metal-organic papers
Acta Cryst.(2005). E61, m1393–m1395 doi:10.1107/S1600536805019148 Puet al. [Cu
2(CN)4(C5H5N)2]
m1393
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
Poly[dipyridinecopper(II)-
l
-cyano-copper(II)-tri-
l
-cyano]
Xiaohua Pu, Xiaoming Jiang, Yibin Wei, Yanping Li and Pin Yang*
Institute of Molecular Science, Chemical Biology and Molecular Engineering Laboratory of Education Ministry, Shanxi University, Taiyuan, Shanxi 030006, People’s Republic of China
Correspondence e-mail: yangpin@sxu.edu.cn
Key indicators
Single-crystal X-ray study
T= 273 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.030
wRfactor = 0.077
Data-to-parameter ratio = 14.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
The crystal structure of the title compound, [Cu(C5H5N)2
-{Cu(CN)4}]n, comprises [Cu(py)2] 2+
(py is pyridine) and [Cu(CN)4]
2
subunits which are connected through bridging cyano groups to form a three-dimensional network, with Cu
atoms located on sites of symmetry 2/mand 222 for the cation
and anion, respectively.
Comment
The magnetic properties of low-dimensional solids are
presently the subject of intense study.
Tetracyano-nickelates(II) are suitable model compounds for magnetic studies at low temperatures because the tetracyanonickelate anion may bridge paramagnetic ions partially coordinated with amine ligands and thus form molecular, one-, two- and three-dimensional structures. In contrast, the use of tetra-cyanocuprate(II) as a bridging unit in a multidimensional structure has rarely been reported. We have designed and
synthesized a novel coordination polymer, poly[
-cyano-tetracyanodipyridinedicopper(II)], [Cu(py)2{Cu(CN)4}]n (py
is pyridine), (I), the structure of which is reported here.
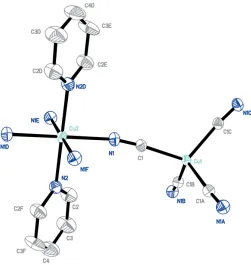
Part of the title complex is shown in Fig. 1 and some features of the molecular geometry are listed in Table 1. The complex consists of a neutral three-dimensional network with
stoichiometry Cu(py)2Cu(CN)4. The structure contains two
types of copper(II) coordination environments. Atom Cu1 in the [Cu(CN)4]
2
unit lies on a position of crystallographic symmetry 222 and is in a slightly distorted tetrahedral coor-dination geometry. The briging cyano groups are all related by
symmetry, with N C—Cu and C N—Cu angles of 174.6 (3)
and 168.8 (3), respectively. Atom Cu2 of the {Cu(py)
2} 2+
unit, lying on a position of symmetry 2/m, is in a slightly distorted
octahedral coordination environment. Four N atoms of the
C N ligand lie in the equatorial plane [Cu—N = 2.144 (3) A˚ ]
and two N atoms of the pyridine ligands are in axial positions
[Cu—N = 2.139 (4) A˚ ]. An extended three-dimensional
structure is formed through the cyano groups acting as
bridging groups. The distance between Cu1 and Cu2 is
5.259 (4) A˚ . In the related cyano-bridged complex
[Cu2(medpt)2Ni(CN)4
-(ClO4)2]2.5H2O [medpt = bis(3-aminopropyl)methylamine],
[Ni(CN)4] 2
subunits coordinate through all four cyano ligands to form a criss-crossed one-dimensional chain of connected square–pyramidal CuIIcations (Majiet al., 2001). In the title complex, the tetrahedral [Cu(CN)4]2 unit
contrib-utes to the formation of this three-dimensional network structure (Fig. 2).
Experimental
All chemicals were of reagent grade, commercially available from the Beijing Chemical Reagents Company of China, and were used without further purification. A pyridine–methanol (5 ml, 5:95 (v/v) solution of CuCl22H2O (0.0511 g, 0.3 mmol) was prepared. This solution was added to a methanol solution (5 ml) of KCN (0.0384 g, 0.59 mmol). The resulting solution was stirred for 24 h, filtered and the filtrate allowed to stand at room temperature. Yellow crystals of the title compound appeared after two weeks of slow evaporation of the solution.
Crystal data
[Cu2(CN)4(C5H5N)2] Mr= 389.36 Orthorhombic,Cccm a= 9.231 (3) A˚
b= 13.375 (4) A˚
c= 13.354 (4) A˚
V= 1648.8 (9) A˚3 Z= 4
Dx= 1.569 Mg m
3
MoKradiation Cell parameters from 4561
reflections
= 2.7–26.7
= 2.58 mm1 T= 273 (2) K Block, yellow 0.400.200.20 mm
Data collection
Bruker SMART CCD diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 2000)
Tmin= 0.404,Tmax= 0.595
3933 measured reflections
806 independent reflections 726 reflections withI> 2(I)
Rint= 0.031
max= 25.5
h=11!10
k=9!16
l=13!16
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.030
wR(F2) = 0.077 S= 1.00 806 reflections 56 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0459P)2
+ 1.7229P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001 max= 0.43 e A˚
[image:2.610.45.296.69.334.2]3 min=0.26 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
Cu1—C1 2.011 (3) Cu2—N2 2.139 (4)
Cu2—N1 2.144 (3) N1—C1 1.144 (4)
C1i
—Cu1—C1 105.29 (17) C1ii
—Cu1—C1 113.68 (17) C1iii
—Cu1—C1 109.55 (17) N2iv
—Cu2—N2 180 N2—Cu2—N1v
90.67 (10) N2—Cu2—N1 89.33 (10)
N1—Cu2—N1 89.23 (14) N1v
—Cu2—N1 90.77 (14) N1—Cu2—N1iv
180 C1—N1—Cu2 168.8 (3) N1—C1—Cu1 174.6 (3)
Symmetry codes: (i)xþ2;y;zþ1
2; (ii)x;yþ1;zþ 1
2; (iii)xþ2;yþ1;z; (iv)
xþ5 2;yþ
1
2;zþ1; (v)xþ 5 2;yþ
1 2;z.
metal-organic papers
m1394
Puet al. [Cu2(CN)4(C5H5N)2] Acta Cryst.(2005). E61, m1393–m1395
Figure 1
A view of part of the title compound. H atoms have been omitted for clarity. Ellipsoids are drawn at the 30% probability level. [Symmetry codes: (A)x+ 2,y+ 1,z; (B)x+ 2,y,z+1
2; (C)x,y+ 1,z+ 1 2;
(D)x+5 2,y+
1
2,z+ 1; (E)x+ 5 2,y+
1
2,z; (F)x,y,z+ 1.]
Figure 2
[image:2.610.48.293.394.643.2]H atoms were placed in geometrically idealized positions, with Csp2= 0.93 A˚ , and constrained to ride on their parent atoms, with
Uiso(H) = 1.2Ueq(C).
Data collection:SMART(Bruker, 2000); cell refinement:SAINT
(Bruker, 2000); data reduction: SAINT; program(s) used to solve structure: SHELXS97(Sheldrick, 2000); program(s) used to refine structure: SHELXL97 (Sheldrick, 2000); molecular graphics:
SHELXTL(Sheldrick, 1999) andPLATON(Spek, 2003); software used to prepare material for publication:SHELXTL.
This work was supported financially by the National Natural Science Foundation of China (grant No. 20171031). The
authors are also indebted to Professor Miao-Li Zhu for helpful discussions.
References
Bruker (2000).SMART(Version 5.0) andSAINT(Version 6.02). Bruker AXS Inc., Madison, Wisconsin, USA.
Maji, T. K., Mukherjee, P. S., Mostafa, G., Zangrando, E. & Chaudhuri, N. R. (2001).Chem. Commun.pp. 1368–1369.
Sheldrick, G. M. (1999).SHELXTL/PC. Version 6.10. Bruker AXS Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (2000).SHELXS97,SHELXL97andSADABS. University of Go¨ttingen, Germany.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
metal-organic papers
Acta Cryst.(2005). E61, m1393–m1395 Puet al. [Cu
supporting information
sup-1
Acta Cryst. (2005). E61, m1393–m1395
supporting information
Acta Cryst. (2005). E61, m1393–m1395 [https://doi.org/10.1107/S1600536805019148]
Poly[dipyridinecopper(II)-
µ
-cyano-copper(II)-tri-
µ
-cyano]
Xiaohua Pu, Xiaoming Jiang, Yibin Wei, Yanping Li and Pin Yang
Poly[µ-cyano-tetracyanodipyridinedicopper(II)]
Crystal data
[Cu2(CN)4(C5H5N)2]
Mr = 389.36
Orthorhombic, Cccm
Hall symbol: -C 2 2 c
a = 9.231 (3) Å
b = 13.375 (4) Å
c = 13.354 (4) Å
V = 1648.8 (9) Å3
Z = 4
F(000) = 776
Dx = 1.569 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 4561 reflections
θ = 2.7–26.7°
µ = 2.58 mm−1
T = 273 K Block, yellow
0.40 × 0.20 × 0.20 mm
Data collection
Bruker SMART CCD diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 2000)
Tmin = 0.404, Tmax = 0.595
3933 measured reflections 806 independent reflections 726 reflections with I > 2σ(I)
Rint = 0.031
θmax = 25.5°, θmin = 2.7°
h = −11→10
k = −9→16
l = −13→16
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.030
wR(F2) = 0.077
S = 1.00 806 reflections 56 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0459P)2 + 1.7229P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.43 e Å−3 Δρmin = −0.26 e Å−3
Special details
supporting information
sup-2
Acta Cryst. (2005). E61, m1393–m1395
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Cu1 1.0000 0.5000 0.2500 0.0292 (2)
Cu2 1.2500 0.2500 0.5000 0.0316 (2)
N1 1.1774 (3) 0.3525 (2) 0.3873 (2) 0.0451 (7)
N2 1.0402 (4) 0.1821 (3) 0.5000 0.0439 (9)
C1 1.1191 (3) 0.4088 (2) 0.3368 (2) 0.0380 (7)
C2 0.9716 (4) 0.1615 (3) 0.4162 (3) 0.0664 (11)
H2 1.0179 0.1751 0.3559 0.080*
C3 0.8357 (5) 0.1211 (4) 0.4134 (4) 0.0894 (16)
H3 0.7909 0.1083 0.3524 0.107*
C4 0.7675 (7) 0.1001 (6) 0.5000 0.089 (2)
H4 0.6755 0.0717 0.5000 0.107*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Cu1 0.0358 (4) 0.0257 (4) 0.0261 (4) 0.000 0.000 0.000 Cu2 0.0353 (4) 0.0312 (4) 0.0281 (4) 0.0042 (3) 0.000 0.000 N1 0.0521 (16) 0.0404 (15) 0.0426 (15) 0.0023 (13) −0.0030 (12) 0.0074 (13) N2 0.047 (2) 0.042 (2) 0.043 (2) −0.0018 (18) 0.000 0.000 C1 0.0428 (16) 0.0374 (16) 0.0339 (15) −0.0021 (14) −0.0011 (13) 0.0023 (14) C2 0.066 (2) 0.081 (3) 0.052 (2) −0.016 (2) −0.0049 (18) −0.005 (2) C3 0.081 (3) 0.106 (4) 0.081 (3) −0.046 (3) −0.022 (2) −0.006 (3) C4 0.067 (4) 0.092 (5) 0.108 (5) −0.038 (4) 0.000 0.000
Geometric parameters (Å, º)
Cu1—C1i 2.011 (3) N1—C1 1.144 (4)
Cu1—C1ii 2.011 (3) N2—C2 1.315 (4)
Cu1—C1iii 2.011 (3) N2—C2v 1.315 (4)
Cu1—C1 2.011 (3) C2—C3 1.366 (6)
Cu2—N2iv 2.139 (4) C2—H2 0.9300
Cu2—N2 2.139 (4) C3—C4 1.347 (6)
Cu2—N1v 2.144 (3) C3—H3 0.9300
Cu2—N1vi 2.144 (3) C4—C3v 1.347 (6)
Cu2—N1 2.144 (3) C4—H4 0.9300
Cu2—N1iv 2.144 (3)
supporting information
sup-3
Acta Cryst. (2005). E61, m1393–m1395
C1i—Cu1—C1 105.29 (17) C1—N1—Cu2 168.8 (3)
C1ii—Cu1—C1 113.68 (17) C2—N2—C2v 116.7 (4)
C1iii—Cu1—C1 109.55 (17) C2—N2—Cu2 121.6 (2)
N2iv—Cu2—N2 180.0 C2v—N2—Cu2 121.6 (2)
N2iv—Cu2—N1v 90.67 (10) N1—C1—Cu1 174.6 (3)
N2—Cu2—N1v 89.33 (10) N2—C2—C3 123.2 (4)
N2iv—Cu2—N1vi 89.33 (10) N2—C2—H2 118.4
N2—Cu2—N1vi 90.67 (10) C3—C2—H2 118.4
N1v—Cu2—N1vi 180.0 C4—C3—C2 119.2 (5)
N2iv—Cu2—N1 90.67 (10) C4—C3—H3 120.4
N2—Cu2—N1 89.33 (10) C2—C3—H3 120.4
N1v—Cu2—N1 89.23 (14) C3v—C4—C3 118.4 (6)
N1vi—Cu2—N1 90.77 (14) C3v—C4—H4 120.8
N2iv—Cu2—N1iv 89.33 (10) C3—C4—H4 120.8
N2—Cu2—N1iv 90.67 (10)
N2iv—Cu2—N1—C1 −133.9 (14) N1v—Cu2—N2—C2v −44.4 (4)
N2—Cu2—N1—C1 46.1 (14) N1vi—Cu2—N2—C2v 135.6 (4)
N1v—Cu2—N1—C1 −43.2 (14) N1—Cu2—N2—C2v −133.7 (4)
N1vi—Cu2—N1—C1 136.8 (14) N1iv—Cu2—N2—C2v 46.3 (4)
N1v—Cu2—N2—C2 133.7 (4) C2v—N2—C2—C3 0.3 (8)
N1vi—Cu2—N2—C2 −46.3 (4) Cu2—N2—C2—C3 −177.9 (4)
N1—Cu2—N2—C2 44.4 (4) N2—C2—C3—C4 −0.6 (9)
N1iv—Cu2—N2—C2 −135.6 (4) C2—C3—C4—C3v 0.9 (12)