Analysis in Early Stage Triple-Negative Breast Cancer Treated
With Mastectomy Without Adjuvant Radiotherapy: Patterns of
Failure and Prognostic Factors
Xingxing Chen, MD1,2†; Xiaoli Yu, MD1,2†; Jiayi Chen, MD1,2; Zhen Zhang, MD1,2; Jeffrey Tuan3; Zhimin Shao, MD4; Xiaomao Guo, MD1,2; and Yan Feng, MD1,2
BACKGROUND:The objective of this study was to evaluate and identify patterns of failure and prognostic factors for locoregional re-currence (LRR) that could justify postmastectomy radiotherapy after modified radical mastectomy in patients with early stage triple-negative breast cancer. METHODS:Between January 2000 and July 2007, the authors retrospectively analyzed 390 patients who had triple-negative breast cancer with T1/T2 tumors and from zero to 3 positive lymph nodes (pathologic T1-T2N0-N1) who under-went modified radical mastectomy without postmastectomy radiotherapy at the author’s institution. The 5-year cumulative incidence for events was calculated using Kaplan-Meier analysis, and subgroups were compared using the log-rank test. Multivariate analysis was performed using a Cox proportional hazards model.RESULTS:Overall, 86.4% of patients received chemotherapy. At a median follow-up of 60.5 months, the 5-year cumulative rates of local recurrence, regional recurrence, LRR, and distant metastasis were 5.4%, 4.7%, 8%, and 13.4%, respectively. On multivariate analysis, age<50 years, the presence of lymphovascular invasion, grade 3 tumor, and 3 involved lymph nodes were associated significantly with an increased risk of LRR. The 5-year LRR rate for patients who had 0 or 1 risk factor, 2 risk factors, and 3 or 4 risk factors was 4.2%, 25.2%, and 81% (P<.0001), respectively. The presence of lymphovascu-lar invasion and having 3 involved lymph nodes were statistically significant predictors of regional recurrence, and the patients who had regional recurrence had a significantly greater risk of distant metastases compared with patients who had local recurrence (59.1% vs 20.9%;P < .0001).CONCLUSIONS:Several risk factors were identified in this study that correlated independently with a greater incidence of LRR in patients who had early stage triple-negative breast cancer. The current results indicated that postmastec-tomy radiotherapy should be considered for those patients who have 2 or more of these factors.Cancer2013;119:2366-74.VC 2013 American Cancer Society.
KEYWORDS:breast cancer; triple-negative; molecular subtype; mastectomy; postmastectomy radiotherapy; locoregional recurrence; pattern of failure; risk factors.
INTRODUCTION
Breast cancer is a heterogeneous disease that encompasses 5 different intrinsic molecular subtypes, each with distinct
prog-nostic and predictive features.1Among these subtypes, triple-negative breast cancer (TNBC), which is negative for
estro-gen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), has recently
drawn substantial attention because of its aggressive behavior, high risk of early relapse, and poor overall survival.2
Cur-rently, there are no proven effective therapies for this subtype of breast cancer, making it a clinical challenge for optimal patient management.
Postmastectomy radiotherapy (PMRT) is an important strategy for the local management of breast cancer after
sur-gery and/or chemotherapy. Large randomized trials3-5in patients with tumors of all molecular subtypes have
demon-strated that PMRT improves locoregional control and overall survival in patients who have high-risk breast cancer after systemic chemotherapy. Therefore, the results from those trials support the current consensus guidelines recommending
PMRT for patients who have high disease burden, defined as tumors>5 cm in greatest dimension and4 involved
axil-lary lymph nodes (ALNs).6,7In patients who have early stage breast cancer, defined as lymph node-negative disease or
stage II disease with 1 to 3 positive lymph nodes, there are data demonstrating that PMRT reduces LRR rates to a similar
Corresponding author:Xiaomao Guo, MD, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, 270 Dong An Road, Shanghai 200032, China; Fax: (011) 8621-64174774; guoxiaomao188@gmail.com
1
Department of Radiation Oncology, Hospital of Fudan University, Shanghai, China;2
Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China;3
Department of Radiation Oncology, National Cancer Center Singapore, Singapore;4
Department of Breast Cancer Surgery, Hospital of Fudan University Shanghai, China.
The first 2 authors contributed equally to this work.
DOI:10.1002/cncr.28085,Received:November 17, 2012;Revised:February 26, 2013;Accepted:March 4, 2013,Published onlineApril 10, 2013 in Wiley Online Library (wileyonlinelibrary.com)
degree (by approximately 66%). However, compared with patients who have advanced disease, the absolute reduction in LRR with PMRT for patients with early stage breast cancer is much smaller, because their baseline
LRR rate is relatively low.8,9It is generally accepted that
the survival benefit from PMRT is proportional to the reduction in LRR; therefore, the use of PMRT in early stage breast cancer remains controversial, because only a small absolute magnitude of benefit can be expected.
It is worth noting that the indications described above are based solely on tumor (T) classification and lymph node (N) status, without considering the biologic heterogeneity of breast cancer. More studies since have demonstrated that patients who have
TNBC are at a higher risk of LRR,10,11 including
those who have very early disease.12,13 Moreover, in
a recent article by Abdulkarim et al14 reporting
exclusively on TNBC, the highest LRR rates were observed in patients who underwent modified radical mastectomy without PMRT compared with those who underwent modified radical mastectomy and received PMRT or breast-conserving therapy. This highlights the need to re-evaluate prognostic factors in early stage TNBC (ETNBC) and the utility of PMRT.
Previous studies assessing locoregional outcomes of patients with TNBC who underwent modified radical mas-tectomy have been limited by small patient numbers, and data specific to patients with ETNBC are even rarer. A bet-ter understanding of patbet-terns of recurrence and prognostic factors in ETNBC is urgently needed to aid decision mak-ing for adjuvant therapy, and especially for PMRT.
In this retrospective study, we investigated the recur-rence patterns and predictive factors for LRR in a large ho-mogenous series of patients with TNBC who were diagnosed with pathologic T1-T2 tumors and zero to 3 positive lymph nodes (pT1-T2N0-N1) and who under-went modified radical mastectomy without receiving PMRT at a single institution. Our objective was to define a subset of patients at sufficiently high risk of LRR who could benefit from PMRT.
MATERIALS AND METHODS
Patients
From January 1, 2000 to July 31, 2007, 5194 consecutive patients with stage I to III breast cancer who underwent modified radical mastectomy were entered into our insti-tutional database. The definition of TNBC was based on immunohistochemical staining for ER, PR, and HER2,
which was routinely performed in the pathology
depart-ment of our hospital as previously described.15The
pres-ence of 1% nuclear staining for ER and PR was
categorized as negative, whereas immunohistochemical
HER2 expression from 0 to 21or nonamplified HER2
according to fluorescence in situ hybridization analysis was considered HER2-negative. Patients who were nega-tive for ER, PR, and HER2 had their tumors defined as TNBC. In total, 709 patients were registered in the data-base as patients with TNBC. Of these, 509 patients had pT1-T2N0-N1 disease. Patients who had distant metasta-sis (DM) at diagnometasta-sis, those who had carcinoma in situ, those who received neoadjuvant chemotherapy or PMRT, and those who had less than 1 month of follow-up were excluded from analysis. The remaining 390 patients were included in the current investigation. Review of data for this investigation was approved by the Institutional Review Board of our hospital. Clinicopathologic informa-tion for all patients was recorded in a computerized data-base at accrual and included details about patient, tumor, and treatment characteristics and clinical outcomes. Patients were followed every 3 months during the first 2 years and then every 6 months during next years after the completion of treatment.
Statistical Analysis
Local recurrence (LR) was defined as a recurrence in the ipsilateral chest wall (CW). Regional recurrence (RR) was defined as a recurrence involving the ipsilateral axillary, supraclavicular, intraclavicular, or internal mammary lymph nodes (IMNs). LRR was defined as LR and/or RR. Recurrence at any other site was considered DM, includ-ing secondary malignancy in the contralateral breast or other organs. Local and regional events were recorded regardless of their relation in time to DM. The endpoints were calculated from the date of surgery to the date of the occurrence of the event of interest. In the absence of an event of interest, the observation time was censored at the date on which follow-up ended.
The 5-year cumulative incidence for failures was cal-culated using Kaplan-Meier analysis, and subgroups were compared using the log-rank test. Cox proportional haz-ards models were used to estimate hazard ratios (HRs) and to correlate between outcomes and established risk variables, including age, tumor size, ALN, LVI, grade, and chemotherapy. All statistical analyses were carried out using the STATA statistical software package (version
11.0; Stata Corporation, College Station, Tex). AllP
val-ues were 2-sided, andP.05 was considered statistically
RESULTS
Clinical Features
Patient, tumor, and treatment characteristics for all 390 patients are listed in Table 1. The median follow-up was 60.5 months (range, 1-139 months), the median age was 53 years (range, 31-90 years), and the median tumor size was 2.0 cm (range, 0.3-5.0 cm). Overall, 307 patients (78.7%) had negative (N0) ALNs, 44 patients (11.3%) had 1 positive ALN, 27 patients (6.9%) had 2 positive ALNs, and 12 patients (3.1%) had 3 positive ALNs. All patients underwent modified radical mastectomy, includ-ing level I to II axillary dissection. The median number of ALNs removed was 15 (range, 1-33 ALNs removed). Eleven patients (2.8%) underwent immediate breast reconstruction. Three hundred thirty-seven patients (86.4%) received adjuvant chemotherapy, consisting of an anthracycline-based regimen in 267 patients; cyclo-phosphamide, methotrexate, and 5-fluorouracil in 67 patients; and other regimens in 3 patients. None of the study participants received PMRT, adjuvant endocrine therapy, or trastuzumab.
Locoregional Recurrence
Thirty-one patients (7.9%) had LRR (Table 2). The 5-year cumulative incidence of LRR was 80%. The sites of LRR in 31 patients were the CW in 13 patients (41.9%), supraclavicular/infraclavicular lymph nodes (SILNs) in 9 patients (29%), IMNs in 2 patients (6.5%), and multiple synchronous locations in 7 patients (22.6%; CW and IMNs; CW and SILNs; CW, SILNs, and IMNs; and CW, SILNs, IMNs, and axilla regions).
Table 2 presents the 5-year crude and cumulative rates of LRR, LR, and RR stratified by patient, tumor, and treatment characteristics. On univariate analysis of
LRR, age<50 years, the presence of LVI, grade 3 tumor,
and 3 positive ALNs were associated significantly with a
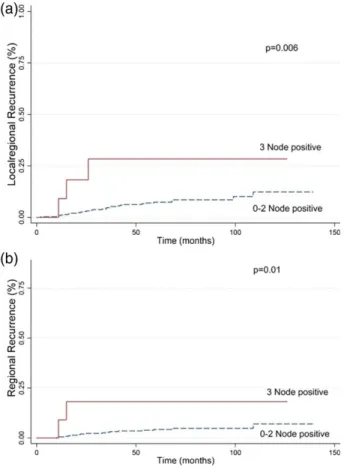
higher risk of LRR (allP<.05) (Fig. 1a). Multivariate
Cox regression analysis revealed that age<50 years (HR,
4.82; 95% confidence interval [CI], 1.35-17.1;
P5.015), 3 positive ALNs (HR, 8.76; 95% CI,
2.04-37.57;P5.003), the presence of LVI (HR, 26.05; 95%
CI, 9.53-71.16;P<.001), and grade 3 tumor (HR, 2.87;
95% CI, 1.05-7.82; P5.039) were statistically
signifi-cant, independent predictors of LRR (Table 3). Local Recurrence
Twenty patients (5.3%) developed LR (Table 2). The 5-year cumulative LR rate was 5.4%. Factors that adversely affected the LR rate on univariate analysis included age
<50 years, the presence of LVI, grade 3 tumor, and
im-mediate reconstruction (allP<.05). Two of 20 patients
who developed LR (18.2%) underwent immediate breast reconstruction. Multivariate Cox regression analysis
indi-cated 2 significant independent predictors of LR: age<50
years (HR, 9.56; 95% CI, 1.18-77.02;P5.034) and the
presence of LVI (HR, 26.45; 95% CI, 7.74-90.43;
P<.001) (Table 3).
TABLE 1. Patient, Tumor, and Treatment Characteristics Characteristic No. of Patients (%) All patients 390 (100) Age, y <50 139 (35.6) 50 251 (64.4) Menopausal status Premenopausal 174 (44.6) Postmenopausal 216 (55.4) Family history Moderate-strong 60 (15.4) No/unknown 330 (84.6) Laterality Left 208 (53.3) Right 179 (45.9) Bilateral 3 (0.8) Pathologic type Ductal 330 (84.6) Others 60 (15.4) Stage I 162 (41.5) IIA 181 (46.4) IIB 47 (12.1) Tumor size, cm 2.0 198 (50.8) 2.1-5.0 192 (49.2) LVI Yes 38 (9.7) No 252 (64.6) Unknown 100 (25.6) Grade 1-2 272 (69.7) 3 60 (15.4) Unknown 58 (14.9)
No. of excised lymph nodes
1-5 10 (2.6)
6-10 86 (22.1)
11-15 173 (44.4)
16 120 (30.8)
No. of positive lymph nodes
0 307 (78.7) 1 44 (11.3) 2 27 (6.9) 3 12 (3.1) Immediately reconstruction Yes 11 (2.8) No 379 (97.2) Chemotherapy Anthracyline 267 (68.5) CMF 67 (17.2) Others 3 (0.8) No 53 (13.6)
Abbreviations: CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; LVI, lymphovascular invasion.
Regional Recurrence
Eighteen patients (4.6%) developed RR (Table 2). The 5-year cumulative RR rate was 4.7%. On univariate analy-sis of RR, the presence of LVI, grade 3 tumor, and 3 posi-tive ALNs were associated with increased RR (all
P<.05). Patients who had 3 positive ALNs had a
signifi-cantly increased RR rate compared with those who had 1 positive ALN, 2 positive ALNs, or from 0 to 2 positive
ALNs (all P<.05) (Fig. 1b). The 5-year RR rate was
18.2% in patients who had 3 positive ALNs versus 2.4% in patients who had 1 positive ALN, 0% in patients who had 2 positive ALNs, and 4.3% in patients who had from
0 to 2 positive ALNs. On multivariate analysis, 3 positive
ALNs (HR, 7.13; 95% CI, 1.22-41.57;P5.029) and the
presence of LVI (HR, 10.19; 95% CI, 2.89-35.88;
P<.001) remained significant predictors of increased RR
(Table 3).
Distant Metastasis
In total, 52 patients (13.3%) developed DM, and the 5-year cumulative DM rate was 13.4%. On univariate analysis, positive ALNs and the presence of LVI were
associated with an increased risk of DM (bothP<.05).
On multivariable analysis, the presence of LVI was the TABLE 2.Crude Rates and 5-Year Kaplan-Meier Rates of Local Recurrence, Regional Recurrence, and Locoregional Recurrence According to Patient, Tumor, and Treatment Characteristics
LR RR LRR Characteristic Crude Rate: No. (%) 5-Year Rate, % P Crude Rate: No. (%) 5-Year Rate, % P Crude Rate: No. (%) 5-Year Rate, % P All patients 20 (5.3) 5.4 — 18 (4.6) 4.7 — 31 (7.9) 8 — Age, y .04 .08 .04 <50 3 (2.2) 1.6 3 (2.2) 1.5 6 (4.3) 3.1 50 17 (6.8) 7.4 15 (6) 6.5 25 (10) 10.7 Laterality .12 .25 .04 Left 14 (6.7) 7.8 12 (5.8) 5.6 22 (10.6) Right 6 (3.4) 2.6 6 (3.4) 3.7 9 (5) Pathologic type .53 .25 .85 Ductal 16 (4.8) 5.2 17 (5.2) 5.1 26 (7.9) 7.8 Others 4 (6.7) 6.6 0 (0) 2.5 5 (8.3) 9.1 Tumor size, cm .31 .56 .48 2.0 8 (4) 4.8 8 (4) 4.3 14 (7.1) 7.9 2.1-5.0 12 (6.3) 5.9 10 (5.2) 5.1 17 (8.9) 8.1 LVI .00 .00 .00 Yes 12 (31.6) 25.6 10 (26.3) 15.6 17 (44.7) 33.9 No 5 (2) 2.3 6 (2.4) 2.9 9 (3.6) 4.2 Grade .003 .02 .006 1-2 12 (4.4) 4.1 11 (4) 3.8 20 (7.4) 6.6 3 8 (13.3) 16.7 6 (10) 12.2 10 (16.7) 21
Lymph node status .07 .74 .16
N0 13 (4.2) 4.4 15 (4.9) 4.9 22 (7.2) 7.1
N1 7 (8.4) 9.1 3 (3.6) 3.9 9 (10.8) 11.5
No. of positive lymph nodes .28 .06 .03
0 13(4.2) 4.4 15 (4.9) 4.9 22 (7.2) 7.1 1 3 (6.8) 5.2 1 (2.3) 2.4 3 (6.8) 5.2 2 3 (11.1) 13.7 0 (0) 0 3 (11.1) 13.7 3 1 (8.3) 12.5 2 (16.7) 18.2 3 (25) 28.4 0-2 vs 3 .40 .01 .006 0-2 19 (5) 5.2 16 (4.2) 4.3 28 (7.4) 7.4 0 vs 1 .31 .03 .004 0 vs 2 .10 .25 .41 0 vs 3 .36 .51 .9 1 vs 3 .72 .04 .05 2 vs 3 .99 .03 .19 Reconstruction .01 .51 .07 Yes 2 (18.2) 25.9 18(4.7) 0 2 (18.2) 7.6 No 18 (4.7) 4.9 0(0.0) 5.4 29 (7.7) 25.9 Chemotherapy .63 .75 .52 Yes 18 (5.3) 5.6 16(4.7) 5.2 28 (8.3) 8.7 No 2 (3.8) 3.9 2(3.8) 2 3 (5.7) 3.9
Abbreviations: CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; LR, local recurrence; LRR, locoregional recurrence; LVI, lymphovascular invasion; RR, regional recurrence.
only significant adverse factor for DM (HR, 12.69; 95%
CI, 5.12-31.40;P<.001) (Table 3). DM occurred in 17
of 31 LRR patients. For patients with LRR, the 5-year DM risk was 46.6%. Patients who developed RR had a significantly greater risk of 5-year DM compared with
patients who developed LR (59.1% vs 20.9%;P<.0001)
(Fig. 2).There were higher rates of visceral metastases compared with bone or other metastases (32.9% and 12.9%, respectively).
Locoregional Recurrence Rates According to Different Numbers of Risk Factors
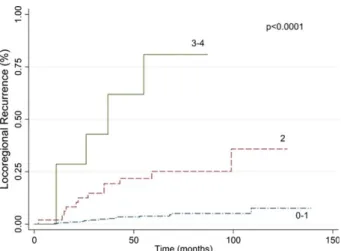
We calculated the 5-year cumulative LRR rates using combinations of 4 significant factors identified on
multi-variate analysis: age<50 years, 3 positive ALNs, the
pres-ence of LVI, and grade 3 tumor (Table 3). The 5-year LRR rate for patients who had 0 or 1 risk factor, 2 risk factors, and 3 or 4 risk factors was 4.2%, 25.2%, and
81%, respectively (P<.0001) (Fig. 3).
DISCUSSION
The current study demonstrated that younger age (<50
years), the presence of LVI, grade 3 tumor, and 3 positive ALNs are associated with a greater risk of LRR among
Figure 1.The risk of (a) locoregional recurrence and (b) re-gional recurrence is illustrated according to the number of positive lymph nodes.
TABLE 3. Multivariate Analysis of Predictors for Local Recurrence, Regional Recurrence, Locoregional Recurrence and Distant Metastases
LR RR LRR DM
Variable HR (95% CI) P HR (95% CI) P HR (95% CI) P HR (95% CI) P
Age:<50 y vs50 y 9.56 (1.18-77.02) .034 3.16 (0.66-15.12) .148 4.82 (1.35-17.1) .015 2.07 (0.72-5.92) .172
Pathologic tumor classification: pT2 vs pT1
1.12 (0.35-3.58) .847 0.33 (0.08-1.32) .118 0.63 (0.24-1.63) .34 0.53 (0.20-1.36) .191
No. of positive lymph nodes: 3 vs 0-2
3.37 (0.34-32.78) .294 7.13 (1.22-41.57) .029 8.76 (2.04-37.57) .003 1.01 (0.11-8.67) .993
LVI status: Positive vs negative 26.45 (7.74-90.43) .000 10.19 (2.89-35.88) .000 26.05 (9.53-71.16) .000 12.69 (5.12-31.40) .000
Grade: 3 vs 1-2 2.76 (0.79-9.55) .108 2.25 (0.58-8.63) .237 2.87 (1.05-7.82) .039 0.58 (0.13-2.57) .482
Chemotherapy: Yes vs no 0.87 (0.21-3.45) .846 0.85 (0.17-4.17) .851 0.91 (0.28-2.86) .87 1.02 (0.32-3.19) .967
Abbreviations: CI, confidence interval; DM, distant metastases; HR, hazard ratio; LR, local recurrence; LRR, locoregional recurrence; LVI, lymphovascular inva-sion; RR, regional recurrence.
Figure 2.The risk of distant metastasis is illustrated accord-ing to the site of locoregional recurrence (LRR). RR indicates regional recurrence; LR, local recurrence.
patients with pT1-T2N0-N1 TNBC who undergo modi-fied radical mastectomy and receive chemotherapy with-out PMRT. When these factors were examined in combination, patients who had 2 or more of them had a
significantly higher 5-year LRR rate of >25%. In
addition, the presence of 3 positive ALNs conferred a sig-nificantly higher 5-year RR risk of 18% compared with the presence of 1, 2, or 0 to 2 positive ALNs. These char-acteristics should be considered when recommending PMRT in ETNBC.
The selection criteria for PMRT have largely been derived from randomized controlled trials in which the baseline risk of LRR was relatively low; only small abso-lute improvements have been documented in patients with pN0-N1 disease (5-year baseline LRR rate: pN0, 6
%; pN1, 11%-26%).8,9This has made the indication for
PMRT in early stage breast cancer uncertain. It should be highlighted that the patients enrolled on those large trials had heterogeneous molecular subtypes, and no parameters other than tumor classification and lymph node status were analyzed for prognostic value. Although accumulat-ing data have identified additional clinicopathologic factors—young age, high-grade tumors, the presence of LVI, and negative ER status are predictors of higher LRR
in early stage breast cancer16,17, molecular characteristics
still were not considered in those studies. This appears be an oversimplification in the new era, in which individual therapy based on biologic characteristics has been increas-ingly accepted.
Recently, biologic molecular subtypes based on ER, PR, and HER2 have been identified as important, inde-pendent prognostic and predictive biomarkers in breast cancer. For the moment, the incorporation of molecular
subtypes into the tailoring of systemic treatment has become the standard of care, whereas the therapeutic implications of molecular subtypes on local management are minimal. Multiple studies have indicated a strong prognostic relation between patients with TNBC and increased LRR, and this also applied to early stage breast
cancer.10-13,18 In patients with ETNBC, Abdulkarim
et al14recently reported that the LRR risk in the modified
radical mastectomy without PMRT group was 2.5 times higher than in the breast-conserving therapy group among
those with T1-T2N0 disease (4% vs 10%; P5.027).
This has sparked an intense debate about re-evaluating the role of PMRT for ETNBC, a subgroup for which it was previously assumed that PMRT was not clearly indicated. Therefore, further investigation regarding the indications for PMRT in patients with TNBC is warranted. To our knowledge, the current study is the first to evaluate the patterns of failure and predictive factors for LRR exclu-sively in patient with TNBC who have pT1-T2N0-N1 disease after undergoing modified radical mastectomy, and we believe our results may help clinicians in deciding how to tailor PMRT for this specific population.
In our study, the baseline LRR rate was 8% for the entire study population. This LRR rate is comparable to
that reported in other subtypes with early stage disease,19
whereas it is relatively lower compared with the 10% to 11.6% LRR rate in patients with pT1-T2N0-N1 TNBC,
as reported in previous studies.14,20 This slightly lower
rate LRR rate in our study may be attributed in part to aggressive axillary clearance, with a mean number of 15 lymph nodes (range, 1-33 lymph nodes) dissected in our series. In the context of multiple adverse prognostic fac-tors, our data concur with previous studies of breast cancer in all subtypes indicating that the LRR rate was suffi-ciently high enough to warrant PMRT in the presence of
2 or more adverse factors (5-year LRR rate, >25%). In
particular, the adverse clinicopathologic factors identified in our series that correlated with a high LRR rate were age
<50 years, 3 positive ALNs, the presence of LVI, and
grade 3 tumor.
An important finding from our study was that the presence of 3 positive ALNs was a poor predictor of both LRR and RR compared with negative ALNs or 1 or 2 pos-itive ALNs, indicating that the absolute number of meta-static lymph nodes, rather than grouped together as “1 to 3” metastatic lymph nodes, may have definitive prognos-tic value in ETNBC. Although the inverse relation between the number of ALNs dissected and prognosis are
well established in breast cancer,21data on the association
between ALNs and LRR in early stage breast cancer still
Figure 3. The risk of locoregional recurrence is illustrated according to the number of risk factors.
are rare. Rakha et al reported the outcomes of women with pT1-T2N0-N1 breast cancer who underwent modi-fied radical mastectomy or received breast-conserving
therapy22and reported that 3 positive ALNs were
associ-ated with poorer breast cancer-specific survival compared with 0, 1, or 2 positive ALNs. Sharma et al analyzed 1091 patients with pT1-T2N0-N1 breast cancer who under-went modified radical mastectomy without PMRT and reported that the 10-year LRR rate was significantly higher in the group that had 2 positive ALNs compared with the group that had negative lymph nodes (2.1% vs
7.9%;P5.003).16 In our study, patients with ETNBC
who had 3 positive ALNs had an increased LRR rate of 28.4% and a higher RR rate of 18.2% compared with the patients who had 0 to 2 positive ALNs without PMRT, indicating that patients who have ETNBC with 3 positive ALNs may benefit from comprehensive PMRT, including both the CW and the regional lymph nodes.
The current results confirm previously published
data demonstrating that age<50 years, the presence of
LVI, and grade 3 tumor are associated with increased
LRR in patients with pT1-T2N0-N1 breast cancer.16,17
Conversely, in the current study, risk factors like pT2 dis-ease and systemic therapy, which were previously reported as significant predictors for LRR in the modified radical
mastectomy setting,16revealed no correlation.
Our observation of 13 CW relapses among 31 LRR events is concordant with previous reports that the CW is
the most common site of postmastectomy LRR.23
Fur-thermore, our 5-year RR rate of 4.7% is in keeping with previous reports of an RR rate of 5% to 6% in patients
with pT1-T2N0-N1 disease.23Consistent with previous
publications on TNBC, the patterns of DM recorded in our series confirmed that there were increased rates of vis-ceral metastases (lung, liver, and brain), compared with
bone metastasis, among patients with ETNBC.24
In the current study, the overall rate of DM was sim-ilar to that in early stage breast cancers of all subtypes, as
previously reported.25The presence of LVI was the only
independent prognostic factor for DM, highlighting the substantial influence it has on the risk of DM, especially in patients with ETNBC. In patients without LVI, the
risk of DM was predominantly <10% at 5 years. The
prognostic value of positive ALN status on DM in early stage breast cancer has been supported by previous
stud-ies.26However, in our study of ETNBC, although
posi-tive ALN status was associated with increased risk of DM in univariate analysis, it was no longer a significant dis-criminator in multivariate analysis. Other factors that were identified previously as significant prognostic factors
for DM in early stage breast cancer,25such as high grade
and large tumor size, were not associated with risk of DM in our study.
The 5-year DM rate for patients with LRR in our study was 46.6%, which is similar to the 51.4% rate previ-ously reported in patients who had TNBC tumors with
LRR27and significantly higher compared with the rate in
patients with non-TNBC tumors (9.2%). Our data also demonstrated a higher DM rate for patients who devel-oped LRR versus those who did not develop LRR. Specifi-cally, when stratified according to the localization of the initial LRR sites, the highest risk for DM was observed among patients who had both LR and RR, followed by patients who had RR alone, and patients who had LR alone. In accordance with other studies, this finding indi-cates that, in addition to the profound adverse effects on quality of life, LRR after modified radical mastectomy also may be a harbinger of secondary dissemination,
espe-cially for those with RR.28More important, a higher risk
of subsequent events and death in patients with TNBC at LRR was observed compared with the risk for patients
who had other subtypes in the study by Montagna et al.28
From this point of view, avoiding LRR can have substan-tial benefit, particularly for patients with TNBC; identify-ing a subgroup of patients at sufficient high risk of LRR to warrant PMRT may be an effective strategy to reduce LRR while sparing treatment-related toxicities. Moreover, in patients who develop LRR, more intensive systemic therapy should be considered in patients with RR than in those with LR alone.
In contemporary clinical practice, patients with pT1-T2N0-N1 breast cancer, including all subtypes, have been perceived as a low-risk to intermediate-risk popula-tion that does not routinely receive PMRT. The current study focused on patients with TNBC who had pT1-T2N0-N1 disease, demonstrating that there is a subgroup of patients having a high risk of LRR when several clinico-pathologic variables are present, despite that the overall LRR rate for the entire population is relatively low. It has been suggested that definite baseline LRR risks should be
categorized into groups with high risk (>20%), moderate
risk (10%-20%), and low risk (<10%) of LRR to guide
the selection of patients for PMRT.29Accordingly, in our
study, when risk factors were examined individually, women who had 3 positive ALNs or the presence of LVI
alone experienced a 5-year LRR risk>25%, which should
merit consideration of PMRT. In the concomitant pres-ence of 2 or more risk factors, a much higher 5-year LRR rate of 25.2% to 81% was observed, which warrants strong consideration of PMRT. The risk factors for LRR
and the increased LRR risks with the presence of those fac-tors in our study were similar to the risk facfac-tors reported previously in patients with early stage breast cancer after modified radical mastectomy without radiotherapy
regardless of breast cancer subtype.19 Our results may
have a considerable impact on managing the selection of adjuvant therapy for this special patient cohort.
There are certain limitations of our study. First, the current study had inherent selective biases because of its retrospective nature. Although we endeavored to mitigate several risk variables by controlling them in the multivari-able model, it is possible that other unmeasured/unknown confounders that were not taken into account may have influenced our results. Second, there may be the potential for misclassification of patients with TNBC using
immu-nohistochemistry alone. The presence of 21 HER2
expression could not be re-evaluated using the fluores-cence in situ hybridization technique for most patients in our cohort. In an effort to further validate the current findings, we conducted a separate analysis in which we
excluded the 72 patients (18.5%) who had 21 HER2
expression. That subset analysis produced the same results as our previous findings. Finally, the number of patients
who had 2 positive ALNs (n527; 6.9%) and 3 positive
ALNs (n512; 3.1%) disease was very small in our study,
and the results for these patients need to be interpreted with caution. A larger sample size and longer follow-up may help validate the current outcomes and better define subgroups of patients who can benefit from PMRT with respect to survival. Despite these limitations, the results from our study suggest that not all patients who had pT1-T2N0-N1 TNBC were truly at low risk, and an assessment of multiple risk factors may help predict LRR.
In conclusion, our population-based study has identified a subset of patients with pT1-T2N0-N1
TNBC in which the rate of LRR is >25%, which is
in contrast to the 8% incidence for the entire
popu-lation. The risk factors younger age (<50 years), the
presence of LVI, and grade 3 tumor or 3 positive ALNs are significant for postmastectomy LRR, and patients who have 2 or more of these factors have a 5-year LRR rate of 25.2% to 81%, a risk that would be considered sufficiently high to justify the addition of PMRT for these patients to optimize locoregional control and improve survival. Finally, the relatively high incidence of RR in our study raises questions about the inclusion of regional areas in the radiation field for patients who have 3 positive ALNs. Prospec-tive research is warranted to confirm the role of PMRT in this special patient population.
FUNDING SUPPORT
This study was supported in part by the National Natural Science Foundation of China (grant 81072164).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
1. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implica-tions.Proc Natl Acad Sci U S A. 2001;98:10869-10874.
2. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast can-cer: clinical features and patterns of recurrence. Clin Cancer Res.
2007;13(15 pt 1):4429-4434.
3. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radio-therapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial.N Engl J Med.1997;337:949-955.
4. Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial.J Natl Cancer Inst.2005;97:116-126.
5. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local re-currence and 15-year survival: an overview of the randomised trials.
Lancet.2005;366:2087-2106.
6. Carlson RW, Anderson BO, Burstein HJ, et al. Breast cancer.J Natl Compr Canc Netw.2005;3:238-289.
7. Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology.J Clin Oncol.2001;19:1539-1569.
8. Favourable and unfavourable effects on long-term survival of radio-therapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000; 355:1757-1770.
9. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmas-tectomy irradiation limited to patients with 4 or more positive nodes, as recommended in international consensus reports? A sub-group analysis of the DBCG 82 b&c randomized trials.Radiother Oncol.2007;82:247-253.
10. Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group.J Clin Oncol. 2008; 26:1419-1426.
11. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse.J Clin Oncol.2010;28:1684-1691.
12. Kwon JH, Kim YJ, Lee KW, et al. Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carci-noma of 1 cm or less [serial online].BMC Cancer.2010;10:557. 13. Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in
women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat.2011;127:713-720.
14. Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-T2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy.J Clin Oncol.2011;29:2852-2858. 15. Yin WJ, Lu JS, Di GH, et al. Clinicopathological features of the
triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat.2009;115:325-333.
16. Sharma R, Bedrosian I, Lucci A, et al. Present-day locoregional control in patients with T1 or T2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol.2010;17:2899-2908.
17. Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and 1 to 3 positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys.2005;61:1337-1347.
18. Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a 5-biomarker panel.J Clin Oncol.2009;27:4701-4708.
19. Trovo M, Durofil E, Polesel J, et al. Locoregional failure in early-stage breast cancer patients treated with radical mastectomy and adjuvant systemic therapy: which patients benefit from postmastec-tomy irradiation [serial online]? Int J Radiat Oncol Biol Phys.
2012;83:e153-e157.
20. Wang SL, Li YX, Song YW, et al. Triple-negative or HER2-positive status predicts higher rates of locoregional recurrence in node-positive breast cancer patients after mastectomy. Int J Radiat Oncol Biol Phys.2011;80:1095-1101.
21. Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. Ten-year pathologic and clinical prognostic discriminants. Cancer.
1993;71:2507-2514.
22. Rakha EA, Morgan D, Macmillan D. The prognostic significance of early stage lymph node positivity in operable invasive breast carci-noma: number or stage.J Clin Pathol.2012;65:624-630.
23. Truong PT, Olivotto IA, Speers CH, Wai ES, Berthelet E, Kader HA. A positive margin is not always an indication for radiotherapy after mastectomy in early breast cancer.Int J Radiat Oncol Biol Phys.
2004;58:797-804.
24. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes.J Clin Oncol.2010;28:3271-3277.
25. Yildirim E, Berberoglu U. Can a subgroup of node-negative breast carcinoma patients with T1-2 tumor who may benefit from postmas-tectomy radiotherapy be identified? Int J Radiat Oncol Biol Phys.
2007;68:1024-1029.
26. Yildirim E, Berberoglu U. Local recurrence in breast carcinoma patients with T(1-2) and 1-3 positive nodes: indications for radio-therapy.Eur J Surg Oncol.2007;33:28-32.
27. Parikh RR, Housman D, Yang Q, Toppmeyer D, Wilson LD, Haffty BG. Prognostic value of triple-negative phenotype at the time of locally recurrent, conservatively treated breast cancer.Int J Radiat Oncol Biol Phys.2008;72:1056-1063.
28. Montagna E, Bagnardi V, Rotmensz N, et al. Breast cancer subtypes and outcome after local and regional relapse.Ann Oncol. 2012;23: 324-331.
29. Olivotto IA, Truong PT, Chua B. Postmastectomy radiation ther-apy: who needs it?J Clin Oncol.2004;22:4237-4239.