organic papers
Acta Cryst.(2005). E61, o2547–o2548 doi:10.1107/S1600536805022142 Zhaoet al. C
12H8N4S
o2547
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
A new polymorph of
2,5-bis(4-pyridyl)-1,3,4-thia-diazole
Xiao-Jun Zhao, Hua Cai and Miao Du*
College of Chemistry and Life Science, Tianjin Normal University, Tianjin 300074, People’s Republic of China
Correspondence e-mail: dumiao@public.tpt.tj.cn
Key indicators
Single-crystal X-ray study T= 293 K
Mean(C–C) = 0.003 A˚ Rfactor = 0.032 wRfactor = 0.101
Data-to-parameter ratio = 12.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
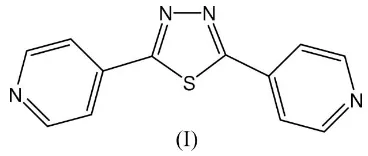
A new polymorph of 2,5-bis(4-pyridyl)-1,3,4-thiadiazole, C12H8N4S, obtained under hydrothermal conditions, is
described. In this structure, the two terminal pyridyl groups are inclined to the central thiadiazole ring with dihedral angles of 6.47 (2) and 29.95 (5), and the dihedral angle between the
pyridyl ring planes is 23.65 (6). An intermolecular C—H N
weak interaction is observed. The structural differences of two polymorphs of 2,5-bis(4-pyridyl)-1,3,4-thiadiazole are also discussed.
Comment
Recently, we have reported the crystal structure of 2,5-bis(4-pyridyl)-1,3,4-thiadiazole [form A, monoclinic, C2/c, with lattice parameters ofa= 26.179 (9) A˚ ,b= 5.8223 (17) A˚ ,c=
7.169 (3) A˚ and = 105.855 (9)], which is obtained by
recrystallizing the commercial product from a hot CH3OH/
H2O solution (Duet al., 2004). During our efforts to
investi-gate the assembly of metal-organic coordination frameworks based on this bent dipyridyl linker, a new polymorph (formB), (I), was generated accidentally under hydrothermal condi-tions; the crystal structure of (I) is described in this paper.
The molecular structure of (I), shown in Fig. 1, contains no crystallographically imposed symmetry; however, a crystal-lographic twofold axis was detected in the structure of
poly-morph A. The two pyridyl rings make dihedral angles of
6.47 (2) and 29.95 (5)with the central thiadiazole system, and
the dihedral angle between them is 23.65 (6). The
corre-sponding values in polymorph A are 21.8 (2), 21.8 (2) and
[image:1.610.239.423.428.506.2] [image:1.610.209.457.631.719.2]Received 23 June 2005 Accepted 11 July 2005 Online 16 July 2005
Figure 1
43.0 (2), respectively. Selected bond lengths and angles are
listed in Table 1. For the pyridyl rings, the C—N bond lengths (mean value 1.329 A˚ ), are within the range of values normally
considered standard for single C—N (1.47 A˚ ) and double
C N (1.28 A˚ ) bonds. The C—N bond lengths in the
thia-diazole ring are 1.302 (2) and 1.298 (2) A˚ . All these bond
geometries are comparable to those in polymorph A.
Addi-tionally, the angle between the center of the thiadiazole ring and the two pyridyl N-atom donors is 161, and the separation
of these two N atoms is 10.785 (2) A˚ . The corresponding parameters in polymorphAare 156and 10.763 (4) A˚ .
No significant interactions, such as hydrogen bonds or–
stacking, are observed in polymorph A. Notably, analysis of the crystal packing of this structure (polymorph B) suggests weak C2—H2 N4 intermolecular interactions, resulting in a one-dimensional chain along the [100] direction (Fig. 2). The relevant hydrogen-bonding parameters are listed in Table 2. Additionally, the interplanar distance between two layers of molecules is 3.5–3.8 A˚ , and the centroid-to-centroid distance is about 3.743 A˚ , indicating the existence of aromatic stacking
interactions. Examination of this structure with PLATON
(Spek, 2003) reveals no solvent-accessible voids in the unit cell.
Experimental
A mixture of Zn(OAc)24H2O (21.8 mg, 0.1 mmol), isophthalic acid
(16.8 mg, 0.10 mmol), and 2,5-bis(4-pyridyl)-1,3,4-thiadiazole (23.5 mg, 0.1 mmol) in water (10 ml) was heated at 433 K for 3 d in a sealed Teflon-lined stainless steel vessel (20 ml) under autogenous pressure. After the reaction mixture was slowly cooled to room temperature at a rate of 5 K h1, pale-yellow lamellar single crystals
suitable for X-ray diffraction were produced.
Crystal data
C12H8N4S
Mr= 240.28
Monoclinic,P21=c
a= 13.395 (2) A˚
b= 7.1833 (11) A˚
c= 11.4151 (18) A˚
= 104.948 (2)
V= 1061.2 (3) A˚3
Z= 4
Dx= 1.504 Mg m3
MoKradiation Cell parameters from 1940
reflections
= 3.2–27.3
= 0.28 mm1
T= 293 (2) K Plate, pale yellow 0.300.240.08 mm
Data collection
Bruker APEX-II CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin= 0.770,Tmax= 1.000 5414 measured reflections
1862 independent reflections 1515 reflections withI> 2(I)
Rint= 0.023
max= 25.0
h=15!15
k=8!4
l=13!13
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.032
wR(F2) = 0.102
S= 1.07 1862 reflections 155 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0679P)2 + 0.0172P]
whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.23 e A˚3 min=0.22 e A˚3
[image:2.610.316.565.71.156.2]Extinction correction:SHELXL97
Table 1
Selected geometric parameters (A˚ ,).
S1—C6 1.7141 (17)
S1—C7 1.7182 (18)
N1—C3 1.325 (2)
N1—C2 1.337 (2)
N2—C6 1.302 (2)
N2—N3 1.365 (2)
N3—C7 1.298 (2)
N4—C11 1.325 (3)
N4—C10 1.330 (3)
C6—S1—C7 86.79 (8)
C3—N1—C2 116.62 (16)
C6—N2—N3 112.18 (15)
C7—N3—N2 112.76 (15)
C11—N4—C10 116.29 (16)
C2—C1—C5 119.13 (16)
N1—C2—C1 123.72 (17)
N1—C3—C4 124.03 (17)
C3—C4—C5 118.91 (16)
C4—C5—C1 117.60 (16)
N2—C6—S1 114.29 (14)
N3—C7—S1 113.96 (13)
C9—C8—C12 117.51 (16)
C8—C9—C10 118.96 (18)
N4—C10—C9 124.13 (18)
N4—C11—C12 124.33 (17)
C11—C12—C8 118.76 (17)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C2—H2 N4i
0.93 2.57 3.361 (3) 143
Symmetry code: (i)xþ1;y;z.
Although all H atoms were visible in difference Fourier maps, they were placed in geometrically calculated positions with C—H = 0.93 A˚ and included in the final refinement in the riding-model approxima-tion, with displacement parameters derived from their parent atoms [Uiso(H) = 1.2Ueq(C)].
Data collection:APEX-II(Bruker, 2003); cell refinement: APEX-II; data reduction:SAINT(Bruker, 2001); program(s) used to solve structure:SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
SHELXTL (Bruker, 2001); software used to prepare material for publication:SHELXTL.
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (grant No. 20401012), the Key Project of Tianjin Natural Science Foun-dation (grant No. 043804111) and Tianjin Normal University (to MD).
References
Bruker (2001). SAINT and SHELXTL. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (2003).APEX-II. Bruker AXS Inc., Madison, Wisconsin, USA. Du, M., Zhao, X.-J. & Li, C.-P. (2004).Acta Cryst.E60, o706–o707. Sheldrick, G. M. (1996).SADABS. University of Go¨ttingen, Germany. Figure 2
[image:2.610.314.566.232.360.2]supporting information
sup-1 Acta Cryst. (2005). E61, o2547–o2548
supporting information
Acta Cryst. (2005). E61, o2547–o2548 [https://doi.org/10.1107/S1600536805022142]
A new polymorph of 2,5-bis(4-pyridyl)-1,3,4-thiadiazole
Xiao-Jun Zhao, Hua Cai and Miao Du
2,5-bis(4-pyridyl)-1,3,4-thiadiazole
Crystal data
C12H8N4S Mr = 240.28 Monoclinic, P21/c Hall symbol: -P 2ybc a = 13.395 (2) Å b = 7.1833 (11) Å c = 11.4151 (18) Å β = 104.948 (2)° V = 1061.2 (3) Å3 Z = 4
F(000) = 496 Dx = 1.504 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 1940 reflections θ = 3.2–27.3°
µ = 0.28 mm−1 T = 293 K
Lamellar, pale yellow 0.30 × 0.24 × 0.08 mm
Data collection
Bruker APEX-II CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996) Tmin = 0.770, Tmax = 1.000
5414 measured reflections 1862 independent reflections 1515 reflections with I > 2σ(I) Rint = 0.023
θmax = 25.0°, θmin = 3.2° h = −15→15
k = −8→4 l = −13→13
Refinement
Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.032 wR(F2) = 0.102 S = 1.07 1862 reflections 155 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0679P)2 + 0.0172P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.23 e Å−3 Δρmin = −0.22 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
S1 0.70555 (3) 0.13019 (6) 0.87463 (4) 0.0411 (2) N1 1.11893 (11) 0.1743 (2) 0.93303 (14) 0.0464 (4) N2 0.75023 (11) 0.1460 (2) 0.67143 (14) 0.0462 (4) N3 0.64493 (12) 0.1409 (2) 0.64481 (14) 0.0472 (4) N4 0.28675 (12) 0.1179 (2) 0.71677 (16) 0.0506 (4) C1 0.97174 (13) 0.0817 (2) 0.77593 (15) 0.0390 (4)
H1 0.9461 0.0253 0.7008 0.047*
C2 1.07654 (13) 0.0957 (3) 0.82551 (17) 0.0432 (5)
H2 1.1204 0.0477 0.7818 0.052*
C3 1.05425 (13) 0.2411 (2) 0.99306 (16) 0.0453 (5)
H3 1.0821 0.2961 1.0681 0.054*
C4 0.94792 (13) 0.2340 (2) 0.95096 (15) 0.0404 (4)
H4 0.9060 0.2830 0.9968 0.048*
C5 0.90482 (13) 0.1529 (2) 0.83938 (15) 0.0339 (4) C6 0.79231 (13) 0.1427 (2) 0.78771 (16) 0.0347 (4) C7 0.61030 (13) 0.1342 (2) 0.74097 (15) 0.0354 (4) C8 0.49930 (12) 0.1287 (2) 0.73569 (16) 0.0355 (4) C9 0.46184 (15) 0.1391 (2) 0.83724 (18) 0.0462 (5)
H9 0.5069 0.1503 0.9140 0.055*
C10 0.35611 (15) 0.1328 (3) 0.82321 (18) 0.0510 (5)
H10 0.3321 0.1394 0.8926 0.061*
C11 0.32375 (14) 0.1087 (3) 0.61987 (18) 0.0472 (5)
H11 0.2767 0.0991 0.5444 0.057*
C12 0.42750 (14) 0.1123 (2) 0.62359 (17) 0.0420 (4)
H12 0.4491 0.1040 0.5526 0.050*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-3 Acta Cryst. (2005). E61, o2547–o2548
C3 0.0401 (10) 0.0525 (11) 0.0399 (10) −0.0071 (8) 0.0041 (8) −0.0018 (8) C4 0.0382 (10) 0.0443 (10) 0.0403 (9) −0.0003 (7) 0.0131 (7) −0.0036 (7) C5 0.0293 (9) 0.0365 (9) 0.0367 (9) −0.0014 (6) 0.0096 (7) 0.0031 (7) C6 0.0304 (9) 0.0378 (9) 0.0370 (9) −0.0002 (7) 0.0107 (7) −0.0017 (7) C7 0.0303 (9) 0.0390 (9) 0.0375 (9) 0.0009 (7) 0.0097 (7) −0.0024 (7) C8 0.0293 (9) 0.0344 (9) 0.0433 (10) 0.0005 (7) 0.0100 (8) −0.0016 (7) C9 0.0374 (10) 0.0601 (12) 0.0419 (10) −0.0019 (8) 0.0112 (8) −0.0043 (8) C10 0.0427 (11) 0.0654 (13) 0.0513 (12) −0.0027 (9) 0.0239 (10) −0.0040 (9) C11 0.0327 (10) 0.0577 (12) 0.0489 (11) 0.0020 (8) 0.0062 (8) 0.0027 (9) C12 0.0342 (10) 0.0527 (11) 0.0397 (10) 0.0032 (7) 0.0106 (8) 0.0026 (8)
Geometric parameters (Å, º)
S1—C6 1.7141 (17) C3—H3 0.9300
S1—C7 1.7182 (18) C4—C5 1.384 (2)
N1—C3 1.325 (2) C4—H4 0.9300
N1—C2 1.337 (2) C5—C6 1.472 (2)
N2—C6 1.302 (2) C7—C8 1.473 (2)
N2—N3 1.365 (2) C8—C9 1.379 (3)
N3—C7 1.298 (2) C8—C12 1.393 (2)
N4—C11 1.325 (3) C9—C10 1.384 (3)
N4—C10 1.330 (3) C9—H9 0.9300
C1—C2 1.375 (2) C10—H10 0.9300
C1—C5 1.387 (2) C11—C12 1.379 (3)
C1—H1 0.9300 C11—H11 0.9300
C2—H2 0.9300 C12—H12 0.9300
C3—C4 1.382 (2)
C6—S1—C7 86.79 (8) N2—C6—S1 114.29 (14)
C3—N1—C2 116.62 (16) C5—C6—S1 123.21 (13)
C6—N2—N3 112.18 (15) N3—C7—C8 122.92 (16)
C7—N3—N2 112.76 (15) N3—C7—S1 113.96 (13)
C11—N4—C10 116.29 (16) C8—C7—S1 123.12 (13)
C2—C1—C5 119.13 (16) C9—C8—C12 117.51 (16)
C2—C1—H1 120.4 C9—C8—C7 123.13 (16)
C5—C1—H1 120.4 C12—C8—C7 119.36 (16)
N1—C2—C1 123.72 (17) C8—C9—C10 118.96 (18)
N1—C2—H2 118.1 C8—C9—H9 120.5
C1—C2—H2 118.1 C10—C9—H9 120.5
N1—C3—C4 124.03 (17) N4—C10—C9 124.13 (18)
N1—C3—H3 118.0 N4—C10—H10 117.9
C4—C3—H3 118.0 C9—C10—H10 117.9
C3—C4—C5 118.91 (16) N4—C11—C12 124.33 (17)
C3—C4—H4 120.5 N4—C11—H11 117.8
C5—C4—H4 120.5 C12—C11—H11 117.8
C4—C5—C1 117.60 (16) C11—C12—C8 118.76 (17)
C4—C5—C6 122.05 (15) C11—C12—H12 120.6
N2—C6—C5 122.49 (16)
C6—N2—N3—C7 0.1 (2) N2—N3—C7—C8 179.64 (14)
C3—N1—C2—C1 0.1 (3) N2—N3—C7—S1 −0.87 (19)
C5—C1—C2—N1 0.2 (3) C6—S1—C7—N3 1.04 (13)
C2—N1—C3—C4 −0.2 (3) C6—S1—C7—C8 −179.48 (13) N1—C3—C4—C5 0.0 (3) N3—C7—C8—C9 −173.72 (16)
C3—C4—C5—C1 0.3 (2) S1—C7—C8—C9 6.8 (2)
C3—C4—C5—C6 −179.06 (15) N3—C7—C8—C12 6.3 (2) C2—C1—C5—C4 −0.4 (2) S1—C7—C8—C12 −173.16 (13) C2—C1—C5—C6 179.00 (15) C12—C8—C9—C10 0.0 (2) N3—N2—C6—C5 −178.05 (14) C7—C8—C9—C10 −179.97 (15) N3—N2—C6—S1 0.71 (18) C11—N4—C10—C9 0.1 (3) C4—C5—C6—N2 149.13 (17) C8—C9—C10—N4 −0.3 (3) C1—C5—C6—N2 −30.2 (2) C10—N4—C11—C12 0.5 (3) C4—C5—C6—S1 −29.5 (2) N4—C11—C12—C8 −0.7 (3) C1—C5—C6—S1 151.11 (14) C9—C8—C12—C11 0.4 (2) C7—S1—C6—N2 −0.98 (13) C7—C8—C12—C11 −179.56 (15) C7—S1—C6—C5 177.77 (13)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C2—H2···N4i 0.93 2.57 3.361 (3) 143
![Figure 2Crystal packing of (I), showing the one-dimensional chain along the [100]direction](https://thumb-us.123doks.com/thumbv2/123dok_us/706330.574194/2.610.316.565.71.156/figure-crystal-packing-i-showing-dimensional-chain-direction.webp)