Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
4,4
000-Dibenzyl-5,5
000-(pyridine-2,6-diyl)-bis(3,4-dihydro-2
H
-1,2,4-triazole-3-thione)
Sibel Demir,aMuharrem Dinc¸er,aAhmet C¸etin,bOsman Dayancand Ahmet Cansızb*
aDepartment of Physics, Arts and Sciences
Faculty, Ondokuz Mayıs University, 55139 Samsun, Turkey,bDepartment of Chemistry, Arts
and Sciences Faculty, Fırat University, 23119 Elazıg˘, Turkey, andcDepartment of Chemistry, Arts and Sciences Faculty, Ege University, 35100 I´zmir, Turkey
Correspondence e-mail: sibeld@omu.edu.tr
Key indicators
Single-crystal X-ray study
T= 296 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.038
wRfactor = 0.093
Data-to-parameter ratio = 18.2
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 27 March 2006 Accepted 4 April 2006
#2006 International Union of Crystallography
The title compound, C23H19N7S2, adopts the ketoamine
tautomeric form and displays C—H N hydrogen-bonding interactions. There are two independent molecules in the asymmetric unit.
Comment
1,2,4-Triazole and its derivatives represent one of the most biologically active classes of compounds, possessing a wide spectrum of activities, including antibacterial, antifungal, antiviral, anti-inflammatory, anticonvulstant, antidepressant, antihypertensive, analgaesic and hypoglycaemic properties (Abbas et al., 2005; Koparır et al., 2005; Holla et al., 1998). Carboxylic acid hydrazides are condensed with carbon disul-fide in ethanolic potassium hydroxide to yield potassium 3-aroyldithiocarbazates, which are cyclized with hydrazine to the triazole (Cansız et al., 2004; Reid et al., 1976). In addition, there are some studies of the electronic structures and thiol– thione tautomeric equilibrium of heterocyclic thione deriva-tives (Koparıret al., 2005; Coyaniset al., 2002). In the present study, 4,40-dibenzyl-5,50 -(pyridine-2,6-diyl)bis(2,4-dihydro-3H-1,2,4-triazole-3-thione), (2), was synthesized by the reaction of benzyl isothiocynate and pyridine-2,6-dicarbohydrazide through 5,50-pyridine-2,6-diylbis(N -phenylhydrazinecarbo-thioamide), (1). Base-catalysed intramolecular dehydrative cyclization of this intermediate furnished the thione in good yield (80%). The reaction sequence depicted in the scheme was followed to obtain compound (2).
in (2) is 1.302 (2)A˚ , in agreement with those in similar compounds.
Experimental
A mixture of pyridine-2,6-dicarbohydrazide (0.01 mol) and the appropriate benzyl isothiocynate (0.01 mol) in absolute ethanol (100 ml) was refluxed for 8 h. The solid material obtained on cooling was filtered off, washed with diethy ether, dried and crystallized from ethanol (yield 80%; m.p 391–392 K) to give (1). To synthesize compound (2), a mixture of compound (1) (0.479 g, 1 mmol) and sodium hydroxide (40 mg, 1 mmol, as a 2Nsolution) was refluxed with stirring for 4 h. After cooling, the solution was acidified with hydrochloric acid and the resulting precipitate was filtered off and then crystallized from a mixture of methanol–dioxane (2:1) (yield: 0.37 g, 80%; m.p. 531 K). IR (KBr,, cm1): 3130–3020 (aryl CH), 2950–2910 (aliphatic CH), 2945–2762–2560 (S—H); 1H NMR
(400 MHz, DMSO-d6,, p.p.m.): 14.29 (br, 2H, 2SH), 8.05 (t,J=
5.70 Hz, 1H, Pr-CH), 7.90 (d,J= 6.00 Hz, 2H, Pr-CH), 7.18–7.30 (m, 10H, 2Ph-CH), 5.17 (s, 4H, 2N—CH2—Ph).
Crystal data
C23H19N7S2
Mr= 457.57
Triclinic,P1
a= 10.6469 (7) A˚
b= 10.8127 (7) A˚
c= 21.9501 (13) A˚
= 89.500 (5)
= 89.496 (5)
= 61.774 (5)
V= 2226.3 (2) A˚3
Z= 4
Dx= 1.365 Mg m 3
MoKradiation
= 0.27 mm1
T= 296 K Prism, colourless 0.620.480.26 mm
Data collection
Stoe IPDS-2 diffractometer
!scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002)
Tmin= 0.853,Tmax= 0.934
32857 measured reflections 10510 independent reflections 6295 reflections withI> 2(I)
Rint= 0.094 max= 28.0
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.038
wR(F2) = 0.093
S= 0.90 10510 reflections 578 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0353P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.18 e A˚ 3
min=0.26 e A˚ 3
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C17—H17B N4 0.97 2.44 3.099 (2) 125 C40—H40A N11 0.97 2.49 3.054 (2) 117
H atoms were positioned geometrically, with C—H = 0.93–0.97A˚ and N—H = 0.86A˚ , and refined using a riding model, withUiso(H) =
1.2Ueq(carrier).
Data collection: X-AREA (Stoe & Cie, 2002); cell refinement:
X-AREA; data reduction:X-RED32(Stoe & Cie, 2002); program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: ORTEP-3 (Farrugia, 1997); software used to prepare material for publication: WinGX (Farrugia, 1999) and PLATON
(Spek, 2003).
AC¸ is greateful to TUBITAK-BAYG for assistance in supporting the synthesis of compound (2). This study was supported financially by the Research Centre of Ondokuz Mayıs University (Project No. F-366).
References
Abbas, A. A. & Khalil, N. S. A. M. (2005).Nucleosides Nucleotides Nucleic Acids,24, 1353–1372.
Cansız, A., Koparır, M. & Demirdag˘, A. (2004).Molecules,9, 204–212. Coyanis, E. M., Vedova, D. C. O., Has, A. & Winter, M. (2002).J. Fluorine
Chem.117, 185–192.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Holla, B. S., Sarojini, B. K. & Gonsalves, R. (1998).Il Farmaco,53, 395–398. Koparır, M., C¸ etin, A. & Cansız, A. (2005).Molecules,10, 475–480. Reid, J. R. & Heindel, N. D. (1976).J. Heterocycl. Chem.13, 925–926. Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of
Go¨ttingen, Germany.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
Stoe & Cie (2002).X-AREA(Version 1.18) andX-RED32(Version 1.04). Stoe & Cie, Darmstadt, Germany.
organic papers
Acta Cryst.(2006). E62, o1822–o1823 Demiret al. C
[image:2.610.316.563.74.246.2] [image:2.610.314.563.292.492.2]23H19N7S2
o1823
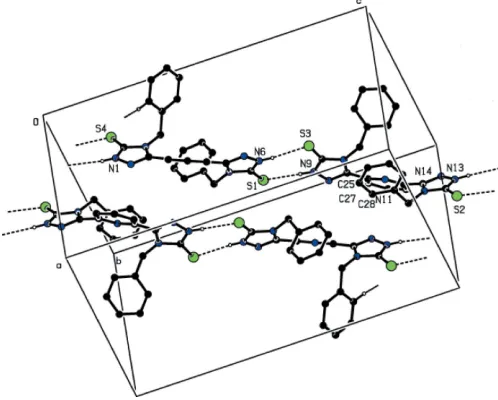
Figure 1The asymmetric unit of (2), showing the atomic numbering scheme. Displacement ellipsoids of non-H atoms are drawn at the 30% probability level and H atoms have been omitted for clarity.
Figure 2
supporting information
Acta Cryst. (2006). E62, o1822–o1823 [https://doi.org/10.1107/S1600536806012323]
4,4
′
-Dibenzyl-5,5
′
-(pyridine-2,6-diyl)bis(3,4-dihydro-2
H
-1,2,4-triazole-3-thione)
Sibel Demir, Muharrem Din
ç
er, Ahmet
Ç
etin, Osman Dayan and Ahmet Cans
ı
z
4,4′-Dibenzyl-5,5′-(pyridine-2,6-diyl)bis(2,4-dihydro-3H-1,2,4-triazole- 3-thione)
Crystal data
C23H19N7S2
Mr = 457.57
Triclinic, P1 Hall symbol: -P 1
a = 10.6469 (7) Å
b = 10.8127 (7) Å
c = 21.9501 (13) Å
α = 89.500 (5)°
β = 89.496 (5)°
γ = 61.774 (5)°
V = 2226.3 (2) Å3
Z = 4
F(000) = 952
Dx = 1.365 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 29144 reflections
θ = 1.9–28.0°
µ = 0.27 mm−1
T = 296 K Prism, colorless 0.62 × 0.48 × 0.26 mm
Data collection
Stoe IPDS-2 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 6.67 pixels mm-1 rotation method scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002)
Tmin = 0.853, Tmax = 0.934
32857 measured reflections 10510 independent reflections 6295 reflections with I > 2σ(I)
Rint = 0.094
θmax = 28.0°, θmin = 1.9°
h = −13→14
k = −13→14
l = −28→28
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.038
wR(F2) = 0.093
S = 0.90
10510 reflections 578 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0353P)2] where P = (Fo2 + 2Fc2)/3 (Δ/σ)max = 0.001
supporting information
sup-2 Acta Cryst. (2006). E62, o1822–o1823
Special details
Experimental. 13C NMR (100 MHz, DMSO-d
6): δ 169.80.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.35877 (19) 0.03279 (18) 0.21759 (7) 0.0491 (4) C2 0.51356 (17) 0.00064 (17) 0.29095 (7) 0.0423 (4) C3 0.57809 (17) −0.00332 (18) 0.35081 (7) 0.0439 (4) C4 0.70576 (18) −0.1208 (2) 0.36462 (9) 0.0568 (5)
H4 0.7505 −0.1931 0.3367 0.068*
C5 0.76405 (19) −0.1272 (2) 0.42085 (10) 0.0666 (6)
H5 0.8491 −0.2050 0.4317 0.080*
C6 0.69643 (19) −0.0183 (2) 0.46096 (9) 0.0600 (5)
H6 0.7338 −0.0212 0.4994 0.072*
C7 0.57071 (17) 0.09596 (18) 0.44240 (7) 0.0450 (4) C8 0.49855 (17) 0.21275 (17) 0.48505 (7) 0.0438 (4) C9 0.37558 (18) 0.42663 (18) 0.52355 (7) 0.0457 (4) C10 0.38788 (17) 0.41883 (18) 0.41026 (7) 0.0433 (4)
H10A 0.4650 0.3595 0.3831 0.052*
H10B 0.3894 0.5075 0.4137 0.052*
C11 0.24954 (17) 0.44588 (16) 0.38198 (7) 0.0432 (4) C12 0.2372 (2) 0.4640 (2) 0.31953 (9) 0.0605 (5)
H12 0.3155 0.4545 0.2969 0.073*
C13 0.1125 (3) 0.4955 (2) 0.28994 (11) 0.0783 (7)
H13 0.1068 0.5067 0.2478 0.094*
C14 −0.0025 (2) 0.5102 (2) 0.32275 (13) 0.0781 (7)
H14 −0.0879 0.5338 0.3031 0.094*
C15 0.0064 (2) 0.4906 (2) 0.38477 (12) 0.0750 (6)
H15 −0.0723 0.4988 0.4068 0.090*
C16 0.13397 (19) 0.4580 (2) 0.41493 (9) 0.0600 (5)
H16 0.1403 0.4447 0.4569 0.072*
C17 0.24705 (17) 0.13548 (17) 0.31686 (7) 0.0445 (4)
H17A 0.1699 0.2075 0.2934 0.053*
H17B 0.2711 0.1819 0.3488 0.053*
C18 0.19561 (16) 0.04174 (18) 0.34558 (8) 0.0456 (4) C19 0.2500 (2) −0.0972 (2) 0.33189 (11) 0.0682 (6)
H19 0.3221 −0.1369 0.3028 0.082*
C20 0.2004 (3) −0.1794 (3) 0.36014 (18) 0.1104 (12)
C21 0.0933 (3) −0.1197 (4) 0.40330 (19) 0.1317 (16)
H21 0.0610 −0.1750 0.4239 0.158*
C22 0.0357 (3) 0.0178 (4) 0.41559 (16) 0.1181 (13)
H22 −0.0394 0.0579 0.4433 0.142*
C23 0.0859 (2) 0.0999 (3) 0.38791 (10) 0.0735 (6)
H23 0.0464 0.1946 0.3975 0.088*
C24 0.32903 (17) 0.54370 (18) 0.72197 (7) 0.0468 (4) C25 0.24611 (17) 0.75879 (17) 0.75844 (7) 0.0451 (4) C26 0.20098 (17) 0.87892 (18) 0.79925 (7) 0.0458 (4) C27 0.09363 (19) 1.0083 (2) 0.78098 (8) 0.0587 (5)
H27 0.0493 1.0197 0.7435 0.070*
C28 0.0544 (2) 1.1193 (2) 0.81971 (10) 0.0724 (6)
H28 −0.0163 1.2081 0.8084 0.087*
C29 0.1200 (2) 1.09905 (19) 0.87536 (9) 0.0626 (5)
H29 0.0943 1.1730 0.9024 0.075*
C30 0.22570 (17) 0.96500 (17) 0.88987 (7) 0.0458 (4) C31 0.29220 (17) 0.93889 (17) 0.95018 (7) 0.0441 (4) C32 0.45115 (18) 0.86880 (17) 1.02453 (7) 0.0454 (4) C33 0.55313 (17) 0.78742 (17) 0.91998 (7) 0.0445 (4)
H33A 0.6350 0.7928 0.9360 0.053*
H33B 0.5268 0.8404 0.8821 0.053*
C34 0.59719 (15) 0.63681 (16) 0.90597 (7) 0.0403 (3) C35 0.6178 (2) 0.53950 (19) 0.95148 (8) 0.0566 (5)
H35 0.5998 0.5681 0.9919 0.068*
C36 0.6646 (2) 0.4007 (2) 0.93725 (10) 0.0656 (5)
H36 0.6782 0.3366 0.9683 0.079*
C37 0.69145 (19) 0.3554 (2) 0.87834 (9) 0.0577 (5)
H37 0.7225 0.2615 0.8692 0.069*
C38 0.67191 (19) 0.4504 (2) 0.83298 (9) 0.0583 (5)
H38 0.6902 0.4208 0.7927 0.070*
C39 0.62514 (18) 0.58994 (19) 0.84665 (8) 0.0504 (4)
H39 0.6123 0.6533 0.8153 0.061*
C40 0.32098 (16) 0.55541 (17) 0.83610 (7) 0.0437 (4)
H40A 0.3632 0.5977 0.8622 0.052*
H40B 0.3880 0.4562 0.8330 0.052*
C41 0.18665 (16) 0.57132 (17) 0.86572 (7) 0.0441 (4) C42 0.1254 (2) 0.4897 (2) 0.84851 (9) 0.0612 (5)
H42 0.1626 0.4295 0.8155 0.073*
C43 0.0088 (2) 0.4964 (3) 0.87995 (12) 0.0778 (7)
H43 −0.0320 0.4411 0.8680 0.093*
C44 −0.0460 (2) 0.5846 (3) 0.92857 (12) 0.0769 (7)
H44 −0.1238 0.5886 0.9498 0.092*
C45 0.0118 (2) 0.6655 (3) 0.94580 (11) 0.0785 (7)
H45 −0.0263 0.7256 0.9788 0.094*
C46 0.1282 (2) 0.6597 (2) 0.91451 (9) 0.0605 (5)
H46 0.1672 0.7163 0.9267 0.073*
N1 0.49485 (15) −0.04313 (15) 0.19934 (6) 0.0522 (4)
supporting information
sup-4 Acta Cryst. (2006). E62, o1822–o1823
N2 0.59129 (15) −0.06378 (15) 0.24393 (6) 0.0505 (3) N3 0.37021 (14) 0.06330 (14) 0.27718 (6) 0.0428 (3) N4 0.51030 (13) 0.10436 (14) 0.38826 (6) 0.0428 (3) N5 0.51151 (16) 0.19550 (16) 0.54390 (6) 0.0545 (4) N6 0.43463 (16) 0.32697 (15) 0.56643 (6) 0.0548 (4)
H666 0.4247 0.3447 0.6048 0.066*
N7 0.41479 (13) 0.35217 (14) 0.47038 (6) 0.0414 (3) N8 0.30061 (13) 0.61953 (14) 0.77463 (6) 0.0417 (3) N9 0.28925 (16) 0.64183 (16) 0.67807 (6) 0.0556 (4)
H9 0.2953 0.6224 0.6399 0.067*
N10 0.23853 (17) 0.77471 (16) 0.69928 (7) 0.0574 (4) N11 0.26771 (14) 0.85655 (14) 0.85265 (6) 0.0434 (3) N12 0.21938 (15) 0.99817 (15) 0.99905 (7) 0.0524 (4) N13 0.31870 (15) 0.95331 (15) 1.04415 (6) 0.0529 (4)
H13A 0.2981 0.9771 1.0817 0.063*
N14 0.43467 (13) 0.85537 (13) 0.96327 (6) 0.0413 (3) S1 0.27793 (5) 0.60008 (5) 0.53146 (2) 0.05506 (12) S2 0.60232 (5) 0.79721 (5) 1.06476 (2) 0.05801 (13) S3 0.39910 (6) 0.37041 (5) 0.71413 (2) 0.06286 (14) S4 0.20956 (6) 0.08270 (7) 0.17815 (2) 0.07435 (17)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C25 0.0509 (9) 0.0484 (10) 0.0346 (8) −0.0223 (8) −0.0041 (7) −0.0060 (7) C26 0.0489 (9) 0.0488 (10) 0.0379 (8) −0.0214 (8) −0.0037 (7) −0.0048 (7) C27 0.0571 (10) 0.0580 (12) 0.0464 (10) −0.0149 (9) −0.0132 (8) −0.0031 (8) C28 0.0677 (12) 0.0543 (12) 0.0634 (13) −0.0024 (10) −0.0163 (10) −0.0077 (10) C29 0.0672 (11) 0.0452 (11) 0.0553 (11) −0.0098 (9) −0.0071 (9) −0.0148 (8) C30 0.0504 (9) 0.0458 (10) 0.0401 (9) −0.0217 (8) −0.0021 (7) −0.0080 (7) C31 0.0522 (9) 0.0395 (9) 0.0406 (9) −0.0215 (7) −0.0017 (7) −0.0079 (7) C32 0.0615 (10) 0.0435 (9) 0.0353 (8) −0.0280 (8) −0.0018 (7) −0.0055 (7) C33 0.0520 (9) 0.0476 (10) 0.0393 (8) −0.0279 (8) 0.0027 (7) −0.0024 (7) C34 0.0392 (8) 0.0436 (9) 0.0391 (8) −0.0206 (7) 0.0014 (6) −0.0039 (7) C35 0.0801 (12) 0.0528 (11) 0.0413 (9) −0.0349 (10) 0.0017 (9) −0.0010 (8) C36 0.0880 (14) 0.0507 (12) 0.0600 (12) −0.0345 (10) 0.0000 (10) 0.0057 (9) C37 0.0563 (10) 0.0461 (11) 0.0680 (13) −0.0221 (8) 0.0090 (9) −0.0114 (9) C38 0.0620 (11) 0.0595 (12) 0.0510 (11) −0.0267 (9) 0.0153 (8) −0.0166 (9) C39 0.0557 (10) 0.0521 (11) 0.0432 (9) −0.0253 (8) 0.0088 (7) −0.0043 (8) C40 0.0466 (8) 0.0479 (10) 0.0329 (8) −0.0193 (7) −0.0069 (6) −0.0014 (7) C41 0.0458 (8) 0.0446 (9) 0.0358 (8) −0.0164 (7) −0.0077 (7) 0.0039 (7) C42 0.0655 (11) 0.0730 (13) 0.0513 (11) −0.0376 (10) −0.0112 (9) −0.0025 (9) C43 0.0701 (13) 0.0999 (18) 0.0810 (17) −0.0547 (13) −0.0189 (12) 0.0185 (14) C44 0.0531 (12) 0.0915 (18) 0.0769 (16) −0.0268 (12) −0.0038 (11) 0.0223 (14) C45 0.0776 (14) 0.0749 (15) 0.0637 (14) −0.0206 (12) 0.0231 (11) −0.0035 (11) C46 0.0742 (12) 0.0589 (12) 0.0487 (11) −0.0318 (10) 0.0109 (9) −0.0099 (9) N1 0.0668 (9) 0.0596 (9) 0.0312 (7) −0.0308 (8) 0.0055 (6) −0.0090 (6) N2 0.0573 (8) 0.0572 (9) 0.0399 (8) −0.0296 (7) 0.0070 (6) −0.0114 (6) N3 0.0482 (7) 0.0458 (8) 0.0350 (7) −0.0226 (6) 0.0027 (5) −0.0060 (6) N4 0.0470 (7) 0.0478 (8) 0.0360 (7) −0.0243 (6) 0.0010 (5) −0.0077 (6) N5 0.0734 (9) 0.0523 (9) 0.0352 (7) −0.0273 (7) −0.0057 (7) −0.0050 (6) N6 0.0784 (10) 0.0541 (9) 0.0303 (7) −0.0299 (8) −0.0018 (7) −0.0075 (6) N7 0.0501 (7) 0.0492 (8) 0.0291 (6) −0.0268 (6) −0.0010 (5) −0.0046 (5) N8 0.0462 (7) 0.0466 (8) 0.0290 (6) −0.0192 (6) −0.0024 (5) −0.0060 (5) N9 0.0782 (10) 0.0570 (9) 0.0300 (7) −0.0306 (8) 0.0004 (7) −0.0099 (6) N10 0.0808 (10) 0.0542 (9) 0.0368 (8) −0.0315 (8) −0.0039 (7) −0.0060 (6) N11 0.0496 (7) 0.0434 (8) 0.0358 (7) −0.0207 (6) −0.0023 (6) −0.0075 (6) N12 0.0563 (8) 0.0547 (9) 0.0417 (8) −0.0223 (7) 0.0001 (6) −0.0138 (6) N13 0.0631 (9) 0.0581 (9) 0.0346 (7) −0.0262 (7) −0.0011 (6) −0.0113 (6) N14 0.0504 (7) 0.0404 (7) 0.0342 (7) −0.0222 (6) −0.0014 (5) −0.0055 (5) S1 0.0724 (3) 0.0522 (3) 0.0391 (2) −0.0280 (2) −0.0015 (2) −0.00893 (18) S2 0.0660 (3) 0.0629 (3) 0.0430 (2) −0.0284 (2) −0.0111 (2) −0.0063 (2) S3 0.0793 (3) 0.0493 (3) 0.0429 (2) −0.0162 (2) −0.0030 (2) −0.01385 (19) S4 0.0663 (3) 0.0988 (4) 0.0402 (2) −0.0242 (3) −0.0078 (2) −0.0103 (2)
Geometric parameters (Å, º)
C1—N1 1.343 (2) C25—N8 1.378 (2)
C1—N3 1.372 (2) C25—C26 1.464 (2)
C1—S4 1.6641 (18) C26—N11 1.336 (2)
C2—N2 1.299 (2) C26—C27 1.382 (2)
supporting information
sup-6 Acta Cryst. (2006). E62, o1822–o1823
C2—C3 1.479 (2) C27—H27 0.9300
C3—N4 1.328 (2) C28—C29 1.376 (3)
C3—C4 1.386 (2) C28—H28 0.9300
C4—C5 1.373 (3) C29—C30 1.387 (2)
C4—H4 0.9300 C29—H29 0.9300
C5—C6 1.373 (3) C30—N11 1.325 (2)
C5—H5 0.9300 C30—C31 1.468 (2)
C6—C7 1.386 (2) C31—N12 1.300 (2)
C6—H6 0.9300 C31—N14 1.381 (2)
C7—N4 1.338 (2) C32—N13 1.337 (2)
C7—C8 1.467 (2) C32—N14 1.375 (2)
C8—N5 1.303 (2) C32—S2 1.6746 (17)
C8—N7 1.377 (2) C33—N14 1.466 (2)
C9—N6 1.339 (2) C33—C34 1.500 (2)
C9—N7 1.369 (2) C33—H33A 0.9700
C9—S1 1.6693 (18) C33—H33B 0.9700
C10—N7 1.4621 (18) C34—C39 1.379 (2)
C10—C11 1.498 (2) C34—C35 1.386 (2)
C10—H10A 0.9700 C35—C36 1.377 (3)
C10—H10B 0.9700 C35—H35 0.9300
C11—C16 1.374 (2) C36—C37 1.366 (3)
C11—C12 1.382 (2) C36—H36 0.9300
C12—C13 1.372 (3) C37—C38 1.369 (3)
C12—H12 0.9300 C37—H37 0.9300
C13—C14 1.359 (3) C38—C39 1.382 (3)
C13—H13 0.9300 C38—H38 0.9300
C14—C15 1.373 (3) C39—H39 0.9300
C14—H14 0.9300 C40—N8 1.4816 (19)
C15—C16 1.403 (3) C40—C41 1.500 (2)
C15—H15 0.9300 C40—H40A 0.9700
C16—H16 0.9300 C40—H40B 0.9700
C17—N3 1.453 (2) C41—C46 1.375 (2)
C17—C18 1.494 (2) C41—C42 1.379 (3)
C17—H17A 0.9700 C42—C43 1.386 (3)
C17—H17B 0.9700 C42—H42 0.9300
C18—C19 1.365 (3) C43—C44 1.367 (4)
C18—C23 1.385 (3) C43—H43 0.9300
C19—C20 1.372 (3) C44—C45 1.345 (4)
C19—H19 0.9300 C44—H44 0.9300
C20—C21 1.382 (5) C45—C46 1.386 (3)
C20—H20 0.9300 C45—H45 0.9300
C21—C22 1.342 (5) C46—H46 0.9300
C21—H21 0.9300 N1—N2 1.3635 (19)
C22—C23 1.370 (4) N1—H1 0.8600
C22—H22 0.9300 N5—N6 1.356 (2)
C23—H23 0.9300 N6—H666 0.8600
C24—N9 1.341 (2) N9—N10 1.359 (2)
C24—S3 1.6668 (18) N12—N13 1.3632 (19)
C25—N10 1.307 (2) N13—H13A 0.8600
N1—C1—N3 103.62 (14) C28—C29—C30 117.96 (18)
N1—C1—S4 129.19 (13) C28—C29—H29 121.0
N3—C1—S4 127.19 (13) C30—C29—H29 121.0
N2—C2—N3 111.17 (14) N11—C30—C29 123.41 (16) N2—C2—C3 121.54 (15) N11—C30—C31 117.48 (14) N3—C2—C3 127.22 (14) C29—C30—C31 119.07 (16) N4—C3—C4 123.92 (16) N12—C31—N14 111.23 (14) N4—C3—C2 118.50 (14) N12—C31—C30 121.99 (15) C4—C3—C2 117.58 (15) N14—C31—C30 126.76 (15) C5—C4—C3 117.90 (17) N13—C32—N14 103.80 (13)
C5—C4—H4 121.1 N13—C32—S2 128.24 (13)
C3—C4—H4 121.1 N14—C32—S2 127.95 (13)
C6—C5—C4 119.82 (17) N14—C33—C34 115.72 (13)
C6—C5—H5 120.1 N14—C33—H33A 108.4
C4—C5—H5 120.1 C34—C33—H33A 108.4
C5—C6—C7 117.93 (17) N14—C33—H33B 108.4
C5—C6—H6 121.0 C34—C33—H33B 108.4
C7—C6—H6 121.0 H33A—C33—H33B 107.4
N4—C7—C6 123.63 (16) C39—C34—C35 117.93 (16) N4—C7—C8 118.30 (14) C39—C34—C33 120.09 (15) C6—C7—C8 118.06 (15) C35—C34—C33 121.91 (15) N5—C8—N7 110.77 (15) C36—C35—C34 120.41 (17)
N5—C8—C7 122.37 (15) C36—C35—H35 119.8
N7—C8—C7 126.82 (14) C34—C35—H35 119.8
N6—C9—N7 103.21 (14) C37—C36—C35 121.23 (18)
N6—C9—S1 129.34 (13) C37—C36—H36 119.4
N7—C9—S1 127.44 (12) C35—C36—H36 119.4
N7—C10—C11 114.87 (13) C36—C37—C38 118.93 (18)
N7—C10—H10A 108.5 C36—C37—H37 120.5
C11—C10—H10A 108.5 C38—C37—H37 120.5
N7—C10—H10B 108.5 C37—C38—C39 120.43 (18)
C11—C10—H10B 108.5 C37—C38—H38 119.8
H10A—C10—H10B 107.5 C39—C38—H38 119.8
C16—C11—C12 118.71 (16) C34—C39—C38 121.07 (17) C16—C11—C10 123.59 (15) C34—C39—H39 119.5 C12—C11—C10 117.67 (15) C38—C39—H39 119.5 C13—C12—C11 121.8 (2) N8—C40—C41 114.36 (12)
C13—C12—H12 119.1 N8—C40—H40A 108.7
C11—C12—H12 119.1 C41—C40—H40A 108.7
C14—C13—C12 119.4 (2) N8—C40—H40B 108.7
C14—C13—H13 120.3 C41—C40—H40B 108.7
C12—C13—H13 120.3 H40A—C40—H40B 107.6
C13—C14—C15 120.52 (19) C46—C41—C42 118.04 (17)
C13—C14—H14 119.7 C46—C41—C40 120.41 (16)
supporting information
sup-8 Acta Cryst. (2006). E62, o1822–o1823
C14—C15—C16 120.0 (2) C41—C42—C43 120.7 (2)
C14—C15—H15 120.0 C41—C42—H42 119.7
C16—C15—H15 120.0 C43—C42—H42 119.7
C11—C16—C15 119.55 (19) C44—C43—C42 119.8 (2)
C11—C16—H16 120.2 C44—C43—H43 120.1
C15—C16—H16 120.2 C42—C43—H43 120.1
N3—C17—C18 114.13 (14) C45—C44—C43 120.4 (2)
N3—C17—H17A 108.7 C45—C44—H44 119.8
C18—C17—H17A 108.7 C43—C44—H44 119.8
N3—C17—H17B 108.7 C44—C45—C46 120.2 (2)
C18—C17—H17B 108.7 C44—C45—H45 119.9
H17A—C17—H17B 107.6 C46—C45—H45 119.9
C19—C18—C23 118.56 (19) C41—C46—C45 120.9 (2) C19—C18—C17 123.52 (16) C41—C46—H46 119.5 C23—C18—C17 117.92 (17) C45—C46—H46 119.5
C18—C19—C20 121.3 (2) C1—N1—N2 113.48 (14)
C18—C19—H19 119.4 C1—N1—H1 123.3
C20—C19—H19 119.4 N2—N1—H1 123.3
C19—C20—C21 119.1 (3) C2—N2—N1 104.27 (13)
C19—C20—H20 120.5 C1—N3—C2 107.44 (13)
C21—C20—H20 120.5 C1—N3—C17 122.62 (13)
C22—C21—C20 120.1 (3) C2—N3—C17 129.58 (13)
C22—C21—H21 119.9 C3—N4—C7 116.78 (14)
C20—C21—H21 119.9 C8—N5—N6 104.16 (13)
C21—C22—C23 120.9 (3) C9—N6—N5 113.97 (13)
C21—C22—H22 119.5 C9—N6—H666 123.0
C23—C22—H22 119.5 N5—N6—H666 123.0
C22—C23—C18 120.0 (3) C9—N7—C8 107.86 (13)
C22—C23—H23 120.0 C9—N7—C10 123.05 (14)
C18—C23—H23 120.0 C8—N7—C10 128.39 (14)
N9—C24—N8 103.53 (14) C24—N8—C25 107.48 (13) N9—C24—S3 128.13 (13) C24—N8—C40 123.17 (14) N8—C24—S3 128.33 (13) C25—N8—C40 129.28 (13) N10—C25—N8 111.23 (15) C24—N9—N10 113.99 (14)
N10—C25—C26 121.46 (15) C24—N9—H9 123.0
N8—C25—C26 127.31 (14) N10—N9—H9 123.0
N11—C26—C27 123.49 (16) C25—N10—N9 103.76 (14) N11—C26—C25 117.33 (14) C30—N11—C26 117.30 (14) C27—C26—C25 119.18 (15) C31—N12—N13 104.17 (13) C28—C27—C26 118.03 (17) C32—N13—N12 113.61 (14)
C28—C27—H27 121.0 C32—N13—H13A 123.2
C26—C27—H27 121.0 N12—N13—H13A 123.2
C27—C28—C29 119.78 (17) C32—N14—C31 107.12 (13)
C27—C28—H28 120.1 C32—N14—C33 124.23 (13)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C17—H17B···N4 0.97 2.44 3.099 (2) 125