Public Assessment Report
Scientific discussion
Lercanidipinhydrochlorid “Sandoz”
10 mg and 20 mg film-coated tablets
(Lercanidipine hydrochloride)
DK/H/2360/001-002/DC
1 December 2015
I.
INTRODUCTION
Based on the review of the quality, safety and efficacy data, the Member States have granted a marketing authorisation for Lercanidipinhydrochlorid ”Sandoz” 10 mg and 20 mg film-coated tablets, from Sandoz A/S.
The product is indicated for the treatment of mild to moderate essential hypertension. A comprehensive description of the indications and posology is given in the SmPC.
Lercanidipine is a calcium antagonist of the dihydropyridine group and inhibits the transmembrane influx of calcium into cardiac and smooth muscle. The mechanism of its antihypertensive action is due to a direct relaxant effect on vascular smooth muscle thus lowering total peripheral resistance. Despite its short pharmacokinetic plasma half-life, lercanidipine is endowed with a prolonged antihypertensive activity because of its high membrane partition coefficient, and is devoid of negative inotropic effects due to its high vascular selectivity.
This decentralised procedure concerns a generic application claiming essential similarity with the reference product Zanidip 10 mg and 20 mg film-coated tablets, which has been registered in Denmark by Meda A/S since 1997 and 2003, respectively.
The marketing authorisation is granted based on article 10.1 of Directive 2001/83/EC.
II.
QUALITY ASPECTS
II.1
Introduction
Each film-coated tablet contains 10 mg or 20 mg of lercanidipine hydrochloride equivalent to 9.4 mg and 18.8 mg of lercanidipine.
The 10 mg tablets are yellow film-coated tablets of round biconvex shape (diameter 6.5 mm), scored on one side, marked 'L' on the other side.
The 20 mg tablets are pink film-coated tablets of round biconvex shape (diameter 8.5 mm), scored on one side, marked 'L' on the other side.
The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. The tablets are packed in PVdC/PVC//Aluminium blisters in pack-sizes of 7, 10, 14, 20, 28, 30, 35, 42 (20 mg only), 50, 56, 60, 98, or 100 film-coated tablets. However, not all pack sizes may be marketed. The tablet core contains: Magnesium stearate (E572); povidone K-30 (E1201); sodium starch glycolate (Type A); lactose monohydrate and cellulose, microcrystalline (E460).
The film-coating consists of: Macrogol 3350 (E 1521); polyvinyl alcohol, partly hydrolysed; talc (E553b); titanium dioxide (E 171); iron oxide, yellow (E 172) and iron oxide, red (E 172) (20 mg only). The RMS has been assured that acceptable standards of GMP (see Directive 2003/94/EC) are in place for this product type at all sites responsible for the manufacturing of the active substance as well as for the manufacturing and assembly of this product prior to granting its national authorisation.
II.2
Drug Substance
The active substance lercanidipine hydrochloride is not described in the European Pharmacopoeia. It is a yellow powder soluble in methanol. Lercanidipine hydrochloride, as reported in literature, exhibits polymorphism.
INN: Lercanidipine hydrochloride
Chemical name(s): 1,4 – Dihydro – 2,6 – dimethyl – 4 – (3 – nitrophenyl) – 3,5 – pyridinedicarboxylic acid – 2 –[(3,3 – diphenylpropyl)methylamino] – 1,1 –dimethylethyl methyl ester hydrochloride Or
(±)-1,4 – Dihydro – 2,6 – dimethyl – 4 – (3 –nitrophenyl) pyridine – 3,5 – dicarboxylic acid – 2 –[N-(3,3 – diphenylpropyl)-N-methylamino] – 1,1 – dimethylethyl methyl diester hydrochloride
Structure:
Molecular formula: C36H41N3O6, HCl Molecular mass: 648.24
The documentation on the drug substance is presented as a EDMF.
Stability studies have been performed with the drug substance. No significant changes in any parameters were observed. According to the stability studies presented, an appropriate retest period has been set.
II.3
Medicinal Product
The development of the product has been described, the choice of excipients is justified and their functions explained.
The product specifications cover appropriate parameters for this dosage form. Validations of the analytical methods have been presented. Batch analysis has been performed on two batches for each strength. The batch analysis results show that the finished products meet the specifications proposed. The conditions used in the stability studies are according to the ICH stability guideline. The control tests and specifications for the drug product are adequately drawn up.
A shelf life of 2 years with the storage conditions “Do not store above 25 °C. Store in original packaging in order to protect from moisture” has been accepted.
has not provided additional studies and further studies are not required. Overview based on literature review is, thus, appropriate.
The non-clinical overview report refers 45 publications up to year 2011. The non-clinical overview on the pre-clinical pharmacology, pharmacokinetics and toxicology is adequate.
III.2 Ecotoxicity/environmental risk assessment (ERA)
Since Lercanidipinhydrochlorid ”Sandoz” is intended for generic substitution, this will not lead to an increased exposure to the environment. An environmental risk assessment is therefore not deemed necessary.
IV.
CLINICAL ASPECTS
IV.1
Introduction
Lercanidipine hydrochloride is a well-known active substance with established efficacy and tolerability. As lercanidipine hydrochloride is a widely used, well-known active substance, the MAH has not provided additional studies (apart from a supportive bioequivalence study referenced below) and further studies are not required. Overview based on literature review is, thus, appropriate.
The clinical overview on the clinical pharmacology, efficacy and safety is adequate.
IV.2
Pharmacokinetics
Biowaiver
The application concerns 2 dosage strengths, 10 mg and 20 mg. The study was carried out with the 20 mg strength. Justification for biowaiver for the 10 mg strength was provided. Biowaiver criteria cf. Guideline on the Investigation of Bioequivalence (CPMP/EWP/QWP/1401/98 Rev.1/Corr*) section 4.1.6 were met.
Bioequivalence
To support the application, the MAH submitted as report one bioequivalence study, in which Lercanidipinhydrochlorid “Sandoz” 20 mg coated tablets was compared with Corifeo 20 mg film-coated tablets, UCB GmbH, Germany.
The study was an open-label, randomized, two-treatment, two-sequence, four-period, replicated, crossover, single-dose bioavailability study conducted under fasting conditions with a wash out period of 7 days between the two administrations. 20 mg was administered in each period.
34 healthy male subjects participated in the study. 27 subjects completed the study. The primary variables for conclusion of bioequivalence were: AUC0-t, AUC0-∞ and Cmax in.
The 90% confidence intervals of the ratio (test/reference) of least-squares means for log-transformed AUC0-t and Cmax should be within 80% and 125% for (+)-S-lercanidipine in order to conclude bioequivalence.
Results
Table 1. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, tmax median, range) for (+)-S-lercanidipine
Treatment AUC0-t pg/ml/h ( SD) AUC0-∞ pg/ml/h (SD) Cmax pg/ml (SD) tmax h T1/2 h ( SD) Test 1 28511.25 (13798.35) 29617.09 (14297.03) 6492.27 (3457.56) 1.75 (1.00-4.50) 11.43 (6.24) Test 2 37988.78 (13728.61) 39817.15 (14380.10) 8539.63 (2630.70) 1.75 (1.00-3.50) 16.41 (4.74) Mean Test 32681.50 (12628.17) (13143.31) 34105.35 (2765.24) 7410.64 (1.00-3.125) 1.75 (4.50) 13.65 Reference 1 29925.76 (9748.86) 30936.80 (10099.82) 6265.17 (2433.89) 2.00 (1.00-4.50) 11.12 (5.59) Reference 2 35928.27 (11323.70) 37505.26 (11687.31) 7542.28 (2743.56) 1.75 (1.00-3.50) 15.00 (7.02) Mean Reference 32662.15 (9826.31) 33932.55 (10152.74) 6883.88 (2157.51) 2.00 (1.00-3.50) 12.85 (4.71) *Ratio (90% CI) 86-104 86-105 89-116
AUC0-∞ area under the plasma concentration-time curve from time zero to infinity AUC0-t area under the plasma concentration-time curve from time zero to t hours
Cmax maximum plasma concentration Tmax time for maximum concentration T1/2 half-life
* log-transformed values
Table 2. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, tmax median, range) for (-)-R-lercanidipine
Treatment AUC0-t pg/ml/h ( SD) AUC0-∞ pg/ml/h (SD) Cmax pg/ml (SD) tmax h T1/2 h (SD) Test 1 30489.17 (15250.61) (15971.83) 31906.50 (3620.81) 6500.63 (1.00-4.50) 1.75 (5.15) 15.58 Test 2 40515.06 (17441.50) (18530.66) 43052.15 (3351.32) 8503.46 (1.00-3.50) 1.75 (3.55) 19.48 Mean Test 35047.85 (14702.06) 36964.40 (15558.95) 7425.89 (3095.46) 1.75 (1.00-3.125) 17.44 (3.35) Reference 1 30986.28 (10281.27) (10588.38) 32695.46 (2188.06) 6001.10 (1.00-5.00) 2.00 (5.05) 16.22 Reference 2 37387.39 (13094.64) (13798.96) 39451.42 (2615.91) 7273.37 (1.25-3.50) 1.75 (6.53) 17.19 Mean Reference 33969.64 (10923.09) (11345.03) 35850.95 (2058.11) 6641.61 (1.25-3.75) 2.125 (4.72) 16.55 *Ratio (90% CI) 87-108 87-107 90-118
AUC0-∞ area under the plasma concentration-time curve from time zero to infinity AUC0-t area under the plasma concentration-time curve from time zero to t hours
Pharmacokinetic conclusion
90% confidence intervals of the ratio (test/reference) of least-squares means for log-transformed AUC 0-t and Cmax were within 80% and 125% for (+)-S-lercanidipine and for (-)-R-lercanidipine.
The 90% CI of AUC0-t and Cmax demonstrate bioequivalence between the test product,
Lercanidipinhydrochlorid “Sandoz” 20 mg film-coated tablets, and the reference product, Corifeo 20 mg film-coated tablets.
The results from the 20 mg film-coated tablet can be extrapolated to the 10 mg film-coated tablet, according to conditions in Note for Guidance on the Investigation of Bioavailability and Bioequivalence CPMP/EWP/QWP/1401/98, section 5.4.
The RMS has been assured that the bioequivalence study has been conducted in accordance with acceptable standards of Good Clinical Practice (GCP, see Directive 2005/28/EC) and Good Laboratory Practice (GLP, see Directives 2004/9/EC and 2004/10/EC).
IV.3
Risk Management Plan
The MAH has submitted a risk management plan, in accordance with the requirements of Directive 2001/83/EC as amended, describing the pharmacovigilance activities and interventions designed to identify, characterise, prevent or minimise risks relating to Lercanidipinhydrochlorid “Sandoz”.
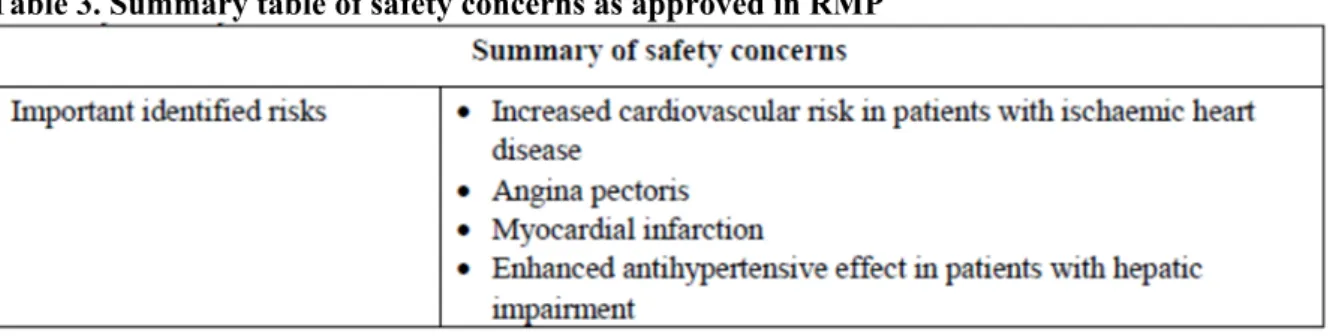
The following summary list of safety concerns with no additional pharmacovigilance measures or risk minimisation measures has been agreed:
Table 3. Summary table of safety concerns as approved in RMP
V.
USER CONSULTATION
A user consultation with target patient groups on the package information leaflet (PIL) has been performed on the basis of a bridging report making reference to Zanidip Novum 8 mg and 16 mg film-coated tablets, UK/H/0132/003-004/DC.
The bridging report submitted by the MAH has been found acceptable.
VI.
OVERALL CONCLUSION, BENEFIT/RISK ASSESSMENT AND
RECOMMENDATION
Lercanidipinhydrochlorid ”Sandoz” 10 mg and 20 mg film-coated tablets has a proven chemical-pharmaceutical quality and is a generic form of Zanidip. Zanidip is a well-known medicinal product with an established favourable efficacy and safety profile.
Bioequivalence has been shown to be in compliance with the requirements of European guidance documents.
The MAH has presented a risk management plan summarising the safety concerns. There are no additional pharmacovigilance or risk minimisation measures.
Agreement between Member States was reached during a written procedure. There was no discussion in the CMD(h). The Concerned Member States, on the basis of the data submitted, considered that essential similarity has been demonstrated for Lercanidipinhydrochlorid ”Sandoz” with the reference product, and have therefore granted a marketing authorisation. The decentralised procedure was finalised on 11 February 2015. Lercanidipinhydrochlorid ”Sandoz” was authorised in Denmark on 11 March 2015.
According to the List of Union reference dates and frequency of submission of periodic safety update reports (PSURs), no routine PSURs are required for this product.
The date for the first renewal will be: 11 February 2020.
The following post-approval commitments have been made during the procedure:
The MAH shall perform the required pharmacovigilance activities and interventions detailed in the agreed RMP presented in Module 1.8.2 of the Marketing Authorisation and any agreed subsequent updates of the RMP.
An updated RMP should be submitted: - At the request of the RMS;
- Whenever the risk management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit/risk profile or as the result of an important (pharmacovigilance or risk minimisation) milestone being reached.
If the dates for submission of a PSUR and the update of a RMP coincide, they can be submitted at the same time.