IJP (2018), Vol. 5, Issue 6 (Research Article)
Received on 12 February 2018; received in revised form, 26 March 2018; accepted, 30 March 2018; published 01 June 2018
IN-VITRO FREE RADICAL SCAVENGING ACTIVITY OF APOROSA LINDLEYANA BAILL.
S. Ramakrishnan * 1 and R. Venkataraman 2
Department of Biotechnology 1, Department of Chemistry 2, Sri Paramakalyani College, Alwarkurichi - 627412, Tamil Nadu, India.
ABSTRACT: The free radical scavenging activity of ethanolic root extract of Aporosa lindleyana (EREAL) was evaluated in-vitro with the spectrophotometric method based on the inhibition of hydroxyl radical, the reduction of stable DPPH free radical, inhibition of superoxide anion radical, total antioxidant activity and reducing power. Preliminary phytochemical screening showed the presence of phenolic compounds and flavonoids. EREAL showed significant activities in all free radical scavenging assays compared to standard antioxidants such as ascorbic acid, butylated hydroxyl toluene and vitamin E. In DPPH scavenging assay the IC50 value of EREAL
was 215.51 µg/mL, while vitamin E had 299.11 µg/mL. Furthermore, EREAL showed good free radical scavenging activity on reducing power, superoxide anion radical activity, total antioxidant activity, hydroxyl radical activity and had good reducing power.
INTRODUCTION: In recent years, the extracts of many plants have been screened for their antioxidant activities. Among that Aporosa lindleyana (Family: Euphorbiaceae) is well known for their antioxidant properties and most of its components have been identified 1. The medicinal properties of folk plants are mainly attributed to the presence of flavonoids, but may also be influenced by other organic and inorganic 2. The large generation of free radicals, particularly reactive oxygen species (ROS) play a key role in the progression of pathological disturbances such as cancer, Parkinson disease, etc. 3, 4, 5, 6, 7, 8 Plants are well known for their phytochemical components, especially polyphenols (flavonoids, phenolic acids etc.) responsible for the free radical scavenging and antioxidant activities of plants 9.
QUICK RESPONSE CODE
DOI:
10.13040/IJPSR.0975-8232.IJP.5(6).369-75
The article can be accessed online on
www.ijpjournal.com
DOI link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(6).369-75
ROS are ubiquitous, normally produced as a by product of cellular metabolism. They are capable of damaging biomolecules, provoking an immune response, activating oncogenes and enhancing aging process 10. ROS also play a role in long term complication of diabetes 11, 12, 13.
Aporosa lindleyana is a much branched, evergreen, glabrous tree, grown in India and Sri Lanka. It possesses antioxidant activity 14 & hepatoprotective effect 1 and also showed an anti-hyperglycemic effect 15. Aporosa lindleyana have many medicinal properties such as a diuretic, antiviral and good analgesic activity. Roots are used to treat headache, fever and jaundice, seminal loss and excessive thirst 16, 17, 18.
A review of the literature did not reveal any information on free radical scavenging activity of this plant. So, in this present study, we reported the free radical scavenging activity of ethanolic roots extracts of Aporosa lindleyana (EREAL) was investigated by employing various in-vitro by the DPPH (1, 1-diphenyl, 2-picrylhydrazyl), reducing power and other antioxidant capacity assays.
Keywords:
EREAL, DPPH, Free radical, Ascorbic acid Correspondence to Author: Srinivasan Ramakrishnan
Department of Biotechnology, Sri Paramakalyani College, Alwarkurichi - 627412, Tamil Nadu India.
MATERIALS AND METHODS:
Chemicals: Thiobarbituric acid (TBA), phenazine-methosulphate (PMS), nitroblue tetrazolium (NBT), 5, 5'-dithiobis 2-nitrobenzoic acid (DTNB), L, g-glutamyl-p-nitroanilide, α-tocopherol, 2, 2’-Azino bis- (3- ethylbenzothiazoline- 6- sulfonic acid), (ABTS.+), 1, 1- diphenyl- 2- picryl- hydrazyl (DPPH), 3- (2-pyridyl)- 5, 6-bis (4- phenyl-sulfonic acid), butylated hydroxytoluene (BHT) and other chemicals and solvents were of analytical grade and purchased from S.D. Fine Chemicals, Mumbai, India.
Collection and Processing of Plant Material: The roots of Aporosa lindleyana was collected from Keeriparai, Kanyakumari District, Tamil Nadu during January 2009. The specimen was identified by Dr. V. Chelladurai, Taxonomist, Department of Ayurvedic Sciences, Tirunelveli District. The roots were cleansed and shade dried for a weak and grounded into a uniform powder. 1 gm of plant material was added to 20 ml of aqueous ethanol (20% v/v) for 18 h at room temperature. The extracts were filtered and used for the estimation of total phenols and antioxidant activity.
Phytochemical Screening: The freshly prepared ethanolic roots extracts of Aporosa lindleyana (EREAL) was qualitatively tested for the presence of phytochemicals. Screening of EREAL for its phytocomponents was tested by using the reagents and chemicals such as alkaloids with Dragendorff’s reagent, flavonoids with the use of mg and HCl. Tannins with ferric chloride and potassium dichromate solution and saponins with ability to produce suds. These were identified by color reactions using standard procedures 19.
Free Radical Scavenging Assays: Numerous antioxidant methods and modifications have been proposed to evaluate antioxidant activity and to explain how antioxidants function. Of these, active oxygen species such as OH˙, H2O2 and O2
•-quenching assays, DPPH●, ABTS●+ radical scavenging assays and reducing power assay are most commonly used for the evaluation of antioxidant activities.
Standard Antioxidants: The standard antioxidants used in the study were namely ascorbic acid, alpha-tocopherol, and butylated hydroxy toluene (BHT).
Ascorbic acid was dissolved in double distilled water. α-tocopherol and BHT were dissolved in methanol. A required quantity of EREAL was suspended in 1% CMC (Carboxy methyl cellulose). Then the resultant suspension was taken and used for evaluating the antioxidant activity.
Hydroxyl Radical Scavenging Assay: The
hydroxyl radical scavenging activity of EREAL was determined by the method of Halliwell et al., 1987. 20 In this assay, hydroxyl radicals are produced by the reduction of H2O2 by the transition
metal (iron) in the presence of Ascorbic acid. The generation of hydroxyl radical is detected by its ability to degrade deoxyribose to form products, which on heating with TBA forms a pink color chromogen. Addition of EREAL competes with deoxyribose for hydroxyl radicals and diminishes the color formation.
The incubation mixture in a total volume of 1 mL contained 0.1 mL of buffer, varying volumes of EREAL (10, 20, 40, 80 and 160 µg/mL), 0.2 mL of ferric chloride, 0.1 mL of ascorbic acid, 0.1 mL of EDTA, 0.1 mL of H2O2 and 0.2 mL of
2-deoxyribose. The contents were mixed thoroughly and incubated at room temperature for 60 min and then added 1 mL of TBA and 1 mL of TCA. All the tubes were kept in a boiling water bath for 30 min. The absorbance of the supernatant was read in a spectrophotometer at 535 nm with reagent blank containing water in place of the extract. The efficiency of EREAL was compared with various concentrations (10, 20, 30, 40 and 50 µg) of standard ascorbic acid. Decreased absorbance of the reaction mixture indicated increased hydroxyl radical scavenging activity. The percentage of scavenging was calculated as shown below:
% Scavenging = Control OD - Test OD# / Control OD × 100
# OD – Optical Density
Superoxide Anion Scavenging Activity:
indicated the generation of superoxide anion, which was measured spectrophotometrically at 560 nm. A decrease in the formation of color after the addition of the antioxidant was a measure of its superoxide radical scavenging activity.
To 1 mL of NBT, 1 mL of NADH solution and varying volumes of EREAL (10, 20, 40, 80 and 160 µg/mL) were added and mixed well. The reaction was started by the addition of 100µL of PMS. The reaction mixture was incubated at 30 ºC for 15 min. The absorbance was measured at 560 nm in a spectrophotometer. Incubation with water in place of EREAL was used as blank. BHT was used as a standard for comparison. Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. The percentage of scavenging was calculated as shown below:
% Scavenging = Control OD - Test OD/ Control OD × 100
DPPH● Radical Scavenging Assay: The radical scavenging activity of EREAL against DPPH● was determined spectrophotometrically by the method of Brand Williams et al., 1995. 22 DPPH● is a stable free radical and accepts an electron, or hydrogen radical to become a stable diamagnetic molecule. DPPH● reacts with an antioxidant compound that can donate hydrogen and gets reduced. The change in colour (from deep violet to light yellow) was measured. The intensity of the yellow colour depends on the amount and nature of radical scavenger present. The reaction mixture in a total volume of 3 mL contained 1 mL of DPPH●, various concentrations of EREAL (25, 50, 100, 200 and 250 µg/mL) and made up to 3 mL with water. The tubes were incubated for 10 min at 37 ºC. A blue color chromophore was formed, the absorbance of which was measured at 517 nm. Vitamin E was used as standards for comparison.
% Scavenging = Control OD - Test OD/ Control OD × 100
Evaluation of Total Antioxidant Activity -
(ABTS●+ Radical Cation Decolourization
Assay): The generation of the ABTS●+ radical cation forms the basis of one of the spectrophotometric methods that have been applied for the measurement of the total antioxidant activity of solutions of pure substances 23. The improved technique for the generation of ABTS●+ described here involves the direct production of the
blue / green ABTS●+ chromophore through the reaction between ABTS●+ and potassium persulphate. Addition of EREAL competes with ABTS●+ diminishes the color formation.
ABTS●+ was dissolved in water at a concentration of 7 mM. The stock solution was mixed with 2.45 mM potassium persulphate (final concentration). The mixture was allowed to stand in the dark at room temperature for 12-16 h before use for incomplete oxidation of ABTS●+. The radical was stable in this form for more than two days when stored in the dark at room temperature. The following reagents were added in the following order. The incubation mixture in a total volume of 5 mL contained 0.54 mL of ABTS●+, 0.5 mL of phosphate buffer and varying concentrations of EREAL (10, 20, 40, 80 and 160 µg/mL). The blank contained water in place of EREAL. The absorbance was read in a spectrophotometer at 734 nm and compared with standard BHT.
Reducing Power: The reducing power of EREAL was determined by the method of Oyaizu M, 1986
36
. Substances which have reduction potential react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form a ferric-ferrous complex that has an absorption maximum at 700 nm. Increase in the reduction of ferric to ferrous ion increases the absorbance indicating the reducing ability of EREAL.
Varying concentrations of EREAL (5, 10, 20, 40, 80 µg/mL) in double distilled water was mixed with 2.5 mL of phosphate buffer and 2.5 mL of potassium ferricyanide. The mixture was incubated at 50 °C for 20 min after which, 1.5 mL of TCA was added and centrifuged at 3000 χ g for 10 min. From all the tubes, 0.5 mL of supernatant was mixed with 1 mL of distilled water and 0.5 mL of ferric chloride. The absorbance was measured at 700 nm in a spectrophotometer. BHT was used as a standard for comparison. Increased absorbance of the reaction mixture indicated increasing reducing power. Incubation with water in place of additives was used as the blank.
RESULTS AND DISCUSSION: Preliminary
phenolic compounds, tannins, flavonoids, xanthoproteins, aromatic acids, triterpenoids, proteins, carbohydrates and reducing sugars, which are summarised in Table 1.
TABLE 1: PRELIMINARY PHYTOCHEMICAL
SCREENING OF EREAL
Phytochemicals Ethanol extract
Alkaloids
-Carbohydrates +
Steroids -
Phenolic compounds +
Tannins +
Flavonoids +
Saponin -
Xanthoprotein +
Aromatic acids +
Triterpenoids +
Proteins +
Reducing sugars +
+ = Presence, - = Absence
Phenolic compounds and flavonoids have been reported for its antioxidant action in the biological system 24, 25. The free radical scavenging ability of EREAL was investigated by evaluation of lipid peroxidation or a measurement of free radical scavengers donate hydrogen to free radicals, leading to non-toxic species and therefore to inhibit the propagation of lipid oxidation.
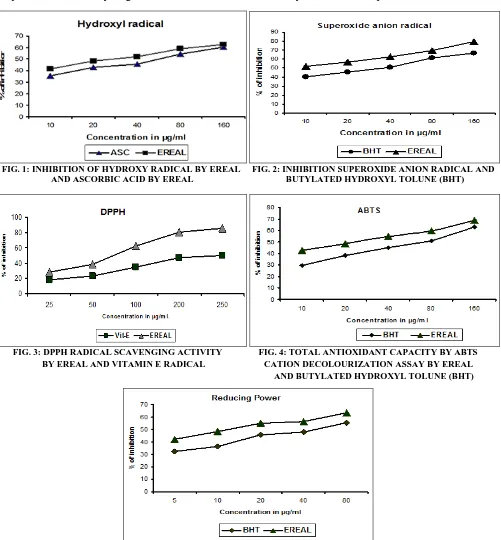
Inhibition of Hydroxyl Radical: The scavenging ability of EREAL on OH● is shown in Fig. 1 and compared with ascorbic acid. Degradation of deoxyribose by OH● releases certain products, which upon heating with TBA under acidic condition would yield a pink color with maximum absorbance at 532 nm. EREAL inhibit OH● formation during the incubation period, and the percentage of inhibition is higher than ascorbic acid at all concentrations. The highly reactive OH● can cause oxidative damage to DNA, lipids, and proteins 26.
Like many other free radicals, OH● can be neutralized if it is provided with a hydrogen atom. The effect of EREAL on the inhibition of free radical-mediated deoxyribose damage was assessed using the Iron (II)-dependent DNA damage assay. The Fenton reaction generates OH● which degrades DNA deoxyribose, using Fe2+ salts as an important catalytic component. Oxygen radicals may attack DNA either in sugar or base, giving rise to a large number of products.
The effect of the EREAL on the Iron (II) dependent deoxyribose damage. EREAL exhibited more pronounced hydroxyl radical scavenging activity compared to ascorbic acid in a dose-dependent manner. In OH● radical scavenging assay the IC50
value of the EREAL was 95.66µg/ml. Phyto-chemical studies of EREAL revealed the presence of phenolic compounds, triterpenoids, squalene and ferulic acid. The scavenging activities of phenolic substances might be due to the active hydrogen donating ability of hydroxyl substitutions 27.
Flavonoids are a group of effective antioxidants which are present abundantly throughout the plant kingdom. Flavonoid and related compound are effective in scavenging DPPH radical 28, hydroxyl radical and in metal-chelating capacity 29. Flavonoids are found to exhibit numerous biological activities like vasodilatory, anti-carcinogenic, anti-inflammatory, antibacterial, immune-stimulating, antiallergic, and antiviral effects 30.
Inhibition of Superoxide Anion Radical: The superoxide anion scavenging ability of EREAL has been presented in Fig. 2. In the PMS–NADH– NBT system, superoxide anion is derived from dissolved oxygen by PMS–NADH coupling reaction and reduces NBT. The decrease of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. The inhibition of O2•- was found
to be concentration dependent and the percentage of inhibition was greater than BHT at all concentrations studied. Superoxide anions are indirectly initiated the lipid oxidation as a result of superoxide and hydrogen peroxide, serving as precursors of singlet oxygen and OH●. In superoxide radical scavenging assay the IC50 value
of the EREAL was 76.16 µg/ml, and BHT was 90.43µg/ml. Inhibition of superoxide generation by EREAL may be due to the presence of terpenoids, squalene, and flavonoids. Robak and Glyglewski, 1988 31 reported that the antioxidant properties of flavonoids are effective mainly via the scavenging of superoxide anion.
radical decreased in the order of EREAL > Vitamin E. The inhibition was found to be concentration dependent. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a
stable diamagnetic molecule. The model of scavenging the stable DPPH● is a widely used method to evaluate antioxidant activities in a relatively short time compared to other methods 32.
FIG. 1: INHIBITION OF HYDROXY RADICAL BY EREAL FIG. 2: INHIBITION SUPEROXIDE ANION RADICAL AND AND ASCORBIC ACID BY EREAL BUTYLATED HYDROXYL TOLUNE (BHT)
FIG. 3: DPPH RADICAL SCAVENGING ACTIVITY FIG. 4: TOTAL ANTIOXIDANT CAPACITY BY ABTS BY EREAL AND VITAMIN E RADICAL CATION DECOLOURIZATION ASSAY BY EREAL AND BUTYLATED HYDROXYL TOLUNE (BHT)
FIG. 5: THE REDUCTIVE CAPABILITY OF EREAL AND BUTYLATED HYDROXYL TOLUENE (BHT) Values are the average of duplicate experiments and represented as mean ± standard deviation. ASC - Ascorbic acid; EREAL - ethanolic Root Extract of Aporosa lindleyana. BHT - Butylated Hydroxy Toluene; EREAL - ethanolic Root Extract of Aporosa lindleyana. Vit. E - Vitamin E; EREAL - ethanolic Root Extract of Aporosa lindleyana.
In DPPH● scavenging assay the IC50 value of the
EREAL was 215.51 µg/ml, and standard Vitamin E was 299.11 µg/ml. The effect of EREAL on
to the reaction between antioxidant molecules and radical, which results in the scavenging of the radical by hydrogen donation. This is visualized as discoloration from purple to yellow.
Total Antioxidant Activity - ABTS Radical Cation Decolourization Assay: Total antioxidant activity of EREAL was determined by ABTS radical cation decolorization assay. This assay assesses the total radical scavenging capacity based on the ability of a compound to scavenge the stable 2, 2’-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical 33. The effects of various concentrations of EREAL (from 10 - 160 µg/mL) on ABTS radical were shown in Fig. 4. EREAL exhibited effective antioxidant activity at all doses. The inhibition was found to be concentration and time-dependent and the antioxidant activity was better than the standard BHT.
In ABTS radical scavenging assay the IC50 value of
the EREAL was 88.33 µg/ml, and standard BHT was 100.22 µg/ml. The antioxidant activity of EREAL might be attributed to the presence of phytochemicals such as terpenoids, squalene, flavonoids, and phenolic compounds. The antioxidant activity of EREAL may be due to the reduction of hydroperoxides, inactivation of free radicals or combinations.
Reducing Ability: Fig. 5 showed the reductive capabilities of EREAL compared to BHT. Like the antioxidant activity, the reducing power of EREAL increased concentration - dependent manner. EREAL showed higher reducing power than the BHT. In reducing power radical scavenging assay, the IC50 value of the EREAL was 46.75 µg/mL and
standard compound 54.94 µg/mL. Antioxidant activity has been reported to be concomitant with the development of reducing power 34. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity 35 reported that the reducing power of tannins prevents liver injury by inhibiting the formation of lipid peroxides. Like antioxidant activity, the reducing power of EREAL was increased with an increased amount of sample.
All concentrations of EREAL showed higher activities than BHT. Based on the data obtained from this study, EREAL exhibits free radical inhibitor or scavenger activity as well as a primary
antioxidant that reacts with free radicals, which may limit free radical damage occurring in the human body. Preventive effect of EREAL was in a dose-dependent manner. The various antioxidant mechanisms of root extract may be attributed to strong hydrogen donating ability and their effectiveness as scavengers of superoxide and free radicals.
Furthermore, the EREAL competes with ABTS●+ or DPPH● and diminishes chromogen formation. Thus, EREAL has a powerful antioxidant activity against various in-vitro oxidative systems and can be used as accessible source of natural antioxidants and a possible food supplement.
CONCLUSION: This present study clearly
concluded that the ethanolic root extract of A. lindleyana possesses antioxidant properties and can serve as a natural source to develop the free radical scavengers and antioxidant agents. These agents are responsible for anti-inflammatory and chemoprotective mechanism.
ACKNOWLEDGEMENT: We are immensely
thankful to The Management and the Principal of Sri Paramakalyani College, Alwarkurichi for providing all the facilities and constant encouragement during the period of work.
CONFLICT OF INTEREST: Nil
REFERENCES:
1. Ramakrishnan S and Venkataraman R: Screening of
antioxidant activity, total phenolics and Gas
Chromatography-Mass Spectrophotometer (GC-MS) study of ethanolic extract of Aporosa lindleyana Baill. African Journal of Biochemistry Research 2011; 5(14): 360-364. 2. Repetto MG and Llesuy SF: Antioxidant properties of
natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res 2002; 35: 523-34.
3. Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS and Leitao SG: Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 2001; 15: 127-130.
4. Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J and Codina C: Comparison between the radical scavenging activities and antioxidant activity of six distilled and non-distilled Mediterranean herbs and aromatic plants. J Agric Food Chem 2002; 50: 6882-6890. 5. Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen
CH, Hwang SY and Lee MH: Free radical scavenging activity of Taiwanese native plants. Phytomedicine 2003; 10: 170-175.
7. Tepe B, Sokmen M, Akpulat HA and Sokmen A: In-vitro
antioxidant activities of the methanol extracts of four
Helichrysum species from Turkey Food Chem 2005; 90:
685-689.
8. Ozgen U, Mavi A, Terzi Z, Yildirim A, Coskun M and Houghton PJ: Antioxidant properties of some medicinal Lamiaceae species. Pharm Biol 2006; 44: 107-112. 9. Heinonen IM, Lehtonen PJ and Hopia AI: Antioxidant
activity of berry and fruit wines and liquors. J Agric Food Chem 1998; 46: 25-31.
10. Devasakayam TPA and Kamat JP: Free radicals and anti-oxidants in human health. EMSI News Let 2000; 23: 3. 11. Sabu MC and Kuttan R: Antidiabetic activity of medicinal
plants and their relationship with their antioxidant property. J Ethnopharmacol 2002; 81: 155-60.
12. Boynes JW: Role of oxidative stress in the development of complication in diabetes. Diabetes 1991; 40: 405-11. 13. Collier A, Wilson R, Bradley H, Thomson JA and Small
M: Free radical activity in type 2 diabetes. Diabetes 1990; 7: 27-30.
14. Badami S, Rai SR and Suresh B: Antioxidant activity of A.
lindleyana root. J Ethnopharmacol 2005; 101: 180-184.
15. Jayakar B and Suresh B: Antihyperglycemic and
hypoglycemic effect of Aporosa lindleyana in normal and alloxan induced diabetic rats. J Ethnopharmacol 2003; 84(2-3): 247-249.
16. Anonymous: Indian Pharmacopoeia, Government of India. Controller of Publication, New Delhi 1985.
17. Chopra RN, Nayar SL and Chopra IC: Glossary of Indian Medicinal plants. CSIR, New Delhi. India 1992; 22. 18. Kirtikar KR and Basu BD: Indian Medicinal plants,
International Books distributors, Book sellers and publishers, Dehradun, India, Vol. III, 1987: 2251.
19. Trease GE and Evans WC: Pharmacognosy, London, Baillieere Tindal, Edition 12th, 1983.
20. Halliwell B, Gutteridge JM and Aruoma OI: The deoxyribose method: A simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987; 165(1): 215-219.
21. Nishimiki M, Rao NA and Yagi K: The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophy Res Commun 1972; 46: 849-853.
22. Brand W, Cuvelier ME and Berset C: Use of a free radical method to evaluate antioxidant activity.
Lebensmittel-wissenschaft und-technologies. Food Science and
Technology 1995; 28: 25-30.
23. Wolfenden BS and Willson RL: Radical cations as reference chromogens in kinetic studies of one-electron
transfer reactions; pulse radiolysis studies of 2, 2’- azinobis-(3-ethylbenzothiazoline-6-sulfonate). J Chem Soc 1982; 24: 805-812.
24. Rice-Evans C, Sampson J, Bramley PM and Holloway DE: Why do we expect carotenoids to be antioxidants in-vivo
Free Radical Res 1997; 26: 381-98.
25. Jorgensen LV, Madsen HL, Thomsen MK, Dragsted LO and Skibsted LH: Regulation of phenolic antioxidants from phenoxyl radicals: An ESR and electrochemical studyof antioxidant hierarchy. Free Radical Res 1999; 30: 207-20.
26. Spencer JPE, Jenner A, Aruoma OI, Evans PJ, Kaur H and Dexter DT: Intense oxidative DNA damage promoted by L-DOPA and its metabolites, implications for neuro-degerative disease. FEBS Letters 1994; 353: 246-251. 27. Aurand LW, Boonme NH and Gidding GG: Superoxide
and singlet oxygen in milk lipid peroxidation. J Dairy Sci 1977; 60: 363-369.
28. Apati P, Szentmihalyi K, Kristo Sz T, Papp I, Vinkler P and Szoke E: Herbal remedies of Solidago, correlation of phytochemical characteristics and antioxidative properties. J Pharmacol Biomed. Analysis 2003; 32: 1045-1053. 29. Lean M, Norrozi M, Kelly L, Burrns J, Talwar D and
Satter N: Dietary flavanoids protect diabetic human lymphocytes against oxidant damage to DNA. Diabetes 1999; 48: 176-181.
30. Middleton E and Kandaswami C: Efects of flavonoids on immune and inflammatory function. Biochem Pharmacol 1992; 43: 1167-1179.
31. Robak J and Gryglewski IR: Flavonoids are scavengers of superoxide anions. Biochem Pharm 1988; 37: 837-841. 32. Soares JR, Dins TCP, Cunha AP and Ameida LM:
Antioxidant activity of some extracts of Thymus zygis. Free Radic Res 1997; 26: 469-478.
33. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M and Rice-Evans C: Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231-1237.
34. Tanaka M, Kuie CW, Nagashima Y and Taguchi T: Application of antioxidative Maillard reaction products from histidine and glucose to saline products. Nippon Suisan Gakkai-Shi 1988; 54: 1409-1414.
35. Mier S, Kaner J, Akiri B and Hadas SP: Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agri Food Chem 1995; 43: 1813-1817.
36. Oyaizu M: Studies on product of browning reaction prepared from glucosamine. Japan J Nutr 1986; 44: 307-315.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
This article can be downloaded to ANDROID OS based mobile. Scan QR Code using Code/Bar Scanner from your mobile. (Scanners are available on Google Playstore)
How to cite this article: