Effect of cigarette smoking on levels of
bioavailable testosterone in healthy men
Katherine M. ENGLISH
*
, Peter J. PUGH
*
, Helen PARRY
*
, Nanette E. SCUTT†,
Kevin S. CHANNER
*
and T. Hugh JONES†‡
*
Department of Cardiology, M Floor, Royal Hallamshire Hospital, Glossop Road, Sheffield S10 2JF, U.K., †Endocrinology and
Cardiovasular Research Group, G Floor, Institute of Endocrinology, The Medical School, Beech Hill Road, Sheffield S10 2RX,
U.K., and ‡Centre for Diabetes and Endocrinology, Barnsley District General Hospital, Gawber Road, Barnsley, South Yorkshire
S75 2EP, U.K.
A
B
S
T
R
A
C
T
The effect of smoking on androgen levels is important given the recent interest in the link between low levels of androgens and the development of cardiovascular disease. Numerous studies examining the effects of cigarette smoking on the levels of total and free testosterone have reported conflicting findings, but there has been no accurate assessment of the effects of cigarette smoking on the levels of bioavailable testosterone [not bound to sex hormone-binding globulin (SHBG)]. We attempted to determine whether smoking affects the level of bioavailable testosterone. We undertook a case-control study of 25 healthy male smokers and 25 healthy never-smokers, matched by age and body mass index. Early morning levels of total, free and bioavailable testosterone, 17β-oestradiol, SHBG and cotinine were determined and compared between the two groups. Levels of total (18.5p4.6 nM versus 15.1p4.9 nM,Pl0.01) and free testosterone (462p91 pM versus 402p93 pM,Pl0.03) were found to be higher in smokers compared with non-smokers respectively, as was SHBG (34.1p12.8 versus 28.1p9.0 nM,Pl
0.06). There were no significant differences in the levels of bioavailable testosterone (3.78p1.59 versus 3.51p1.26 nM,Pl0.49) or 17β-oestradiol (44.5p11.4 versus 42.3p11.5 pM,Pl0.50) between smokers and non-smokers respectively. These data suggest that cigarette smoking has no significant effect on the biologically active fraction of testosterone, but may influence the levels of total and free testosterone through changes in the levels of SHBG.
INTRODUCTION
The results of previous studies describing the effects of cigarette smoking on testosterone levels in human males have been conflicting, demonstrating that testosterone levels are lower [1,2], similar [3–8] or higher [9–14] in healthy male smokers than in matched non-smokers. However, the effects of cigarette smoking on the levels of bioavailable testosterone are unknown.
There is increasing interest surrounding the link between low levels of male sex hormones and coronary
Key words:androgens, bioavailable testosterone, coronary artery disease, oestradiol, smoking, testosterone.
Abbreviation:SHBG, sex hormone-binding globulin.
Correspondence:Dr T. H. Jones, at the Centre for Diabetes and Endocrinology, Barnsley District General Hospital (e-mail hugh.jones!bdgh-tr.trent.nhs.uk).
artery disease in men. A number of studies have reported lower levels of androgens in cases with coronary heart disease compared with disease-free controls [15], this relationship being particularly marked when the levels of bioavailable testosterone are assessed [16]. The effects of an excess of smokers in groups with coronary artery disease compared with controls in these studies may be an important confounding factor, with the demonstrated differences in levels of testosterone being only a conse-quence of cigarette smoking, not a risk factor for coronary heart disease itself.
The aim of the present study was to determine whether the biologically active fraction of testosterone differs in healthy male smokers and non-smokers, since a signifi-cant effect of smoking on androgen levels may confound association studies between levels of testosterone and coronary artery disease.
METHODS
Study design
This was a case-control study undertaken in a teaching hospital with the approval of the local ethics committee. Healthy volunteers were recruited from hospital visitors and staff by posting advertisements in the hospital. Smokers were matched to never-smokers of similar age and body mass index. Subjects were studied between 08.00 h and 09.30 h.
Subjects
A total of 25 male smokers of10 cigarettes\day and 25 males who had never smoked (aged 17–60 years) were recruited. Volunteers were excluded if they had debilitat-ing illness, diabetes mellitus, a history of infertility or hormone-manipulating therapy. Two cases and one control had mild asthma and two controls had hy-pertension. Junior doctors were excluded from the study since previous research [17] suggests they may have abnormal androgen levels.
Measurements
Height and weight were measured, and blood was taken for analysis of levels of serum total testosterone, bioavail-able testosterone, sex hormone-binding globulin (SHBG), 17β-oestradiol and cotinine (a metabolite of nicotine). Free testosterone was calculated as a percentage of the total testosterone by a validated method [18], using the formula :
% Free testosteronel6.11k2.38ilog"![SHBG] C-reactive protein was measured to ensure that no subject was suffering from an acute inflammatory illness, which may have affected hormone levels.
Total testosterone was measured by RIA (Coat-A-Count ; Diagnostic Products Corp., Llanberris, Caernar-fon, U.K.). Percentage bioavailable testosterone was assayed using an adaptation of the method of Tremblay and Dube [19], where $H-labelled testosterone radio-active tracer was measured in the supernatant fraction following ammonium sulphate precipitation of SHBG. The concentration of bioavailable testosterone was then calculated from the percentage of the total steroid. Inter-assay variation was less than 8 % throughout the range of
the assay. SHBG was measured by automated enzyme immunoassay (Immulite ; Diagnostic Products Corpor-ation, Los Angeles, CA, U.S.A.). 17β-Oestradiol was measured using an ELISA assay from DRG Instruments GmbH, Germany. Cotinine was measured using the Cozart serum cotinine microplate enzyme immunoassay (Cozart Bioscience Ltd, Abingdon, Oxford, U.K.), to ensure accuracy of the self-reported smoking\ non-smoking status of our subjects [20]. C-reactive protein was measured using the high-sensitivity Biostat\Diasys kit.
Statistical analysis
We estimated a sample size requirement of 25 people in each group in order to demonstrate a difference of at least one S.D. in androgen levels with 90 % power and 5 % significance. Data were inspected and analysed using the SPSS for Windows 95 computer package. Values for all parameters proved to be normally distributed except those for alcohol intake. Student’s independent samplest test was used to compare mean values between the two groups for parametric data. The Mann–WhitneyUtest was used to analyse non-parametric data. Correlation coefficients were determined using Pearson’s correlation statistic for parametric data. All results are expressed as meanspS.D. unless otherwise stated. Statistical signifi-cance was accepted whenP 0.05.
RESULTS
Baseline characteristics of the two groups are shown in Table 1. Cases were matched with controls for age and body mass index. Smokers had higher alcohol intake than controls, but this difference was not statistically signifi-cant. The large difference in cotinine levels demonstrates the reliability of the self-reported smoking\non-smoking status of our subjects.
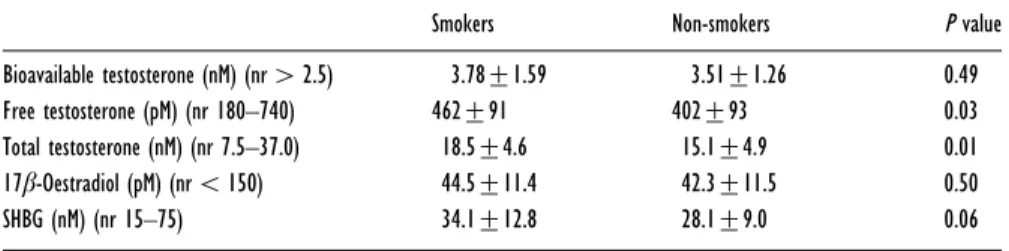
Table 2 shows the hormone profiles of the two groups. Levels of total and free testosterone were significantly higher in smokers compared with non-smokers ; how-ever, the more accurate assessment of biologically active androgen status, bioavailable testosterone, was not sig-nificantly different. SHBG was higher in smokers than in non-smokers ; this difference approached statistical sig-nificance. There was no difference in 17β-oestradiol levels between the two groups.
Serum cotinine levels correlated positively with the reported number of cigarettes smoked daily (rl0.40, P 0.05) and with levels of SHBG (rl0.49,P 0.05), but not with any of the hormone levels analysed. None of the subjects had raised C-reactive protein, suggestive of concurrent acute inflammatory illness.
Table 1 Baseline characteristics of smokers and non-smokers
Results are expressed as meanspS.D.
Smokers Non-smokers P value
Age (years) 35p11 35p11 0.93
Body mass index (kg/m2) 23.2p2.7 24.5p3.1 0.12
Cotinine (ng/ml) 327.2p173.8 2.7p0.5 0.001
Alcohol intake (units/week) 18.8p14.6 11.4p9.8 0.063
Table 2 Hormone levels in smokers and non-smokers
Results are expressed as meanspS.D. Abbreviation : nr, normal range.
Smokers Non-smokers P value
Bioavailable testosterone (nM) (nr2.5) 3.78p1.59 3.51p1.26 0.49 Free testosterone (pM) (nr 180–740) 462p91 402p93 0.03 Total testosterone (nM) (nr 7.5–37.0) 18.5p4.6 15.1p4.9 0.01 17β-Oestradiol (pM) (nr 150) 44.5p11.4 42.3p11.5 0.50 SHBG (nM) (nr 15–75) 34.1p12.8 28.1p9.0 0.06
DISCUSSION
The higher incidence of ischaemic heart disease in men compared with women, and reports of sudden car-diovascular death amongst male athletes abusing anabolic steroids, have led many to assume that androgens are detrimental to the male cardiovascular system [21,22]. However, there is no direct evidence to support this theory, and there are numerous studies to support the contrary view. It has been convincingly demonstrated in male animal models that low androgen levels are associ-ated with an increase in atheroma development, a phenomenon which is reversed by the administration of physiological levels of androgens [23–25]. In addition, a number of case-control studies have described an association between low androgen levels and excess cardiovascular risk in human males [15,26–28]. This relationship is particularly marked when the bioavailable fraction of testosterone is assessed [16]. However, it is well recognized that case-control studies may be easily confounded by other factors. The effects of an excess of smokers in groups with coronary artery disease com-pared with controls in these studies may be an important confounding factor, with the demonstrated differences in levels of testosterone being only a consequence of cigarette smoking, not a risk factor for coronary heart disease itself.
Previous work looking at the effects of smoking on androgen levels has produced conflicting results, which may be explained, in part, by difficulties in the accurate assessment of testosterone levels. Androgens display marked circadian fluctuations in plasma levels, and considerable intra- and inter-individual variability. In addition, much (65–80 %) of the circulating total
tes-tosterone is inactive and tightly bound to SHBG, whereas the biologically active fraction circulates either free (1–3 %) in the circulation or loosely bound (20–35 %) to albumin [29]. Levels of total testosterone can, therefore, be directly affected by changes in levels of SHBG and other plasma proteins. In addition, the direct immuno-assay techniques used most commonly to measure free testosterone are also subject to variation due to differ-ences in SHBG level [30]. Thus the measurement of total or free testosterone alone is insufficient to accurately assess the true impact of smoking on biologically active androgen status.
Two previous studies [1,2] demonstrated significant falls in the levels of total testosterone amongst male smokers compared with non-smokers, but the levels of free, bioavailable testosterone, oestradiol or SHBG were not assessed in these patients. Conversely, many other studies [9–14] have found the levels of total testosterone to be increased amongst male smokers. Two of these studies [10,14] also reported an increase in the levels of free testosterone. However, in all but one of these studies [9], no measurement of SHBG was made, and hence the testosterone levels were not adjusted for differences in plasma protein binding. The one study which did measure SHBG [9] demonstrated a significant increase in SHBG, but no significant change in ‘ albumin-bound testos-terone,’ suggesting the raised total testosterone levels were secondary to the raised SHBG. This is the only previous study to measure ‘ albumin-bound testoster-one ’, an approximation of the bioavailable fraction, and no significant difference was seen. Finally, a number of studies [3–8] have reported no significant differences in the levels of total or free testosterone in male smokers versus non-smokers.
The present study demonstrates that plasma levels of biologically active testosterone, as assessed by the levels of bioavailable testosterone, do not differ between healthy male smokers and non-smokers, whereas levels of total and free testosterone are significantly higher in smokers. This discrepancy is a reflection of the difference in SHBG, which was higher in the smokers than in non-smokers. We have also demonstrated that there was no significant difference in 17β-oestradiol levels, suggesting that the increased SHBG in the smokers is independent of changes in 17β-oestradiol levels.
SHBG was the only variable which significantly correlated with serum cotinine levels, a measure of cigarette smoking. This may indicate that smoking has a greater relative effect on the levels of SHBG than on the hormones themselves, supporting the suggestion that the observed differences in total and free testosterone in this and previous studies are due to changes in plasma binding capacity, rather than an effect of smoking on androgen levels. Plasma levels of SHBG may also be influenced by other clinical factors. Thyrotoxicosis and liver cirrhosis are both associated with elevated SHBG levels, whereas hypothyroidism and obesity have been linked with decreased SHBG levels [31,32]. Although thyroid and liver function tests were not performed in this study, there was no clinical suspicion of thyroid or liver disease in any subject. Furthermore, subjects were matched by body mass index (Table 1).
We conclude that accurate assessment of androgen status should include a measure of the biologically active fraction. In addition, it appears that smoking has no significant effect on the levels of bioavailable testosterone, and causes an increase in the measured levels of total testosterone. There is no evidence to suggest, therefore, that smoking status may be a confounding factor in association studies demonstrating a link between low levels of androgens and coronary heart disease.
ACKNOWLEDGMENTS
We thank Dr Mike Diver, University of Liverpool, for performing the bioavailable testosterone assays, and Mr Martin Loxley, Department of Clinical Chemistry, Royal Hallamshire Hospital, Sheffield, for his expert help in performing the SHBG and total testosterone assays.
REFERENCES
1 Briggs, M. H. (1973) Cigarette smoking and infertility in men. Med. J. Aust.1, 616–617
2 Shaarawy, M. and Mahmoud, K. Z. (1982) Endocrine profile and semen characteristics in male smokers. Fertil. Steril.38, 255–257
3 Klaiber, E. L. and Broverman, D. M. (1988) Dynamics of estradiol and testosterone and seminal fluid indexes in smokers and non-smokers. Fertil. Steril.50, 630–634
4 Hautanen, A., Manttari, M., Kupari, M. et al. (1993) Cigarette smoking is associated with elevated adrenal androgen response to adrenocorticotropin. J. Steroid. Biochem. Mol. Biol.46, 245–251
5 Barrett-Connor, E. and Khaw, K.-T. (1987) Cigarette smoking and increased endogenous estrogen levels in men. Am. J. Epidemiol.126, 187–192
6 Lindholm, J., Winkel, P., Brodthagen, U. and Gyntelberg, F. (1982) Coronary risk factors and plasma sex hormones. Am. J. Med.73, 648–651
7 Meikle, A. W., Bishop, D. T., Stringham, J. D., Ford, M. H. and West, D. W. (1987) Cigarette smoking alters plasma sex-steroid levels. Clin. Res.35, 183A
8 Handelsman, D. J., Conway, A. J., Boylan, L. M. and Turtle, J. R. (1984) Testicular function in potential sperm donors : normal ranges and the effects of smoking and varicocele. Int. J. Androl.7, 369–382
9 Field, A. E., Colditz, G. A., Willett, W. C., Longcope, C. and McKinlay, J. B. (1994) The relation of smoking, age, relative weight and dietary intake to serum adrenal steroids, sex hormones and sex hormone-binding globulin in middle-aged men. J. Clin. Endocrinol. Metab79, 1310–1316
10 Handa, K., Ishii, H., Kono, S. et al. (1997) Behavioural correlates of plasma sex hormones and their relationships with plasma lipids and lipoproteins in Japanese men. Atherosclerosis130, 37–44
11 Vogt, H. J., Heller, W. D. and Borelli, S. (1986) Sperm quality of healthy smokers, ex-smokers, and never smokers. Fertil. Steril.45, 106–110
12 Attia, A. M., El-Dakhly, M. R., Halawa, F. A., Ragab, N. F. and Mossa, M. M. (1989) Cigarette smoking and male reproduction. Arch. Androl.23, 45–49
13 Deslypere, J. P. and Vermeulen, A. (1984) Leydig cell function in normal men : effect of age, lifestyle, residence, diet and activity. J. Clin. Endocrinol. Metab.59, 955–962 14 Dai, W. S., Gutai, J. P., Kuller, L. H. and Cauley, J. A.
(1988) Cigarette smoking and serum sex hormones in men. Am. J. Epidemiol.128, 796–805
15 Alexandersen, P., Haarbo, J. and Christiansen, C. (1996) The relationship of natural androgens to coronary heart disease in males : a review. Atherosclerosis125, 1–13 16 English, K. M., Mandour, O., Steeds, R. P., Diver, M. J.,
Jones, T. H. and Channer, K. S. (2000) Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur. Heart. J.21, 890–894
17 Singer, F. and Zumoff, B. (1992) Subnormal serum testosterone levels in male internal medicine residents. Steroids57, 86–89
18 Nanjee, M. N. and Wheeler, M. J. (1985) Plasma free testosterone – is an index sufficient ? Ann. Clin. Biochem.
22, 387–390
19 Tremblay, R. R. and Dube, J. Y. (1974) Plasma
concentrations of free and non Te-BG bound testosterone in women on oral contraceptives. Contraception10, 599–605
20 Smith, R. F., Mather, H. M. and Ellard, G. A. (1998) Assessment of simple colorimetric procedures to determine smoking status of diabetic subjects. Clin. Chem.44, 275–280
21 British Heart Foundation Statistics Database (1996) British Heart Foundation
22 Sullivan, L., Martinez, C. M., Gennis, P and Gallagher, E. J. (1998) The cardiac toxicity of anabolic steroids. Prog. Cardiovasc. Dis.41, 1–15
23 Alexandersen, P., Haarbo, J., Byrjalsen, I., Lawaetz, H. and Christiansen, C. (1999) Natural androgens inhibit male atherosclerosis: A study in castrated, cholesterol fed rabbits. Circ. Res.84, 813–819
24 Larsen, B. A., Nordestgaard, B. G., Stender, S. and Kjeldsen, K. (1993) Effect of testosterone on atherogenesis in cholesterol-fed rabbits with similar plasma cholesterol levels. Atherosclerosis99, 79–86
25 Bruck, B., Brehme, U., Gugel, N. et al. (1997) Gender specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol.17, 2192–2199
26 Herrington, D. M. (1995) Dehydroepiandrosterone and coronary atherosclerosis. Ann. N.Y. Acad. Sci.774, 271–280
27 Wu, S. Z. and Weng, X. Z. (1993) Therapeutic effects of an androgenic preparation on myocardial ischaemia and cardiac function in 62 elderly male coronary heart disease patients. Chin. Med. J.106, 415–418
28 Zhao, S. P. and Li, X. P. (1998) The association of low plasma testosterone level with coronary artery disease in Chinese men. Int. J. Cardiol.63, 161–164
29 Dunn, J. F., Nisula, B. C. and Rodbard, D. (1981) Transport of steroid hormones : Binding of 21 endogenous steroids to both testosterone-binding globulin and
Received 5 January 2001/8 February 2001; accepted 22 March 2001
corticosteroid binding globulin in human plasma. J. Clin. Endocrinol. Metab.53, 58–68
30 Vermeulen, A., Verdonck, L. and Kaufman, J. M. (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab.84, 3666–3672
31 Rosner, W. (1990) The functions of corticosteroid-binding globulin and sex hormone-binding globulin : recent advances. Endocr. Rev.11, 80–91
32 von Schoultz, B. and Carlstrom, K. (1989) On the regulation of sex-hormone-binding globulin–a challenge of an old dogma and outlines of an alternative mechanism. J. Steroid. Biochem.32, 327–334