Durability of Indium Tin Oxide-Silver-Indium Tin Oxide Films
against Moisture Investigated Through The Wettability
of The Top Oxide Layer
Shi-Wei Chen
1;*1, Ching-Yuan Bai
2;*2, Cho-Chi Jain
1;*1,
Chau-Jie Zhan
1;*1and Chun-Hao Koo
1;*31Department of Materials Science and Engineering, National Taiwan University, Taipei, Taiwan 10617, R. O. China 2Department of Applied Chemistry and Materials Engineering, National Defense University,
Ta-Hsi, Tao-Yuan, Taiwan 32551, R. O. China
With good wettability, the top oxide layer of oxide-Ag-oxide depositions can resist the moisture penetration, and consequently is able to prevent the degradation and enhance the durability of oxide-Ag-oxide depositions. The wettability of the top oxide on the Ag layer is determined according to the contact angle evaluated from the Young’s equation. Before the evaluation, the vOCG’s approach with the three-liquids-method is used to estimate the surface or interface energy of the films. [doi:10.2320/matertrans.MER2007038]
(Received February 15, 2007; Accepted June 6, 2007; Published July 19, 2007)
Keywords: Oxide-Ag-oxide thin films, Wettability, Surface energy, Durability
1. Introduction
In order to meet the requirement of high-performance flat panel displays, various studies have been sought to advance the conductivity of transparent electrodes. The indium tin oxide-silver-indium tin oxide (ITO-Ag-ITO) films1,2) with relatively low resistivity have the potential to replace the single-layer ITO for the application of transparent electrodes, although the transparency of ITO-Ag-ITO is slightly lower than that of ITO. However, several hazy spots will form in the ITO-Ag-ITO films and then reduce their conductivity and transparency after exposure to the moist environment for a long time. The poor durability against moisture limits the application of ITO-Ag-ITO films in flat panel displays.
Recently, E. Ando and M. Miyazaki have proposed the moisture-induced degradation to explain the formation of the hazy spots in the ZnO-Ag-ZnO films.3–5)The degradation is mainly composed of the agglomeration of the silver layer and the peeling and cracking of the top oxide layer, resulting from the moisture penetration through the top oxide layer. All of these defects would cause the scattering of light and the formation of hazy spots. They have also advised two methods to improve the durability of transparent conductive oxide-Ag-oxide films. One is alloying the silver layer to suppress the interface migration of silver,4)and the other is reducing the internal compressive stress of the top oxide layer to prevent the formation of cracks.5) However, the ability of moisture resistance of top oxide layer has not been considered in their suggestion.
In our opinion, the ability of top ITO to resist the moisture penetration could play an important role in the durability of ITO-Ag-ITO films. The top ITO with good wettability would be a continuous film with rare voids or defects.6) This deposition can effectively resist the moisture penetration and prevent the ITO-Ag-ITO films from degradation. Therefore,
this investigation proposes an attitude that determines the wettability of the top ITO according to the contact angle calculated from Young’s equation. Before the determination of wettability, the surface/interface energy of films must be evaluated by the van Oss-Chaudhury-Good (vOCG) ap-proach with three-liquids-method. The method is most recently developed for surface/interface science.7–13)
2. Experimental
In this investigation, multilayer films, ITO-Ag or Ag(Ti)-ITO, were continuously deposited on glass substrates (coring 7059) by magnetron sputtering. The thickness of the top ITO, intermediate Ag or Ag(Ti), and base ITO films is 45, 10, and 45 nm, respectively. ITO films were prepared using a RF power source, but Ag or Ag(Ti) films were produced employing a DC power source at 6:67101Pa pressure under extra pure Ar atmosphere. The working temperature of deposition process is maintained at 298 K or 523 K. Both three-layer films, ITO-Ag or Ag(Ti)-ITO, and two-layer films, Ag or Ag(Ti)-ITO, are deposited under the similar procedure conditions. The doping amount of Ti was kept at 0.5 mass%, within the limitation of solubility in silver.
The contact angle measurement (FTA-125) was used to measure the contact angle by the sessile drop method. Diiodomethane (apolar), Formamide (polar), and DI water (polar) were applied as the probing liquids.
Atomic force microscopy (AFM) was used to measure the surface roughness and the thickness of the deposited films. The experiment of durability against moisture was performed in a testing box with a relative humidity of 95% at 323 K for a period of 72 or 144 hours. It is according to the standard MIL-C-48497. After that, the surface morphologies of samples were observed by optical microscopy (OM, Olympus) and field emission scanning electronic microscopy (FE-SEM, Philips LEO 1530) with X-ray energy dispersive spectrom-etry (EDS), while the depth profile of the elemental con-centration was examined by Auger electron spectroscopy. *1Graduation Student, National Taiwan University
*2Assistant Professor, National Defense University *3Corresponding author, E-mail: chkoo@ntu.edu.tw
3. Results
3.1 Moisture induce degradation of ITO-Ag-ITO films The OM images of the surface of the ITO-Ag-ITO film, deposited at 298 K, exposed to 95% relative humidity and 323 K for 144 hours are shown in Fig. 1(a), in which there are several hazy spots. However, no spot is formed in the same depositions exposed to 50% relative humidity and 368 K for the same period. It indicates that the moisture plays an important role in the durability of the ITO-Ag-ITO film. The moisture penetrating through the pre-existing defects of the top ITO layer would be absorbed in the intermediate Ag layer, and weaken the interface bonding between the top ITO and the Ag layer. This phenomenon enhances the migration of Ag atoms and promotes the agglomeration of the Ag layer, and then causes the peeling of the top ITO. Meanwhile, the cracks are formed on the top ITO due to the relaxation of the compressive internal stress. All of these defects produced by the moisture penetration would cause the scattering of light and the formation of hazy spots.
The mechanism of the formation of hazy spots, mentioned above, could be evidenced from the SEM observation of the hazy spots formed on the ITO-Ag-ITO film after the durability testing, as shown in Fig. 1(b), in which the agglomeration of the Ag layer is presented as the white clusters, based on the EDS analysis. The peeling zone and the retaining zone of ITO, based on the AES depth profile analysis, are also represented in Fig. 1(b).
3.2 Surface energy estimation
The Ag-ITO and the ITO-Ag-ITO films deposited at 298 or 523 K are used to estimate the surface energy. The surface energy is evaluated by using the vOCG’s approach, which applies the Lifshitz–van der Waals and the acid-base theories,11–13) with three-liquids-method. In the approach, both Lifshitz–van der Waals (LW, apolar) and the acid-base (AB, polar) interactions occur in solid surfaces. Thus, the surface energy,S, is the sum of the two components,SLW andSAB:
S¼SLWþ AB
S ð1Þ
in which
SAB¼2 ffiffiffiffiffiffiffiffiffiffiffiffi
þ
SS
q
ð2Þ
where Sþ is the Lewis acid parameter of surface energy (electron acceptor), andSis the Lewis base parameter of surface energy (electron donor). Therefore, three compo-nents, SLW, Sþ and S, are required to determine the surface energy of a solid surface.
Moreover, the interface energy between solid and liquid
ðS-LÞcan be expressed as:
SL¼SþL2
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
LW
S LLW
q 2 ffiffiffiffiffiffiffiffiffiffiffiffi Sþ L q 2 ffiffiffiffiffiffiffiffiffiffiffiffi S þ L q
ð3Þ
and Young’s equation is
S¼LcosþSL ð4Þ
Incorporation of eq. (4) into eq. (3) yields
Lð1þcosÞ ¼2
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
SLWLLW
q þ ffiffiffiffiffiffiffiffiffiffiffiffi Sþ L q þ ffiffiffiffiffiffiffiffiffiffiffiffi S þ L q
ð5Þ
Three probing liquids, Diiodomethane (apolar), Formamide (polar), and DI water (polar), with given surface energy,
[image:2.595.69.269.73.386.2]LLW, Lþ and L, shown in Table 1,10,14) are used to measure the contact angle of the liquids by the sessile drop method. Substituting the measured contact angle and the given surface energy of the liquids into the eq. (5), three simultaneous equations are produced. By solving the three simultaneous equations, the surface energy of the designated solid,SLW,SþandS, can be obtained.
Table 2 lists the measured contact angle and Table 3 lists the evaluated surface energy of Ag (Ag) and top ITO (ITO) layers.
3.3 Estimation of wettability of the top ITO
The wettability of the top ITO on the intermediate Ag layer of ITO-Ag-ITO films can be estimated after the surface energy of Ag (Ag) and top ITO (ITO) layers are evaluated. Introducing these evaluated components of surface energy, as shown in Table 3, into eq. 3, the interface energy (ITO-Ag) can be calculated. Furthermore, the contact angle can be estimated by substituting the interface energy and the surface energy of the top ITO and the Ag layer into Young’s equation (Ag¼ITO
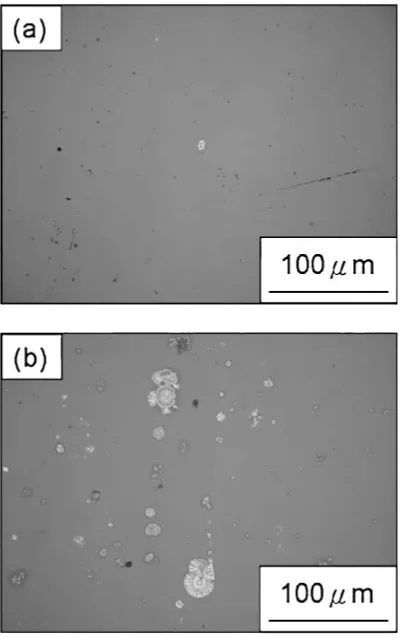
cosþITO-Ag). The calculated results are presented in Table 4. The contact angle of the top ITO, deposited at 298 K, on the Ag layer is smaller than that deposited at 523 K, indicating that the top ITO layer, deposited at 298 K, holds the better wettability than that deposited at 523 K. [image:2.595.303.548.325.433.2]3.4 Durability dependent on wettability of the top ITO The section 3.3 has revealed that the top ITO layer deposited on the Ag layer at 298 K holds the better wettability than that deposited at 523 K. To understand the influence of the wettability of top ITO on the durability against moisture, the ITO-Ag-ITO films deposited at 298 or 523 K were also subjected to the test of durability against moisture for 72 hours. The results are shown in Fig. 2, indicating that the number of hazy spots formed in the ITO-Ag-ITO film deposited at 298 K is fewer than that deposited at 523 K. It is evident that the top ITO with good wettability can more effectively resist the moisture penetration and prevent the degradation induced by moisture, leading ITO-Ag-ITO films with excellent durability against moisture.
Moreover, the surface roughness (Ra) of the Ag layer deposited at 298 K and 523 K is 0.19 nm and 0.49 nm, respectively. The wettability of the top ITO layer may
increase with the decreasing of the surface roughness of the intermediate Ag layer.
3.5 Durability dependent on alloying the intermediate silver layer
The ITO-Ag(Ti)-ITO film deposited at 298 K was also subjected to the test of durability against moisture for 144 hours. After the testing, no hazy spots were produced, and the films maintain high conductivity and transparency. This result had been proposed in our previous study reported in the literature.2)As proposed by Endo andet al.,4)the factor that induced by alloying the intermediate silver layer and leading the ITO-Ag(Ti)-ITO films with excellent durability against moisture could be the inhibition of the agglomeration of the intermediate silver layer.
The mixing enthalpy of the Ag(Ti) solution, proposed by
[image:3.595.56.548.86.156.2]Wei et al.,15)shows a negative quantity, indicating that the
Table 1 The parameters of surface energy of three probing liquids.10;14Þ
Surface energy,
/mJ.m2
Lifshitz–van der Waals component,
LW/mJ.m2
Lewis acid parameter,
þ/mJ.m2
Lewis base parameter,
/mJ.m2
Diiodomethane 50.80 50.80 0.72 0
Formamide 58.00 39.00 2.28 39.60
[image:3.595.48.549.191.309.2]DI Water 72.80 21.80 25.50 25.50
Table 2 The measured contact angle of various layers.
Deposition temperature,
T/K
Contact angle of the diiodomethane,
/degree
Contact angle of the formamide,
/degree
Contact angle of the DI water,
/degree
Ag layer 298 54.99 97.34 105.59
523 42.72 81.91 98.49
The top ITO (on Ag) 298 47.94 89.52 82.41
523 35.03 80.50 76.31
Ag(Ti)layer 298 53.43 90.59 102.42
The top ITO (on Ag(Ti)) 298 56.58 90.98 91.70
Table 3 The evaluated surface energy of various layers.
Deposition temperature,
T/K
Lifshitz–van der Waals component,
LW/mJ.m2
Lewis acid parameter,
þ/mJ.m2
Lewis base parameter,
/mJ.m2
Surface energy,
/mJ.m2
Ag layer 298 28.73 3.24 4.44 36.32
523 35.81 1.17 2.83 39.45
The top ITO (on Ag) 298 28.60 3.51 25.83 47.64
523 34.43 2.86 26.72 51.91
Ag(Ti)layer 298 29.83 1.73 3.66 34.86
The top ITO (on Ag(Ti)) 298 25.91 1.96 13.55 36.22
Table 4 The interface energy (interfacial) and the contact angle () between the top ITO and the Ag or Ag(Ti) layers.
Deposition temperature, T/K
Interface energy,
interfacial/mJ.m2
Contact angle,
/degree
Top ITO on Ag 298 0.44 41.14
Top ITO on Ag 523 4.26 47.32
[image:3.595.48.550.343.461.2] [image:3.595.47.552.496.558.2]bonding energy of Ti atoms is stronger than that of Ag-Ag atoms based on the statistical mixing model.16)Therefore, the addition of Ti into Ag layer is expected to restrain the migration of Ag atoms and suppress the agglomeration of the silver layer.
As shown in the Fig. 3, only Ag and Ag(Ti) films deposited on the ITO substrates were also subjected to the test of durability against moisture for 144 hours. After the testing, there were several cavities formed on the surface of the Ag film. It could be due to the moisture-induced migration of Ag atoms as proposed by E. Ando andet al.4)
On the contrary, the Ag(Ti) film could maintain completive and continuous surface after the testing. It evidences that the doping Ti can effectively restrain the moisture-induced migration of Ag atoms and inhibit the agglomeration of the silver layer. That could lead the improvement of durability of ITO-Ag(Ti)-ITO films.
On the other hand, as shown in the section 3.4, the improved wettability of the top ITO will lead the improved durability of the ITO-Ag-ITO film against moisture. There-fore, it is expected that the wettability of the top ITO might also play a role in the excellent durability of the ITO-AgTi-ITO film. The wettability of the top ITO-AgTi-ITO on the Ag(Ti) layer is also represented in the calculated contact angle. The surface energy of the top ITO and the Ag(Ti) layers is listed in Table 2, and Table 3 presents the calculated contact angle between the top ITO and the Ag(Ti) layers. The contact angle of the top ITO on the Ag(Ti) layer is much smaller than that
on the Ag layer, indicating that the wettability of the top ITO on the Ag(Ti) layer is much better than that on the Ag layer. Therefore, a relatively continuous top ITO film would be formed on the Ag(Ti) layer, and this deposition could prevent moisture penetration effectively. As a result, the good wettability of the top ITO could also contribute to the excellent durability of the ITO-Ag(Ti)-ITO film against moisture.
4. Conclusions
This study proposes the top ITO with improved wettability is intended to resist the moisture penetration more effectively and prevent the moisture-induced degradation, consequently leading ITO-Ag-ITO films with improved durability against moisture. The wettability of the top ITO can be determined according to the contact angle evaluated from the Young’s equation. Before the evaluation, the surface or interface energy of the films can be estimated successfully using the vOCG’s approach with the three-liquid-method.
This study also proposes alloying the intermediate silver layer by doping Ti atoms can lead the ITO-Ag(Ti)-ITO films to exhibit excellent durability against moisture. Except the inhibition of the agglomeration of the intermediate silver layer by alloying, the good wettability of the top ITO would also contribute to the excellent durability of the ITO-Ag(Ti)-ITO films.
Fig. 2 The OM images of the surface of the ITO-Ag-ITO films, deposited at (a) 298 K or (b) 523 K, after the test of durability against moisture for 72 hours.
[image:4.595.69.269.72.391.2] [image:4.595.325.526.72.389.2]REFERENCES
1) M. Sawada, M. Higuchi, S. Kondo and H. Saka: Jpn. J. Appl. Phys.40 (2001) 3332–3336.
2) S. W. Chen, C. H. Koo, H. E. Huang and C. H. Chen: Mater. Trans.46 (2005) 2536–2540.
3) E. Ando and M. Miyazaki: Thin Solid Films351(1999) 308–312. 4) E. Ando, S. Suzuki, N. Aomine, M. Miyazaki and M. Tada: Vacuum59
(2000) 792–799.
5) E. Ando and M. Miyazaki: Thin Solid Films392(2001) 289–293. 6) E. H. E. Wu, S. H. Li, C. W. Chen, G. Li, Z. Xu and Y. Yang: IEEE/
OSA JOURNAL OF DISPLAY TECHNOLOGY1(2005) 105–111. 7) E. Lugscheider, K. Bobzin and M. Moller: Thin Solid Films355–356
(1999) 367–373.
8) A. Abderrahmen, F. F. Romdhane, H. Ben Ouada and A. Gharbi: Mater. Sci. Eng. C26(2006) 538–541.
9) J. K. Chen, F. H. Ko, K. F. Hsieh, C. T. Chou and F. C. Chang: J. Vac. Sci. Technol. B22(2004) 3233–3241.
10) L. H. Lee: Fundamentals of Adhesion, (Plenum, New York, 1991) pp 160–161.
11) C. J. van Oss, R. J. Good and M. K. Chaudhury: J. Colloid Inter. Sci. 111(1986) 378–390.
12) C. J. van Oss, M. K. Chaudhury and R. J. Good: Chem. Rev.88(1988) 927–941.
13) R. J. Good: J. Adhesion Sci. Technol.6(1992) 1269–1275. 14) M. L. Gonza´lez-Martı´n, B. Jan´czuk and L. Labajos-Broncano: J. M.
Bruque, Langmuir13(1997) 5991–5994.
15) P. Wei, L. Rongti, C. Jian, S. Ruifeng and L. Jie: Mater. Sci. Eng. A287 (2000) 72–77.