxxxxxxxxxxx...xxxxxxxxxxxxxx....xxxxxxx
006 4 2 1 1 S30 xxxxxxxxxxxxx....xxxxxxxxxxxxxxx....xxxxxxx
006 4 2 1 1 S31 xxxxxxxxxxxxxx.xx.xxxxxxxxxxxxxx....xxxxxxx
006 4 2 13 11 S3 xxxxxxxxxxxxxxxxx.xxxxxxxxxxxxxx....xxxxxxx
006 4 2 1 1 S32 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx...xxxxxxx
006 4 2 8 8 S29 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx
085 5 3 1 1 S78 xxxxxxxxxxxxxxxxxxx.xx.xxxxxxxxx....xxxxxxx
085 5 3 1 1 S100 xxxxxxxxxxxxxxxx...x.xxxxxxxxx....xxxxxxx
146 8 2 1 1 S28 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxx.x....xxxxxxx
146 8 2 1 1 S206 xxxx.xxxxxxxxxxxxxxxxxxxxxxxxx.x....xxxxxxx
aArbitrary designation.
bDefined by Sreevatsan et al. (22).
674 SOINI ET AL. J. CLIN. MICROBIOL.
on May 15, 2020 by guest
http://jcm.asm.org/
[image:6.612.53.549.502.712.2]Correlation with published spoligotypes. On the basis of published spoligotypes, Sola et al. (21) compiled a table of 69 spoligotypes shared by more than two patients in any region of the world. Twenty-six of the 69 spoligotypes were also identi-fied in Houston (Fig. 1). Four of these (spoligotypes 17, 29, 44, and 67) were spoligotypes previously identified in the Carib-bean and neighboring Central American regions only. One of the Houston patients infected by an organism with a Caribbe-an-specific spoligotype was born in the Caribbean (St. Lucia), but no direct epidemiologic links could be found for the other patients.
A large number of the Houston patients (326 of 1283; 25%) were infected by anM. tuberculosisisolate of spoligotype S1. This is the characteristic pattern of the Beijing family ofM. tuberculosisisolates, which is prevalent in China and neighbor-ing countries but uncommon in Europe and the Caribbean (21, 25). This is also the spoligotype of the W family ofM. tuber-culosisisolates, which is a group of closely related multidrug-resistant organisms that have recently spread from New York City to other U.S. communities and to Paris (1, 4). Our results indicate thatM. tuberculosisisolates with this spoligotype are also common in Houston. In contrast to spoligotype S1, the majority of the spoligotypes identified in Houston have not been described previously. Our spoligotype data thus support the results obtained by IS6110profiling showing that mostM. tuberculosisisolates are confined to a specific geographic loca-tion (28).
ACKNOWLEDGMENT
This research was supported by Public Health Service grant DA-09238 to J.M.M.
REFERENCES
1.Agerton, T. B., S. E. Valway, R. J. Blinkhorn, K. L. Shilkret, R. Reves, W. W.
Schluter, B. Gore, C. J. Pozsik, B. B. Plikaytis, C. Woodley, and I. M.
Onorato.1999. Spread of strain W, a highly drug-resistant strain of
Myco-bacterium tuberculosis, across the United States. Clin. Infect. Dis.29:85–92.
2.Aranaz, A., E. Liebana, A. Mateos, L. Dominguez, D. Vidal, M. Domingo, O.
Gonzolez, E. F. Rodriguez-Ferri, A. E. Bunschoten, J. D. A. van Embden, and
D. Cousins.1996. Spacer oligonucleotide typing ofMycobacterium bovis
strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J. Clin. Microbiol.34:2734–2740.
3.Bauer, J., A. B. Andersen, K. Kremer, and H. Miorner.1999. Usefulness of
spoligotyping to discriminate IS6110low-copy-numberMycobacterium tuber-culosiscomplex strains cultured in Denmark. J. Clin. Microbiol.37:2602– 2606.
4.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey,
S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford,
J. M. Musser, and B. N. Kreiswirth.1996. Origin and interstate spread of a
New York City multidrug-resistantMycobacterium tuberculosisclone family. JAMA275:452–457.
5.Blazquez, J., L. E. Espinosa de los Monteros, S. Samper, C. Martin, A.
Guerrero, J. Cobo, J. van Embden, F. Baquero, and E. Gomez-Mampaso.
1997. Genetic characterization of multidrug-resistantMycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J. Clin. Microbiol.35:1390–1393.
6.Cousins, D., S. Williams, E. Liebana, A. Aranaz, A. Bunschoten, J. van
Embden, and T. Ellis.1998. Evaluation of four DNA typing techniques in
epidemiological investigations of bovine tuberculosis. J. Clin. Microbiol.36:
168–178.
7.Driscoll, J. R., M. A. McGarry, and H. W. Taber.1999. DNA typing of a
nonviable culture ofMycobacterium tuberculosisin a homeless shelter out-break. J. Clin. Microbiol.37:274–275.
8.Goguet de la Salmonie`re, Y.-O., H. M. Li, G. Torrea, A. Bunschoten, J. van
Embden, and B. Gicquel.1997. Evaluation of spoligotyping in a study of the
transmission ofMycobacterium tuberculosis. J. Clin. Microbiol.35:2210–2214.
9.Goyal, M., N. A. Saunders, J. D. A. van Embden, D. B. Young, and R. J.
Shaw.1997. Differentiation ofMycobacterium tuberculosisisolates by
spoli-gotyping and IS6110restriction fragment length polymorphism. J. Clin. Mi-crobiol.35:647–651.
10. Groenen, P. M. A., A. E. Bunschoten, D. van Soolingen, and J. D. A. van
Embden.1993. Nature of DNA polymorphism in the direct repeat cluster of
Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol. Microbiol.10:1057–1065.
11. Gutierrez, M., S. Samper, M. S. Jimenez, J. D. A. van Embden, J. F. G.
Marin, and C. Martin.1997. Identification by spoligotyping of a caprine
genotype inMycobacterium bovisstrains causing human tuberculosis. J. Clin. Microbiol.35:3328–3330.
12. Hennekens, C. H., and J. E. Buring.1987. Epidemiology in disease control,
p. 327–347.InS. L. Mayrent (ed.), Epidemiology in medicine. Little, Brown & Co., Boston, Mass.
13. Horgen, L., C. Sola, A. Devallois, K. S. Goh, and N. Rastogi.1998. Follow up
ofMycobacterium tuberculosistransmission in the French West Indies by IS6110-DNA fingerprinting and DR-based spoligotyping. FEMS Immunol. Med. Microbiol.21:203–212.
[image:7.612.66.548.82.304.2]14. Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S.
TABLE 3. The most common spoligotypes observed in the Houston area Spoligotype
designationa Spoligotype pattern profileIS6110a copy no.IS6110 isolatesNo. of patientsNo. of Geneticgroupb
S1 ...xxxxxxxxxc 002 13 87 78 1
003 20 33 29 1
007 10 54 42 1
015 9 30 24 1
022 14 12 10 1
024 12 21 17 1
027 15 1 1 1
033 21 54 48 1
034 18 3 3 1
035 18 2 2 1
037 17 2 1 1
040 12 1 1 1
059 19 2 2 1
074 13 1 1 1
076 20 2 2 1
145 20 2 1 1
148 20 2 2 1
999 0–23 67 62 1
S12 xxxxxxxxxxxxxxxxx.xxxxxxxxxxxxxx....xx....x 012 5 3 3 2
999 2–5 144 131 2
aArbitrary designation.
bDefined by Sreevatsan et al. (22). cSpoligotype 1 defined by Sola et al. (21).
VOL. 38, 2000 SPOLIGOTYPING OF M. TUBERCULOSIS ISOLATES 675
on May 15, 2020 by guest
http://jcm.asm.org/
Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van
Embden.1997. Simultaneous detection and strain differentiation of
Myco-bacterium tuberculosisfor diagnosis and epidemiology. J. Clin. Microbiol.35:
907–914.
15. Kremer, K., D. van Soolingen, J. van Embden, S. Hughes, J. Inwald, and G.
Hewinson.1998.Mycobacterium microti: more widespread than previously
thought. J. Clin. Microbiol.36:2793–2794.
16. Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. M.
Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley,
M. A. Yakrus, J. M. Musser, and J. D. A. van Embden.1999. Comparison of
methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosiscomplex strains: interlaboratory study of discrim-inatory power and reproducibility. J. Clin. Microbiol.37:2607–2618.
17.Niemann, S., E. Richter, and S. Rusch-Gerdes.1999. Stability of
Mycobac-terium tuberculosisIS6110restriction fragment length polymorphism patterns and spoligotypes determined by analyzing serial isolates from patients with drug-resistant tuberculosis. J. Clin. Microbiol.37:409–412.
18. Pfyffer, G. E., R. Auckenthaler, J. D. A. van Embden, and D. van Soolingen.
1998.Mycobacterium canettii, the smooth variant ofM. tuberculosis, isolated from a Swiss patient exposed in Africa. Emerg. Infect. Dis.4:631–634.
19. Qian, L., J. D. A. van Embden, A. G. M. van der Zanden, E. F. Weltevreden,
H. Duanmu, and J. T. Douglas.1999. Retrospective analysis of the Beijing
family ofMycobacterium tuberculosisin preserved lung tissues. J. Clin. Mi-crobiol.37:471–474.
20. Sola, C., L. Horgen, J. Maisetti, A. Devallois, K. S. Goh, and N. Rastogi.
1998. Spoligotyping followed by double-repetitive-element PCR as rapid alternative to IS6110fingerprinting for epidemiological studies of tubercu-losis. J. Clin. Microbiol.36:1122–1124.
21. Sola, C., A. Devallois, L. Horgen, J. Maisetti, I. Filliol, E. Legrand, and N.
Rastogi.1999. Tuberculosis in the Caribbean: using spacer oligonucleotide
typing to understand strain origin and transmission. Emerg. Infect. Dis.5:
404–414.
22. Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth,
T. S. Whittam, and J. M. Musser.1997. Restricted structural gene
polymor-phism in theMycobacterium tuberculosiscomplex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA94:9869–9874.
23. Taylor, G. M., M. Goyal, A. J. Legge, R. J. Shaw, and D. Young.1999.
Genotypic analysis ofMycobacterium tuberculosisfrom medieval human re-mains. Microbiology145:899–904.
24. van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D.
Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick,
and P. M. Small.1993. Strain identification ofMycobacterium tuberculosisby
DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol.31:406–409.
25. van Soolingen, D., L. Qian, P. E. W. de Haas, J. T. Douglas, H. Traore, F.
Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadwa, and J. D. A. van Embden.
1995. Predominance of a single genotype ofMycobacterium tuberculosisin countries of east Asia. J. Clin. Microbiol.33:3234–3238.
26. van Soolingen, D., A. G. M. van der Zanden, P. E. W. de Haas, G. T.
Noordhoek, A. Kiers, N. A. Foudraine, F. Portaels, A. H. J. Kolk, K. Kremer,
and J. D. A. van Embden.1998. Diagnosis ofMycobacterium microti
infec-tions among humans by using novel genetic markers. J. Clin. Microbiol.36:
1840–1845.
27. Wilson, S. M., S. Goss, and F. Drobnievski.1998. Evaluation of strategies for
molecular fingerprinting for use in the routine work of a mycobacterium reference unit. J. Clin. Microbiol.36:3385–3388.
28. Yang, Z., P. F. Barnes, F. Chaves, K. D. Eisenach, S. E. Weis, J. H. Bates,
and M. D. Cave.1998. Diversity of DNA fingerprints of Mycobacterium
tuberculosisisolates in the United States. J. Clin. Microbiol.36:1003–1007.
29. Zumarraga, M. J., C. Martin, S. Samper, A. Alito, O. Latini, F. Bigi, E. Roxo,
M. E. Cicuta, F. Errico, M. C. Ramos, A. Cataldi, D. van Soolingen, and M. I.
Romano.1999. Usefulness of spoligotyping in molecular epidemiology of
Mycobacterium bovis-related infections in South America. J. Clin. Microbiol.
37:296–303.
676 SOINI ET AL. J. CLIN. MICROBIOL.