Original Article
Integrin β1 mediates 5-fluorouracil chemoresistance
under translational control of eIF4E in colorectal cancer
Zhengchuan Niu*, Pingping Xu*, Dexiang Zhu*, Wentao Tang, Meiling Ji, Qi Lin, Tianyu Liu, Li Ren, Ye Wei, Jianmin Xu
Department of General Surgery, Zhongshan Hospital of Fudan University, Shanghai 200032, China. *Equal
contributors.
Received April 22, 2018; Accepted May 23, 2018; Epub October 1, 2018; Published October 15, 2018
Abstract: Purpose: In recent years, aberrant mRNA translational control has gained much attention as a critical player in the malignant process of tumors. Eukaryotic initiation factor 4E (eIF4E), by binding to the mRNA cap,
can regulate specific protein synthesis, contributing to malignancy in human tumors. However, integrin β1 medi -ated chemoresistance under translational control remains unknown in colorectal cancer. Patients and methods:
The expression relationship between eIF4E and Integrin β1, along with their clinical significance was investigated
in colorectal cancerous tissues of 118 cases using immunohistochemistry. Cell transfection techniques of small interfering RNA (siRNA) and cDNA expression plasmid were applied to investigate the molecular relationship of
integrin β1 and eIF4E and their biological effects on 5FU resistance in SW480 and LoVo cell lines. Results: The ex
-pression of eIF4E and integrin β1 was positively correlated in colorectal cancer, and patients with high ex-pressions of both markers tended to have a worse prognosis according to a Kaplan-Meier survival analysis. Integrin β1 could contribute to 5-fluorouracil (5FU) resistance in colorectal cancer cell lines. Moreover, the protein expression of β1 could be regulated by eIF4E, interestingly, without any change of mRNA expression level. Significantly, Hoechst/PI double staining and an MTT assay proved integrin β1 could contribute to cellular survival and 5FU resistance under translational control of eIF4E in these cells. Conclusion: We conclude that integrin β1 mediated 5FU chemo resis -tance in colorectal cancer could be translationally regulated by eIF4E. Promisingly, targeting key molecules of this translational apparatus may provide an innovative therapeutic strategy for colorectal cancer.
Keywords: Integrin β1, eukaryotic initiation factor-4E, chemo resistance, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignancies globally [1, 2]. Although
fluorouracil-based systemic chemotherapy has
become an integral option in treating advanced colorectal cancer, a considerable number of patients still have disease recurrence and
pro-gression after surgery due to 5FU resistance.
Recently, translational control has been proved to participate in cancer progression and cancer response to stresses [3], which can cause adaptive changes in cancer cells that stimulate the protein expressions in stress-response genes [4]. Within the eukaryotic initiation factor 4F (eIF4F) complex, eIF4E is considered the least common rate-limiting component [5], functioning as a central regulator in
translation-al control [6]. It is capable of selectively binding weak mRNAs, which have a long structure [7-9]. Subsequently, eIF4E can enhance the transla-tion of these mRNAs into proteins associated
with tumor malignancy, including fibroblast
growth factor (FGF-2), vascular endothelial
growth factor (VEGF), c-myc, cyclin D1, matrix
metalloprotease 9 (MMP-9) and others [10, 11].
When cancer cells are exposed to the 5FU che -motherapeutic drug, translational control of rel-evant gene expression is required for cellular
survival to respond to 5FU induced damage.
Recently, a possible connection between trans-lational control and integrins was proposed [12]. Studies proved integrin-dependent trans-lational control could play a critical role in the regulation of tumor malignancy [13]. Among all
integrin signaling can participate in promoting cellular survival and tumor progression [14-17].
In addition, both β1 integrin and eIF4E have
been demonstrated to be involved in
chemo-therapy in different tumors [18-23]. However,
the clinical and biological relationship between
eIF4E and integrin β1 remains obscure. In this
study, we observed the expressions of eIF4E
and integrin β1 in colorectal cancer and further
investigated their implications in cellular apop-tosis and chemo resistance, which may
promis-ingly provide new therapeutic strategies for improving patients’ prognosis.
Materials and methods
Antibody and reagent
Primary antibodies against eIF4E (Epitomics,
Burlingame, USA) and Integrin β1 (Proteintech, Chicago, USA) were used for both
[image:2.612.92.522.95.569.2]immuno-histochemistry staining and Western blotting. Table 1. Association between eIF4E expression, Integrin β1 expression and clinicopathologic vari -ables in colorectal cancer cases
Clinicopathologic Factor
β1 expression
P Value
eIF4E expression
P Value High
n=37 n=62Low Negative n=19 n=45High n=56Low Negative n=17
Gender 0.775 0.278
Male 23 34 11 30 30 8
Female 14 28 8 15 26 9
Age (years) 0.268 0.51
<65 25 33 13 30 31 10
≥65 12 29 6 15 25 7
Tumor Dimeter (cm) 0.039 0.027
>5 21 27 4 25 24 3
≤5 16 35 15 20 32 14
Location 0.19 0.302
Right Colon 5 14 3 6 15 1
Left Colon 15 14 3 13 14 5
Rectum 17 34 13 26 27 11
T stage 0.647 0.024
T1 1 3 2 1 3 2
T2 3 10 3 2 9 5
T3 8 16 3 10 16 1
T4 25 33 11 32 28 9
Lymph node Invasion 0.001 0.002
Negative 15 41 17 19 40 14
Positive 22 21 2 26 16 3
M stage 0.001 0.032
M0 26 58 19 35 51 17
M1 11 4 0 10 5 0
TNM stage <0.001 <0.001
I 1 7 5 1 6 6
II 6 31 12 10 31 8
III 19 20 2 24 14 3
IV 11 4 0 10 5 0
Differentiation 0.289 0.251
Well 7 5 2 7 7 0
Moderate 22 49 15 29 41 16
Besides, antibodies used for the detection of
β-actin (4E8H3), αv (P2W7), β3 (N20) and β6
(C-19) were also used in Western blotting, and they were all purchased from Santa Cruz
Biotechnology (Santa Cruz, USA). 5-Fluorouracil
was obtained from Millipore Sigma (Darmstadt, Germany).
Clinical sample and immunohistochemistry
One hundred eighteen patients who were pathologically diagnosed with colorectal cancer were enrolled in our study between May 2008
and November 2010 at Zhongshan Hospital of Fudan University. All the patients received suc -cessful, radical surgery as their initial treat-ment by the same operating team. Meanwhile, adequate tissue samples and complete clinico-pathologic data were acquired from the patients and archived. Routine postoperative follow-up for the patients was conducted via phones or the outpatient clinic. In all, we collected tissues from 118 patients with an average age of 61.7 years and ranging from 23 to 88 years. The detailed characteristics are shown in Table 1. All patients or relatives involved in this study have signed an informed consent approved by the Ethics Committee of our institution.
For the immunohistochemistry staining of
eIF4E and integrin β1, cancerous tissues from
these patients were used in our study, as described in our previous study [24]. Following
incubation and deparaffinization, sections were
subject to microwave antigen retrieval. The endogenous peroxidase of tissues was
inacti-vated using 3% H2O2. Then sections were blocked with goat serum and incubated with a primary antibody overnight. The next day, they were incubated with a secondary antibody for 30 min. Following DAB and hematoxylin appli-cation, sections were sealed and then observed using a lighted microscope (Olympus, Japan). The semi-quantitative method was used to the evaluate staining results by three individuals [24].
Cell line and culture condition
The human colorectal cancer LoVo, SW620, HCT116, SW480, HT29, and Colo320 cell lines
were provided by American Type Culture
Collection (ATCC, Rockville, MD, USA). All the
cells were maintained as monolayers at 37°C, with 5% CO2 and saturated humidity. The cul-ture medium is comprised of DMEM (Gibco,
USA) with 20 mM HEPES and 10% fetal bovine serum (Gibco, USA). In addition, 100 IU/ml pen -icillin and 100 lg/ml streptomycin (Merck, Germany) were also added into medium. The
5FU resistance colorectal cancer cell line LoVo-R was established successfully from the parent LoVo cell line by exposure to gradient concentrations of 5FU for 12 months [25].
Eventually, the maintenance concentration of
5FU for LoVo-R cells was 15 ug/ml.
Synthesis and transfection of siRNA and plas-mid DNA
To knockdown or upregulate expression of
eIF4E or integrin β1, transfection techniques of
small interfering RNA (siRNA) and plasmid were used in our study. GenePharma (Biosune, Shanghai China) synthesized the siRNA se-
quences against eIF4E and integrin β1 as well
as the negative control siRNA. The plasmid pMD-eIF4E, containing the human eIF4E Gene ORF cDNA clone in a vector, was maintained in our lab. The plasmid DNA or siRNA mixed with LipofectamineTM 2000 reagent (Invitrogen,
Thermo Fisher Scientific Inc., USA) was diluted
in a serum-free medium and then transferred
to 6-well plates coated with fibronectin. The
siRNA sequences against eIF4E were 5’-GCA-
AACCUGCGGCUGAUCUTT-3’ and 5’-GGAUGGU-AUUGAGCCUAUGTT-3’, while the two siRNA se-quences against integrin β1 were 5’-GACUAUU-GAAAUCAAGCUUAUUGGA-3’ and 5’-AGUGAAG-ACAUGGAUGCUUACUGCA-3’ respectively.
Western blot analyses
Cells from the culture dishes were lysed on ice for 30 min. Then proteins were separated by
SDS-PAGE and transferred onto PVDF mem -branes. After blocking for 2 hours, the primary
antibodies against eIF4E and Integrin β1 were
used for incubation overnight at 4°C, followed by the secondary antibody incubation next day. Protein bands were visualized using an enhanced chemiluminescence detection
sys-tem. β-actin was used as the internal control.
ImageJ software was used for Western blot band intensity analysis.
Flow cytometry for apoptosis analysis
Cells with 80% confluence were transfected
with small interfering RNAs. Subsequently,
these cells were exposed to 5FU for 48 h.
manufacturer, the Annexin V-FITC and PI apop -tosis detection kit (BestBio, Shanghai, China) was used for analyzing the apoptosis rate of cells with different treatments. After addition and mixture of the detection solution in a dark environment, the cells in each tube were
mea-sured by flow cytometry.
Cell viability assay
Cell growth and viability was measured using
the Vybrant® MTT Cell Proliferation Assay Kit.
Briefly, harvested cells were seeded in a 96-well
plate, and then treated with varying
[image:4.612.95.520.73.577.2]concentra-tions of 5FU for 48 hours. Subsequently, an
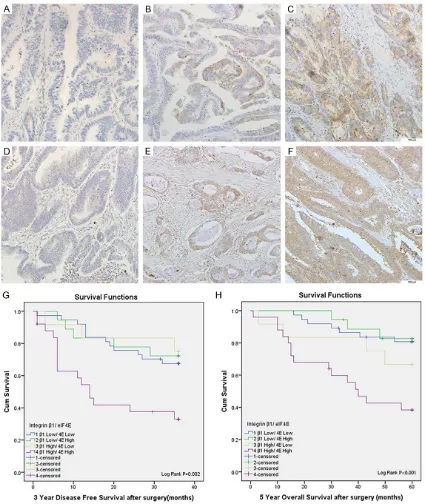
Figure 1. eIF4E and Integrin β1 expressions and patients’ survival in human colorectal cancer. A-C. Were obtained from colorectal cancerous tissues with negative, low and high eIF4E expression respectively (Scale bar 100 μm). D-F. Were obtained from colorectal cancerous tissues with negative, low and high Integrin β1 expression respec
MTT solution was added into each well for measurement. The wavelength of 540 nm was used for absorbance measurement using a Microplate Reader.
RNA preparation and RT-PCR
First, total RNA was isolated from all the cells using TRIzol, obtained from Invitrogen. Next, the PrimeScriptTM RT-PCR Kit (Takara, Japan) was used to perform a reverse transcription reaction and then quantitative PCR on ABI
Prism 7900HT (Applied Biosystems, Foster
City), based on the manufacture’s instruction.
The 2-ΔΔCT method was adopted to analyze
relative fold change of mRNA. Primer sequenc-es used in the prsequenc-esent study were listed as
fol-lows: for human β1 integrin, Forward
5’-CCT-ACTTCTGCACGATGTGATG-3’ and Reverse 5’-CC- TTTGCTACGGTTGGTTACATT-3’; and for eIF4E, Forward 5’-AAACCTGCGGCTGATCTC-3’ and Re- verse 5’-CCACATAGGCTCAATACCATCC-3’. In ad-
dition, the GAPDH was chosen as internal con -trol in present study.
Propidium Iodide (PI) and nuclear staining as-say
Propidium iodide (PI) is a popular red-fluores -cent nuclear and chromosome counterstain. Since PI does not permeate living cells, it is commonly used to detect dead or late
apoptot-ic cells. Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA, and
they are less toxic than DAPI and can ensure a higher viability of stained cells. In our experi-ments, cells were transfected with small
inter-fering RNA followed by exposure to 5FU for 48 h. Then, they were treated with PI and Hoechst
33258 working solution (from Thermo Fisher,
USA) for observing cellular death or late apop
-tosis induced by 5FU. Fluorescence images were taken under an Olympus inverted fluores -cence microscope and analyzed using Image-Pro Plus 6.0 software.
Statistical analysis
SPSS 24.0 software was used to perform all the statistical analyses. The Kaplan-Meier method and log-rank test were applied in sur-vival analyses. Statistical differences were
compared using a one-way ANOVA test, a
Chi-square test, and Fisher’s exact test or post-hoc in our study. P<0.05 was considered
statisti-cally significant.
Results
EIF4E and Integrin β1 expression and clinical significance in colorectal cancer tissues
All the sections from the 118 cases were assessable based on their immunohistochem-istry analysis. For the eIF4E cohort, 85.6% (101/118) cases exhibited a positive expres-sion (Figure 1A-C; Table 1). Among them, 45 patients were categorized into the high eIF4E
expression group; and 56 were defined as low
expression. In addition, the remaining 17 patients’ tissue samples were stained
nega-tively. For integrin β1, 99 patients showed posi -tive staining with posi-tive rate of 83.9%, of
which 37 cases were stained with a high β1
expression (Figure 1D-F; Table 1). In all, 19 patients were negatively stained for Integrin
β1.
Next, the association between eIF4E expres-sion and clinicopathologic variables was ana-lyzed (Table 1). A significant association was
found between high eIF4E and tumor size, T stage, N stage, M stage, or TNM stage (P=0.027, 0.024, 0.002, 0.032 and <0.001 respectively). Moreover, there was no relationship between eIF4E expression and patients’ gender, age, tumor location or differentiation. Similarly, high
β1 expression was related to tumor size, N
stage, M stage, and TNM stage (P=0.039, 0.001, 0.001 and <0.001 respectively), but not T stage (P=0.647). In addition, we didn’t find any association between β1 expression and
tumor location and differentiation. Meaningfully, according to a Spearman correlation analysis,
it was revealed that expression integrin β1 and
eIF4E were moderately positively correlated (r=0.515, P<0.001, Supplementary Table 1).
For the patients with both β1 and eIF4E posi
-tive expression, 92 of 118 cases were stratified
into 4 groups: Group 1, low eIF4E/low integrin
β1 (n=37); Group 2, low high eIF4E/integrin β1 (n=18); Group 3, low eIF4E/high integrin β1
(n=12); and Group 4, high eIF4E/high integrin
β1 (n=25). A Kaplan-Meier survival analysis shown that patients in Group 4 had significantly
shorter 5-year overall survival (OS) and 3-year disease free survival (DFS) than the other groups (P=0.001 and 0.002, respectively, log-rank test) (Figure 1G, 1H). In addition, patients
with high eIF4E or integrin β1 expression only
and <0.001 respectively; Supplementary Figure 1).
The effect of Integrin β1 on 5FU resistance in colorectal cancer cell lines
Firstly, an MTT assay was performed to observe
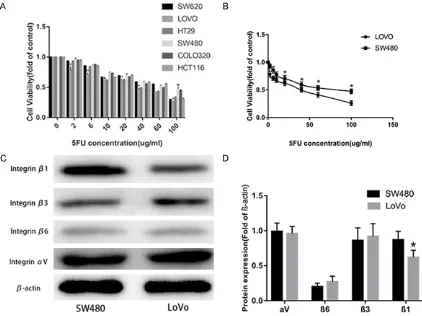
the antitumor potential of 5FU in a panel of
colorectal cancer cell lines. As shown in Figure 2A, the growth of cells exposed to 5FU for 48
hours could be inhibited in a dose-dependent
manner. Apparently, LoVo cells were the most
sensitive cell line, while the least sensitive one was SW480, for which the IC50 was 27.9 and 70.1 ug/ml respectively (Figure 2B).
Then, we investigated expression levels of
inte-grins in SW480 and LoVo cell lines. As was
shown in Figure 2C and 2D, Western blot
analy-sis showed a higher β1 expression in SW480 cells compared to LoVo, but no significant dif
-ferences of β3, β6 and αv expression existed
between the two cell lines.
Next, we sought to explore the effect of β1 on 5FU induced cellular apoptosis inhibition in
cancer cells. Firstly, as shown in Figure 3A, 3B and 3D, SW480 cells transfected with β1-siRNA showed an obvious downregulation of β1
ex-pression at the mRNA and protein levels, but no changes of eIF4E expression were found in
these cells. Besides, LoVo-R cells had higher β1 and eIF4E expressions compared to the LoVo cells in Figure 3C and 3E. Furthermore, we evaluated the cellular apoptosis effect using
the Annexin V and PI detection assays. Then, all the cells were exposed to 50 ug/ml 5FU for 48
hours. As shown in Figure 3F-I and 3M, the
apoptotic rates of β1-siRNA transfected SW480
cells were higher than in the cells treated with the negative control or untreated cells. Similarly,
[image:6.612.94.516.74.390.2]LoVo-R cells showed a lower apoptosis rate
Figure 2. Cell Viability assay for different colorectal cancer cell lines exposed to 5FU. A. The growth of cells exposed to 5FU for 48 hours could be inhibited in a dose dependent manner. LoVo cells were the most sensitive cell line, while the least sensitive one was SW480. B. For LoVo and SW480 cells, the IC50 was 27.9 and 70.1 ug/ml respec
than LoVo cells when exposed to 5FU (Figure 3J-L and 3M). Above all, it was clear that
colorectal cancer cells expressing higher β1
tended to have the ability of pro-survival and
anti-apoptosis when exposed to 5FU.
Expression relationship of eIF4E and β1 in CRC cells treated with eIF4E siRNA or plasmid
We have known that eIF4E can selectively enhance the translation of “weak” mRNAs, encoding proteins associated with malignan- cy. In addition, a positive correlation exists between the protein expressions of eIF4E and
β1 based on our IHC finding. Therefore, we fur
-ther investigated whe-ther β1 mRNAs could also
be translated into proteins under the control of eIF4E in CRC cells.
Firstly, we treated SW480 cells with eIF4E siRNA (Figure 4A-C), which resulted in a sig-
nificantly decreased mRNA expression of eIF4E
at 48 hours by RT-PCR analysis, and then decreased protein expressions of eIF4E at 72
hours in these cells. Interestingly, the protein
expression of β1 decreased accordingly, but there was little change of β1 mRNA expression
for these cells.
Then, we treated LoVo cells with plasmids con -taining eIF4E-cDNA (Figure 4D-F). Correspond-
ingly, eIF4E mRNA expression increased signifi -cantly at 48 hours, followed by increased eIF4E protein expression at 72 hours. Similarly, we
found a significantly increased protein expres
-sion of β1; also, β1 mRNA expres-sion did not
change based on our RT-PCR analysis.
To sum up, it can be safely summarized that
eIF4E upregulates the β1 protein expression, meaningfully, without affecting the β1 mRNA
level.
EIF4E can translationally mediate integrin β1 induced 5FU Chemo resistance of cancer cells
In this part, we investigated the β1 induced
pro-survival and chemo resistance under trans-Figure 3. The effect of Integrin β1 on 5FU resistance in colorectal cancer cell lines SW480 and LoVo. RT-PCR (A) and western blot (B, D) assays were performed to analyze mRNA and protein expression of β1 and eIF4E in SW480 cells when treated with Integrin β1-siRNA (siβ1) or negative control siRNA (siNC). Meanwhile, protein expressions of eIF4E and β1 (C and E) were observed in LoVo and LoVo 5FU-resistant (LoVo-R) cells. (F-M) Flow cytometry analysis showed the apoptosis results in SW480 and LoVo cells exposed to 5FU for 48 hours. Cells in I and L were treated with PBS without 5FU as negative control, while cells in (F-H and J, K) were treated with 5FU. X axis in F-L stands for FITC labeled Annexin V, and Y axis for PI. Shown are the mean and standard deviation of three independent experi -ments. *Represents P<0.05, and *indicates P<0.05.
Figure 4. Expression level of eIF4E and β1 in CRC cell lines when treated with eIF4E siRNA or eIF4E cDNA expression
plasmid. A-C. mRNA and protein expression levels were observed in SW480 cells when treated with eIF4E siRNA
(si4E). D-F. Similarly, mRNA and protein expression levels were examined in LoVo cells when treated with eIF4E
[image:8.612.94.519.175.376.2]lational control of eIF4E in LoVo and SW480
[image:9.612.93.520.71.566.2]cells. Hoechst/PI double staining was performed on the LoVo cells after treatment with 5FU for 48
Figure 5. eIF4E can translationally regulate integrin β1 mediated anti-apoptosis and chemo resistance to 5FU. (A, B) Cellular fluorescence assay was performed to observe the PI positive rate in LoVo cells treated with eIF4E expres
-sion plasmid when exposed to 5FU. Cells on the bottom row of (A) were treated with PBS without 5FU as negative control, while all the other cell in (A) were treated with 5FU. For each group, PI (+) cells were quantified using 10 high-power fields under microscope. (C and F) In MTT assay, 5FU inhibited growth of SW480 and LoVo cells in a dose-dependent way, which showed β1-integrin can protect cells from 5FU induced growth inhibition and apoptosis
regulated by eIF4E. (D and E) Then, On the other hand, Western blot was performed to investigate apoptosis-related
hours. As is shown in Figure 5A and 5B, LoVo
cells transfected with eIF4E plasmid exhibited
a lower percentage of Hoechst (+)/PI (+) cells
under microscopy. Importantly, when cells were
treated with eIF4E plasmid and β1-siRNA simul -taneously, the PI positive cell rate of these cells returned to a level similar to that of the
untreat-ed cells. For the cell viability assay, LoVo and SW480 cells were exposed to various 5FU con -centrations for 48 h. As shown in Figure 5C and 5F, the cellular viability decreased in a dose-dependent manner in these cells. Similarly, cancer cells containing higher eIF4E
expres-sion, such as LoVo cells transfected with eIF4E
plasmid or SW480 cells untreated with eIF4E siRNA, showed a higher cellular survival index at different dose points.
To further investigate whether the Bcl-2 family
and caspases participated in integrin β1 medi
-ated resistance to 5FU, we examined the pro -tein expressions of Bcl-2, Bax and cleaved Caspase 3 in SW480 cells after exposure to
5FU for 48 hours. A Western blot analysis
(Figure 5D and 5E) demonstrated the
expres-sion of eIF4E and β1 was significantly
decreas-ed in SW480 cells treatdecreas-ed with eIF4E siRNA, which could subsequently lead to decreased expression of Bcl-2, and increased expressions of Bax and cleaved caspase-3. These data show that the apoptotic activity at the molecu-lar level was weakened by the downregulation
of β1-integrin expression under translational
control of eIF4E.
Discussion
For advanced colorectal cancer, 5-Fluorouracil
(5FU) based adjuvant chemotherapy has been the first line treatment option following surgery [26, 27]. However, many patients are unable to obtain any survival benefit due to chemo
resistance. Therefore, an elucidation of the underlying molecular mechanisms is required to improve patients’ prognosis. In the present study, we found that the protein expression of
integrin β1 and eIF4E were positively moderate -ly-correlated in colorectal cancer, which could be indicative of poorer prognosis in these pa-
tients. Then, we revealed that integrin β1 could
be upregulated by eIF4E at the translational level, which could protect colorectal cancer
cells from 5-fluorouracil induced apoptosis. The findings provide a new perspective in under
-standing the mechanism of 5FU chemo resis-tance, which may lead to innovative therapies for CRC.
Recently, translational control has gained much attention in exerting a critical effect on cancer development and progression among different cancers [11, 28]. It can allow for coordinated and immediate changes in protein levels when responding to environmental stress like irradia-tion, hypoxia and exposure to chemotherapy drugs [29]. eIF4E, as a rate-limiting element of the eIF4F translation initiation complex, is able to selectively bind “weak” mRNAs containing
long 5’UTR with a complex secondary structure
[12, 30, 31], and thus promote protein synthe-sis to adapt to stress stimuli. Interestingly, the
5’untranslated regions of integrin β1 mRNA possess significant secondary structures with
more than 75% GC content (GENE ID 3688), which met the criteria of “weak mRNA”. Meanwhile, we found that expressions of eIF4E
and integrin β1 were positive correlated in
these patients’ tissues. From this point, a rea-sonable hypothesis may be posed that
transla-tion of integrin β1 mRNA could be regulated by eIF4E, thereby contributing to the 5FU induced
chemo resistance of colon cells. To substanti-ate it, we down and upregulsubstanti-ated the expression of eIF4E in colorectal cancer cells, revealing
that the β1 protein expression changed accord -ingly with eIF4E variation, while mRNA levels did not change obviously during this process. Additionally, when knocking down the
expres-sion of β1 in SW480 cells, there was no any
change for eIF4E mRNA and protein expres-sion. Taken together, we can conclude that eIF4E can regulate the expression of integrin
β1 at the translational level.
Among all the 24 members of integrins from
combinations of 18 α and 8 unique β subunits, the β1 subunit is the most widely expressed
type in cells, which can be involved in the mod-ulation of cellular proliferation and survival under various death-inducing conditions [14, 32-34]. In present study, we found colorectal
cancer cells with a lower expression of β1 tend -ed to have a higher apoptosis rate and a lower
cellular viability under 5FU exposure. Moreover,
suppressing eIF4E expression using 4E-siRNA
could led to downregulation of β1 protein
were transfected with an eIF4E-expressed
plas-mid, these cells showed a higher β1 protein
level with a decreasing apoptotic rate. The pro-apoptosis or death stimuli can trigger the cellular intrinsic apoptosis pathway, which is regulated by the Bcl family and executed by activated caspase-3 [35, 36]. According to our Western blot results, we also found that Bcl2 and Bax and cleaved caspase-3 were involv-
ed in this process following 5FU treatment. These results showed that integrin β1 could protect cells from 5FU induced apoptosis, thus
enabling cancer cells with a chemo-resistance phenotype.
The biological implications of selective transla-tion are clearly important for cancer cells to adapt to stress stimuli. Although some mecha-nisms of selective translation such as IRES and miRNA-mediated control of translation initia-tion are recently getting much atteninitia-tion, the process of how it works remains largely unex-plored [4, 37]. To some degree, the regulation of translation may be both the cause and the
consequence of 5FU induced cellular apoptosis [38, 39]. When exposed to 5FU, the deregula -tion of transla-tion initia-tion in these cells could
regulate the expression of integrin β1, leading
to chemoresistance through an enhanced resistance to apoptosis. Nevertheless, how integrins transmit signaling and regulate gene expression still remain as an open question [40-42]. Encouragingly and interestingly, re- cent studies have revealed integrin-dependent translational control could trigger more compli-cated signaling cascades involving cancer pro-gression than expected [13]. Inspired by these
new findings, our future research will reason -ably focus on further tumor regulatory
mecha-nisms underlying the eIF4E-integrin β1 loop sig
-naling, which may contribute to the identifica -tion of transla-tional machinery and molecular targets for anticancer therapeutics in colorec-tal cancer.
Taken together, expressions of eIF4E and
integ-rin β1 were moderately positively correlated,
and they were associated with patients’ poorer prognosis in colorectal cancer. Moreover,
integ-rin β1 could be regulated by eIF4E at the trans -lational level, which subsequently contributed to cellular survival and anti-apoptosis, leading
to 5FU chemo resistance. Significantly, further
the mechanical investigation of how transla-tional control determines the cellular response
to 5FU may provide a better insight into improv -ing chemo resistance, and ultimately lead to the development of new therapeutic modalities for colorectal cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81402341 and 81602036, 81372315 and 81472228) and the Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive (No. 17DZ2252600). We thank Prof.
Jun Niu from Qilu Hospital of Shandong Uni-versity and TC Wang from Columbia UniUni-versity
for all their assistance with this study. Disclosure of conflict of interest
None.
Address correspondence to: Jianmin Xu, Depart-
ment of General Surgery, Zhongshan Hospital of Fudan University, Building 1, Room 1101, 180
Fen-glin Road, Xuhui District, Shanghai 200032, China.
Tel: +86-21-64041990; Fax:
+86-21-64041990-296; E-mail: xujmin@aliyun.com
References
[1] Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J and Jemal A. Global cancer statis-tics, 2012. CA Cancer J Clin 2015; 65: 87-108. [2] Chen W, Zheng R, Baade PD, Zhang S, Zeng H,
Bray F, Jemal A, Yu XQ and He J. Cancer statis -tics in China, 2015. CA Cancer J Clin 2016; 66: 115-132.
[3] El-Naggar AM and Sorensen PH. Translational
control of aberrant stress responses as a hall-mark of cancer. J Pathol 2018; 244: 650-666. [4] Holcik M and Sonenberg N. Translational con -trol in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6: 318-327.
[5] Hiremath LS, Webb NR and Rhoads RE.
Immunological detection of the messenger RNA cap-binding protein. J Biol Chem 1985; 260: 7843-7849.
[6] Ruggero D, Montanaro L, Ma L, Xu W, Londei P,
Cordon-Cardo C and Pandolfi PP. The transla -tion factor eIF-4E promotes tumor forma-tion and cooperates with c-Myc in lymphomagene-sis. Nat Med 2004; 10: 484-486.
[8] Dai N, Rapley J, Angel M, Yanik MF, Blower MD and Avruch J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ri-bosomal entry. Genes Dev 2011; 25: 1159-1172.
[9] Cawley A and Warwicker J. eIF4E-binding pro-tein regulation of mRNAs with differential
5’-UTR secondary structure: a polyelectrostatic
model for a component of protein-mRNA inter-actions. Nucleic Acids Res 2012; 40: 7666-7675.
[10] De Benedetti A and Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004; 23: 3189-3199.
[11] Truitt ML and Ruggero D. New frontiers in translational control of the cancer genome. Nat Rev Cancer 2016; 16: 288-304.
[12] Enyu L, Zhengchuan N, Jiayong W, Benjia L, Qi S, Ruixi Q, Cheng P, Khan AQ, Wei S and Jun N. Integrin beta6 can be translationally regulated by eukaryotic initiation factor 4E: contributing to colonic tumor malignancy. Tumour Biol 2015; 36: 6541-6550.
[13] Chung J and Kim TH. Integrin-dependent trans -lational control: implication in cancer progres-sion. Microsc Res Tech 2008; 71: 380-386. [14] Naci D, Vuori K and Aoudjit F. Alpha2beta1 in
-tegrin in cancer development and chemoresis-tance. Semin Cancer Biol 2015; 35: 145-153. [15] Poschau M, Dickreuter E, Singh-Muller J,
Zscheppang K, Eke I, Liersch T and Cordes N. EGFR and beta1-integrin targeting differential-ly affect colorectal carcinoma cell radiosensi-tivity and invasion. Radiother Oncol 2015; 116: 510-516.
[16] Song J, Zhang J, Wang J, Wang J, Guo X and Dong W. beta1 integrin mediates colorectal cancer cell proliferation and migration through
regulation of the Hedgehog pathway. Tumour
Biol 2015; 36: 2013-2021.
[17] Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A and Languino LR. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res 2013; 73: 3155-3167.
[18] Li Z, Sun Y, Qu M, Wan H, Cai F and Zhang P.
Inhibiting the MNK-eIF4E-beta-catenin axis in-creases the responsiveness of aggressive breast cancer cells to chemotherapy. Onco- target 2017; 8: 2906-2915.
[19] Wang D, Ma J, Ji X, Xu F and Wei Y. miR-141 regulation of EIF4E expression affects docetax-el chemoresistance of non-small cdocetax-ell lung can-cer. Oncol Rep 2017; 37: 608-616.
[20] Liu T, Li R, Zhao H, Deng J, Long Y, Shuai MT, Li Q, Gu H, Chen YQ and Leng AM. eIF4E pro -motes tumorigenesis and modulates chemo-sensitivity to cisplatin in esophageal squa-mous cell carcinoma. Oncotarget 2016; 7: 66851-66864.
[21] Adesso L, Calabretta S, Barbagallo F, Capurso G, Pilozzi E, Geremia R, Delle Fave G and Sette C. Gemcitabine triggers a pro-survival re-sponse in pancreatic cancer cells through acti-vation of the MNK2/eIF4E pathway. Oncogene 2013; 32: 2848-2857.
[22] Cencic R, Hall DR, Robert F, Du Y, Min J, Li L, Qui M, Lewis I, Kurtkaya S, Dingledine R, Fu H, Kozakov D, Vajda S and Pelletier J. Reversing
chemoresistance by small molecule inhibition of the translation initiation complex eIF4F.
Proc Natl Acad Sci U S A 2011; 108:
1046-1051.
[23] Vallo S, Rutz J, Kautsch M, Winkelmann R, Michaelis M, Wezel F, Bartsch G, Haferkamp
A, Rothweiler F, Blaheta RA and Cinatl J Jr. Blocking integrin beta1 decreases adhesion in chemoresistant urothelial cancer cell lines. Oncol Lett 2017; 14: 5513-5518.
[24] Niu Z, Wang J, Muhammad S, Niu W, Liu E,
Peng C, Liang B, Sun Q, Obo S, He Z, Liu S, Zou
X and Niu J. Protein expression of eIF4E and integrin alphavbeta6 in colon cancer can
pre-dict clinical significance, reveal their correla -tion and imply possible mechanism of interac-tion. Cell Biosci 2014; 4: 23.
[25] Zhang L, Song R, Gu D, Zhang X, Yu B, Liu B and Xie J. The role of GLI1 for 5-Fu resistance in colorectal cancer. Cell Biosci 2017; 7: 17. [26] Maindrault-Goebel F, de Gramont A, Louvet C,
Andre T, Carola E, Gilles V, Lotz JP, Tournigand C, Mabro M, Molitor JL, Artru P, Izrael V and
Krulik M. Evaluation of oxaliplatin dose inten-sity in bimonthly leucovorin and 48-hour
5-fluorouracil continuous infusion regimens
(FOLFOX) in pretreated metastatic colorectal cancer. Oncology Multidisciplinary Research Group (GERCOR). Ann Oncol 2000; 11: 1477-1483.
[27] Masi G, Allegrini G, Cupini S, Marcucci L, Cerri E, Brunetti I, Fontana E, Ricci S, Andreuccetti M and Falcone A. First-line treat-ment of metastatic colorectal cancer with
iri-notecan, oxaliplatin and 5-fluorouracil/leucov -orin (FOLFOXIRI): results of a phase II study
with a simplified biweekly schedule. Ann Oncol
2004; 15: 1766-1772.
[28] Loreni F, Mancino M and Biffo S. Translation factors and ribosomal proteins control tumor onset and progression: how? Oncogene 2014; 33: 2145-2156.
[29] Liwak U, Faye MD and Holcik M. Translation
control in apoptosis. Exp Oncol 2012; 34: 218-230.
[30] Graff JR, Konicek BW, Carter JH and Marcusson
EG. Targeting the eukaryotic translation initia-tion factor 4E for cancer therapy. Cancer Res 2008; 68: 631-634.
[31] Lazaris-Karatzas A, Smith MR, Frederickson
Sonenberg N. Ras mediates translation initia-tion factor 4E-induced malignant transforma-tion. Genes Dev 1992; 6: 1631-1642.
[32] Legate KR, Wickstrom SA and Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 2009; 23: 397-418.
[33] Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello
A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G and Defilippi P. Integrins
and signal transduction. Adv Exp Med Biol 2010; 674: 43-54.
[34] Song J, Zhang J, Wang J, Cao Z, Wang J, Guo X and Dong W. beta1 integrin modulates tumor growth and apoptosis of human colorectal can-cer. Oncol Rep 2014; 32: 302-308.
[35] Cory S and Adams JM. The Bcl2 family: regula-tors of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647-656.
[36] Yang SY, Sales KM, Fuller B, Seifalian AM and Winslet MC. Apoptosis and colorectal cancer: implications for therapy. Trends Mol Med 2009; 15: 225-233.
[37] Fabian MR and Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look un-der the hood of miRISC. Nat Struct Mol Biol 2012; 19: 586-593.
[38] Siddiqui N and Sonenberg N. Signalling to eIF4E in cancer. Biochem Soc Trans 2015; 43: 763-772.
[39] Walters B and Thompson SR. Cap-independent translational control of carcinogenesis. Front Oncol 2016; 6: 128.
[40] Liu Y, Chattopadhyay N, Qin S, Szekeres C,
Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH and Kreidberg JA.
Coordinate integrin and c-Met signaling regu-late Wnt gene expression during epithelial morphogenesis. Development 2009; 136: 843-853.
[41] Pandolfi F, Franza L, Altamura S, Mandolini C,
Cianci R, Ansari A and Kurnick JT. Integrins: in-tegrating the biology and therapy of cell-cell interactions. Clin Ther 2017; 39: 2420-2436. [42] Bouvard D, Pouwels J, De Franceschi N and
Supplementary Table 1. Correlation between integrin β1 expression and eIF4E expression in human
colonic carcinoma tissues (r=0.515, P<0.001, Correlation Spearman)
Integrin β1 Expression eIF4E Expression r P
Negative Low High
Negative 10 7 2
Low 7 37 18 0.515 <0.001
High 0 12 25
Supplementary Figure 1. Kaplan-Meier analysis of patients’ survival according to eIF4E and Integrin β1 expression.
A and B. Patients’ 3-Year disease free survival and 5-Year overall survival after surgery according to EIF4E expres-sion through immunohistochemical staining accordingly; C and D. Patients’ 3-Year disease free survival and 5-Year
overall survival after surgery according to Integrin β1 expression through immunohistochemical staining. Numbers