Platinum is now considered one of the most practical materials for achieving electrochemical conversions (1, 2). In particular it can easily be cleaned to a polished surface, and employed in most electrocatalytic transformations, especially where adatoms deposited at the surface may induce a specific activity (3–13). Applications of the perfectly defined interfaces obtainable with platinum monocrystals are also noteworthy (14, 15). In the field of anodic processes, platinum is known (16) to be electrochemically stable even at very positive potentials. However, when platinum is employed as the cathode material, the situation is more complex (17): platinum indeed possesses a very low overpotential towards proton reduction, and this phenomenon seriously limits the accessi-ble domain for achieving conversions of organic molecules when rather negative potentials are required. In general, beyond –1 V vs. SCE (saturat-ed calomel electrode), hydrogen is frequently evolved (18–20). Thus even in organic solvents the level of moisture strongly limits the cathodic domain.
To circumvent the inconvenience of the low hydrogen evolution overpotential at platinum, the use of solvents of low proton availability has been developed. This may extend the cathodic domain by about 1 V. Given the desirability of reaching extremely negative potentials (e.g. –3 V vs. SCE, known to be easily obtained in organic solvents in the presence of tetraalkylammonium salts if a con-ventional mercury cathode is used), the application of “super-dry” media was developed (21, 22). Significantly dry media have been obtained by maintaining the solvent-electrolyte mixture over drying reagents such as strongly activated alumina. Extremely low moisture levels (often less than 50 ppm of water) have been attained. Unexpectedly, however, the use of platinum cathodes in contact with a super-dry solvent-electrolyte mixture brought to light (23–27) a form of ion insertion of (or reactivity with) the electrolyte (often an alkali metal or tetraalkylammonium salt) at the platinum-solution interface. Thus, in the presence of an electrolyte MX under conditions which effectively prevent hydrogen evolution at the platinum sur-DOI: 10.1595/147106706X152703
The Platinised Platinum Interface
Under Cathodic Polarisation
MORPHOLOGY CHANGES UNDER THE INFLUENCE OF TETRALKYLAMMONIUM SALTS IN SUPER-DRY SOLUTION
By Jacques Simonet
Equipe MaCSE, UMR 6226, Université de Rennes 1, Campus de Beaulieu, 35042 Rennes Cedex, France; E-mail: jacques.simonet@univ-rennes1.fr
face, the formation of a thin “ionometallic” layer of a general structure [Ptn–, M+, MX] could be
demonstrated. Here n depends on the saturation level of the platinum layer and therefore on its maximum degree of reduction. In general, it was found that n = 2 for N,N-dimethylformamide (DMF) containing dissolved alkali metal iodides or bulky tetraalkylammonium halides (NR4+X–),
according to Equation (i):
nPt + e–+ MX → [Pt
n–, M+, MX] (i)
In some respects, such a cathodic reaction of plat-inum with electrolytes – and the subsequent formation of an ionometallic layer – strongly resembles the formation of Zintl phases (28–30) via the reaction of an electropositive alkali metal with most of metalloids (such as Pb, Si or Ge). The classical synthesis of Zintl phases generally involves heating a mixture of these reagents in a closed tantalum or niobium vessel. Another reported method is the reduction of a salt of a post-transition metal in the presence of sodium metal in liquid ammonia (28, 29). Electrochemical methods may also be used (30–35): for example, cathodically polarised lead (or any other post-tran-sition metal) immersed in a solution of a non-electroactive cation (such as K+) may produce a reduced polyanionic form as shown in Equation (ii):
4K++ 4e–+ 9Pb →[Pb
94–, 4K+] (ii)
(Pb is the cathode material.)
Similar phases have already been described (34, 35) with polarised mercury in the presence of tetraalkylammonium ions. However, the implica-tion of the choice of anion in the electrolyte was never considered.
Returning to platinum, the analogous electro-chemical building of a complex layer owing to the involvement of ions concomitantly with the asso-ciated electron motion raises a number of issues (36, 37) about this new kind of interface: its stabil-ity, its conductivstabil-ity, the reversibility of electron storage, the maximum attainable thickness as well as its reducing power. Additional factors to be taken into account include progressive distortion of the platinum lattice induced by the motion of
electrolyte cations, and progressive swelling evi-dently associated with the stoichiometry of insertion into platinum.
The present paper aims to describe in terms of morphologic changes the cathodic behaviour of platinised platinum, which has been found to be much more reactive towards electrolytes than smooth platinum. This study focuses especially on the effect of tetraalkylammonium salts (TAAX) on the electrochemical reduction of platinum. An explanation of the effect of the TAA+cation size on structural changes to the layer is proposed. In particular the behaviour of tetramethylammonium salts (TMAX) appeared anomalous. This behav-iour provides information on the interfacial activity of the TMA+
ion, considered as the smallest non-acidic ammonium cation. Moreover, TMA+cannot form an ylide through the Hoffmann degradation (38, 39). Also at non-reactive cathodes, the TMA+ ion has been reported as by far the most readily electroactive (39), meaning that the electrochemi-cal reactivity of TMA+ could occur at a potential much less negative than those of other NR4+ions,
especially within a potential range where there is no appreciable reduction of residual water (even when present in significant amounts (> 500 ppm)).
Experimental Procedure
Salts and Solvent
In most of the experiments, the concentration of TAAX (X = I, Br, Cl, BF4, ClO4) was 0.1 M. All
salts studied were obtained from Fluka; their puri-ty was at least 99%, and all were employed without any additional purification after being thoroughly dried under vacuum at 60ºC (except for TAAClO4
the moisture levels no higher than 50 ppm.
Electrochemistry
All potentials are given with respect to the sat-urated calomel electrode (SCE), although measurements were obtained with a Ag⎪AgI⎪0.1 M TBAI system in DMF; this electrode, which is particularly suitable for scrupulously dry solutions, has a potential of –0.52 V vs. SCE at 25ºC. For voltammetry, a three-electrode cell was used with-out a separator. The counter electrode was a glassy carbon or graphite rod. Platinum working elec-trodes had a diameter of 1 mm. The working electrodes were polished with Norton polishing papers (grades 02 and 03) or DP Paste M made from monocrystalline diamonds (with particle size appropriate to the finest polishing). After polish-ing, the working electrode was sonicated for five minutes, rinsed twice with alcohol and acetone, and then dried at about 60ºC. Chronocoulometric and ECQM (electrochemical quartz microbalance measurement) procedures have been fully described in a recent paper (40). (Here the elec-trode acts specifically as a base provider, making
possible at its interface chemical reactions catal-ysed by strongly basic media.)
Electrode Plating
Coulometric experiments were carried out with platinised platinum electrodes prepared by deposi-tion of the metal from an aqueous soludeposi-tion of 10 g l–1H
2PtCl6 (Aldrich) in 0.1 M HCl onto a metal
disk (effective area: 0.78 mm2). Gold was plated
under the same conditions. Metal was deposited at a constant current density (30 mA cm–2).
Morphology changes of the platinum layer during charging/discharging cycles were followed by SEM analysis after rinsing each sample with alco-hol and acetone, and sonication to eliminate alumina particles.
Results
Voltammetry for Tetramethylammonium Salts
Figure 1 (curve (b)) summarises the voltammet-ric response of smooth platinum under standard aprotic conditions. In 0.1 M TMAClO4 (as with
other TMA+
salts), a diffusion step (IC) with a
Fig. 1 Voltammetric responses of platinum electrodes in 0.1 M
TMAClO4in DMF. Super-dry
condi-tions. Scan rate: 0.1 V s–1. Effective
electrode area: 0.78 mm2. Potentials
are referred to aqueous SCE: (a) Response of a platinised platinum electrode (two first scans) with a pause of 20 s at –2.8 V. Average thickness of the platinum deposit:
0.25 μm; (b) For comparison, the
half-peak potential of about –2.2 V vs. SCE is observed in the course of the first scan. At more negative potentials (beyond about –2.5 V vs. SCE), the cathodic limit is reached. This limit is attribut-able to electrolyte decomposition. Both the potential and the current for the main cathodic step (IC) vary with the mode of polishing of the platinum and the moisture level of the electrolyte solution. If the solution contains much more than 200 ppm water, step IC is progressively shifted towards less negative potentials and becomes total-ly irreversible. Higher water content leads to a “cathodic wall” attributable to the water reduction. However, the current at step IC depends on the mode of polishing of the platinum electrode. Given particularly careful polishing (always under super-dry conditions), the magnitude of step IC may decrease, and it may even disappear. The nature of the surface, grain boundaries, as well as the possible presence of fractals and activated sites would be expected to favour an interfacial charge-discharge process.
If the platinum interface described above is now platinised galvanostatically so as to produce a much larger active surface, the shape of the voltammetric curves, as shown in Figure 1(a), changes dramatically. For a relatively thick film of electrodeposited platinum (with, for example, an average thickness significantly greater than 0.1 μm), the use of a super-dry solvent-electrolyte enables a quasi-reversible step (IIC), at least in the
cases of TMAI, TMAClO4and TMABF4(Figures
1 and 2). For example, platinised platinum in 0.1 M TMAClO4exhibits a pair of broad main steps (IIC
and IA) whose half-peak potentials are E0.5pc =
–2.0 V and E0.5pa= –1.7 V vs. SCE, respectively. At
scan rates greater than 50 mV s–1, the relative areas
of these two steps are approximately equal. At potentials more negative than –2.3 V vs. SCE, another cathodic step may arise. Its presence apparently depends on the history of the electrode surface and on the amount of platinum galvanos-tatically deposited onto the substrate. Limiting currents for step IIC (iII) do not depend directly, at
a given sweep rate, on the amount of electrode-posited platinum. The platinising procedure shifts the main reduction step towards much less nega-tive potentials, and the rate of the charging process (although not measured here) appears significantly greater at a platinised platinum surface. The shapes of these steps and their current values would sug-gest that only the external part of the deposit – then in contact with the liquid phase – reacts with the components of the electrolyte. With longer electrolysis times, a sudden decay of the cathodic current is observed at very negative potentials (Figures 1 and 2). This may be induced by a self-inhibiting phenomenon. This inhibition is attributable to the weak electronic conductivity of the ionometallic layer formed during the reduction process. Conceivably, compacting associated with a structural change in the platinised layer further
Fig. 2 Electrochemical behaviour of platinised
plat-inum in 0.1 M TMABF4in
DMF. Super-dry conditions. Effective electrode area: 0.78 mm2. Average thickness of the
galvanostatic platinum deposit: 0.3 μm. Scan rate: 0.1 V s–1. Two first scans.
restricts the motion of species participating in this charging phenomenon.
Pauses were introduced into forward voltam-metric scans on both smooth platinum surfaces and substrates covered by very thin, strongly het-erogeneous platings. In these cases, at very negative potentials (as shown in Figure 1(a)), the following scans towards negative potentials may be significantly altered. The total cathodic current is then strongly increased, and step IC completely disappears. Recurrent scanning and/or pauses in scanning would be expected to contribute to changes in the nature of the platinum surface. Thus the presence of only the pair of steps IIC and IA would suggest that smooth platinum zones at the electrode surface have disappeared under cathodic treatment. This could correlate with the observation that the presence of steps IC and IIC together depends on the conditions prevailing dur-ing the platdur-ing, and on the amount of electricity passed. Step IIC is observed alone in some cases; this could be attributable to changes in the plating homogeneity.
For timescales much longer than those current-ly used for voltammetric experiments, the charging processes remained reversible, but generally showed current efficiencies lower than 60%. Figure 3 shows the two distinct branches in the chronocoulometric response of a platinised plat-inum microelectrode in a 0.1 M solution of TMAClO4 in DMF. The very steep slope at the
beginning of the discharge process is notable; it is
associated with the thinness of the reduced film at the interface.
Voltammetry for Other Tetraalkylammonium Salts
Voltammetric data obtained with bulky TAA+ salts (from tetra-n-butyl- to tetra-n-octylammoni-um salts) indicated much less reversibility than that already described for TMAX salts. The cathodic step IIIC (Figure 4) attributable to the reduction of platinum in the presence of the salt is shifted to more negative potentials. The corresponding anodic step IIIA is shifted in the anodic direction. Half-peak potentials are –2.4 V and –1.5 V vs. SCE respectively. The current of the anodic step is rel-atively small. At significantly more positive potentials, two reversible steps H1 and H2 may appear, even under super-dry conditions. These are readily assigned to the reversible anodic oxida-tion of hydrogen formed at very negative potentials at the polycrystalline platinum surface during the forward scan. The steps could not be induced merely by residual water reduction beyond –2.8 V. These steps were not observed with TMAX salts. Their presence tended to sup-port the concept of a “probase” cathode (40) previously proposed for tetramethylammonium salts. On the other hand, it would be expected that large TAA+ ions under the conditions given here would produce hydrogen evolution, leading to reductive degradation of the salt. This may be regarded as a kind of cathodic Hoffmann reaction,
Fig. 3 Chronocoulometric curve for a platinised platinum
electrode of area 0.78 mm2in
0.1 M TMAClO4in DMF.
Superdry conditions. Plating thickness: 0.2 μm. Charge at
–2.3 V vs. SCE for 10 s
fol-lowed by discharge at –0.5 V
in which the free electron plays the role of a base as shown in Equation (iii):
R3N+CH2–CH2R′+ e–
→ R3N + CH2=CHR′+ ½H2 (iii)
In a parallel process, the expected two-electron cathodic cleavage of TAA+(forming the anion R–) may trigger the classical Hoffmann degradation (protonation of the anion R–by the salt) – a partial explanation for the poor reversibility of the charg-ing process. In overall terms, this type of degradation leads to a broadly similar product dis-tribution, with the formation of a tertiary amine and an alkene. Only the hydrogen evolution (as proposed in Equation (iii)) appears specific to the behaviour of TAA+at the platinum interface.
Microcoulometry
Coulometric measurements were carried out at platinised interfaces in super-dry DMF-TAAX solutions. The conditions were close to those shown in Figure 3 (i.e. reduction beyond –2.4 V and oxidation at about –0.8 V), having reached sat-uration of the deposited layer. Only the amount of electricity recovered during the oxidation process
was taken into account. With n-butyl-, tetra-n-hexyl- and tetra-n-octylammonium halides, the stoichiometry was found to correspond to one electron per two atoms of platinum. This suggests a formula for the complex of the following form (Equation (iv)):
[Pt2–, TAA+, TAAX] (iv)
A large series of TMAX salts was tested (with X = ClO4–, BF4–, I–, Br–and Cl–), as well as the
corre-sponding sulfate salt (Figure 5). It was confirmed that bare platinum as such does not rapidly accu-mulate a high charge within the timescale necessary for such processes (e.g. a few minutes). All coulo-metric experiments were conducted in very carefully dried DMF-electrolyte solutions. Complete dryness and neutrality of the platinised platinum electrode were ensured by rinsing with alcohol and/or DMF with the addition of Me4NOH, followed by double rinsing with
ace-tone and drying. The gradient of the plot in Figure 5 reveals that four platinum atoms (abscissa) are correlated with the charge of one electron (ordi-nate), which supports the conclusion that the stoichiometry of the phase, after saturation within
Fig. 4 Repeated voltammetric scans at a platinised platinum electrode of effective area 0.78
mm2in 0.1 M TBAClO
4in
DMF. Super-dry conditions. Average thickness of the
plat-inum deposit: 0.4 μm. Scan
rate: 0.5 V s–1. Potentials are
the timescale of charging, can be written as follows (Equation (v)):
[Pt4–, TMA+, (TMAX)m] (v)
Analysis of platinum microdeposits onto the gold substrate (via the EQCM technique) confirmed that m = 1.
Morphology Changes to Large Electrodes on Macroelectrolysis
A large number of potentiostatic electrolyses were performed in two-compartment cells on pla-tinised sheets of commercial platinum in the presence of tetramethylammonium salts (TMAClO4, TMABF4 and TMAI), tetra-n-butyl
ammonium iodide (TBAI), tetra-n-hexylammon-ium bromide (ThexABr) and tetra-n-octyl-ammonium bromide (ToctABr). Experimental conditions were as described for the voltammetric and coulometric studies. The main point of these experiments was to use super-dry solvent-elec-trolyte; the addition of activated alumina to the cell appeared crucial to this. In order to compare the
surface modifications and draw conclusions, the following parameters were considered:
–
the initial thickness of the platinum deposit;–
the applied potential for the reduction;–
the theoretical amount of electricity per unit area required to charge (partially or totally) the platinised layer.After cathodic polarisation of the platinised samples, two alternative modes of oxidation were used and compared. The sample was either simply exposed to air after the electrolysis, or the sample was kept in the cell at a potential generally between –0.5 and +0.3 V vs. SCE until the current decayed completely. The present study describes only mor-phology changes caused by contact with air at the completion of the reduction process. Surface modifications were generally controlled so as to occur without any noticeable mass change. However, when thick deposits are over-reduced for a long time, charging of the platinum layer could possibly lead to a local collapse of the origi-nal plating. Figure 6 shows the origiorigi-nal appearance of a platinised platinum sample before any cathod-ic treatment in the presence of salts.
A few results have been selected as being the most representative from a large number of exper-iments demonstrating the huge modification of platinum surfaces by TAA+
salts under cathodic polarisation.
Modification of Platinum Films
In the absence of a visible degradation of the film (which was easily checked with a magnifying glass and confirmed by the absence of mass
Fig. 5 Microcoulometry of platinised platinum (area: 0.8 mm2) for different plating thicknesses. Charging at
–2.2 V vs. SCE (reduction for 100 s, allowing full
satura-tion of the deposit) and then oxidasatura-tion at 0 V until nil current. Qdis the quantity of electricity recovered during
discharge. Qf/4 is one quarter of the quantity of
electrici-ty required to convert PtIVto Pt0. Five TMAX salts were
tested at 0.1 M concentration in DMF: +(X = Cl),
•
(X= ClO4), Δ(X = Br), {{(X = BF4), (X = I)
Fig. 6 SEM image of a platinised layer galvanostatically deposited onto smooth platinum from H2PtCl6solution in
0.1 M HCl . Current density: 5 mA cm–2. Total quantity of
changes), the amount of electrodeposited platinum could be related to the amount of electricity neces-sary to achieve the deposit. For example, by using the initial platinised layer shown in Figure 6, the original deposit was made with a quantity of elec-tricity of 1.6 C cm–2(total reduction of PtIV), which
corresponds approximately to an average thickness of the deposit of δ= 0.4 μm. The reduction of the deposit at –2 V vs. SCE in the presence of TMAClO4was performed until an amount of
elec-tricity of 0.8 C cm–2was passed. This theoretically
corresponds to twice the amount of electricity required for total saturation of the layer, given that the charging yield would be about 50% platinum. The stoichiometry is given by Equation (v) with m = 1. Cathodic polarisation was followed by oxi-dation, simply by contact of the samples with air during rinsing. The samples were then thoroughly sonicated. This electrochemical treatment resulted in a complex coverage consisting of more or less
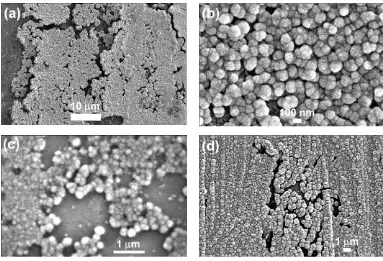
touching platinum spheroids, with average diame-ter depending on the thickness of the initial layer. The average diameter is estimated at about 0.5 μm for the structure shown in Figure 7(a). Apart from the zones of spheroids, large flat zones were attrib-uted to the original smooth platinum substrate.
Similar morphologies were observed with pla-tinised films, in particular with TBAI (See Figures 7(b) and 7(c)) and ToctABr (Figure 7(d)). However, with thicker platinised films, and with large amounts of electricity involved in the reduc-tion process, the layer swells to different extents. (The layer swells by transformation into spheres during the cathodic reaction of platinum with bulky salts, as indicated by the stoichiometry of the ionometallic complexes.)
The case of some TMA+salts is, however, more complex. These afford relatively limited swelling in terms of the stoichiometry given by Equation (v). Primary spheres are covered by smaller spheroids,
Fig. 7 Reduction of thin platinised film in the presence of TAAX salts in DMF under super-dry conditions. After
reduction, samples were rinsed and sonicated in contact with air: (a) Reduction in 0.1 M TMAClO4of film of mean
thickness 0.2 μm) at –2.5 V. Total amount of electricity: 0.8 C cm–2. Oxidation by air; (b) and (c) Reduction in 0.1 M
TBAI of a platinum deposit of 0.22 mg cm–2onto platinum substrate. Electrolysis at –2.5 V vs. SCE. Quantity of
elec-tricity: 1.7 C cm–2. No mass loss after oxidation by air, sonication and rinsing; (d) Reduction in 0.1 M
tetra-n-octylammonium bromide. Deposit of platinum: 0.30 mg cm–2. Electrolysis at –2.5 V. Quantity of electricity
giving the overall morphology the appearance of a layer of blackberry-like structures. The uniform volume of all the spheres is also striking. (See Figure 8 for the case of TMAI.)
The swelling may sometimes become chaotic, particularly where large amounts of electricity are involved. Large, regularly swollen cauliflower-like structures then arise (Figure 8). These are reminis-cent of the structures observed during the anodic polymerisation of pyrrole or thiophene (41, 42), in which the polymers are rendered electrically con-ductive by the progressive insertion of anions as “dopants” inside the polymer matrix. In the pre-sent work, cauliflower and macrospherical structures were found, principally with thick pla-tinised layers (Figures 9(a), 9(b) and 9(c)). During the oxidation of the platinised layer, movement of the salt out of the platinum causes a shrinkage, as clearly evidenced by the formation of large cracks (see Figure 9(d)) and large zones of uncovered
platinum substrate (Figure 7(c)). The shrinkage of cathodically generated spheres under oxidation by air, due to the expulsion of ions, is strongly expect-ed to yield almost empty structures. By contrast, the anodic oxidation of spheroid layers leads to profound changes, until a quasi-flattening of the platinum surface occurs (43) through the bursting of the spheres.
Discussion
The use of tetraalkylammonium salts as elec-trolytes in aprotic (super-dry) DMF can undoubtedly lead to the reversible charging of pla-tinised layers electrochemically deposited onto substrates such as platinum, glassy carbon or gold. The finely divided structure of platinised deposits apparently enhances the cathodic reactivity of plat-inum. Under these conditions, tetraalkyl-ammonium salts (R4NX with R bulkier than Me)
react cathodically with the platinum surface, but at potentials significantly more negative than those observed with R = Me. At such potentials, signifi-cant reduction of residual water may occur, and the charging process observed in conjunction with hydrogen evolution is always poorly reversible in terms of charge recovery.
By contrast, cyclic voltammetric experiments carried out at platinised layers in the presence of cathodically more reactive TMA+salts provide evi-dence for a quasi-reversible charge process. Thus, under super-dry conditions and for rather thick platinum deposits, the quantity of electricity specifically involved in the charging process was found to be an increasing function of the scan rate. Electrochemical charging is definitely first associ-ated with the external part of the platinised layer. However, with sufficiently high currents and long electrolysis times, the platinum layer may become saturated with the electrolyte salt. The charge recovered during the re-oxidation process reaches a limit, and becomes proportional to the amount of deposited platinum. Thus a simple calculation performed for several TMA+ salts demonstrates that, at least with thin platinum films, the charging process involves four atoms of platinum for each transferred electron.
All the ionometallic complexes obtained under
Fig. 8 Reduction (E = –2.5 V vs. SCE) of platinised
platinum in 0.1 M TMAI. Thickness of platinum deposit:
0.2 μm. Moderate quantity of charge corresponding to
similar conditions with bulky NR4+salts and alkali
metal iodides exhibit reducing properties. They can therefore produce anion-radicals from π-acceptors such as fluorenone and 1,4-dinitrobenzene. Similarly, the reducing power of the layer was recently used to cleave diazonium cations, making possible the chemical functionalisation of the plat-inum surface (44).
With TMAX, as stated above, the structure of the platinum layer at the saturation stage is quite different from those previously found (37) with bulky NR4+ (such as tetra-n-butyl-, tetra-n-hexyl-,
and tetra-n-octylammonium salts). This difference seems to be due to the smaller size of the TMA+ cation producing a change in the spatial structure of the ionometallic complex. After cathodic polar-isation of platinum and air exposure of the interface obtained in the presence of NR4+cations,
SEM images most frequently revealed either cauli-flower-like structures or layers of spheres,
depending on such experimental parameters as the thickness of the platinised layer and the amount of electricity involved in the reduction process. The appearance of spheres of uniform size, as well as huge changes to the morphology of the original platinum layer are highly intriguing. Does the for-mation of spheres or swollen structures occur during the reduction process or later when the sur-face is oxidised by contact with air? Recent in situ electrochemical scanning tunnelling microscopy (EC-STM) experiments in this laboratory (45) revealed, particularly in the case of tetra-n-buty-lammonium iodide, that the reversible swelling of platinum monocrystals can be followed using cyclic voltammetry over relatively short durations. The swelling of platinum was found to be directly related to the cathodic reaction of surface platinum atoms with the electrolyte. The complete restora-tion of the surface at the end of the reverse scan was highly effective, at least for short reaction
Fig. 9 Formation of more chaotic structures of cauliflower-like morphology. (a) Reduction of a platinised layer of
thickness 0.2 μm in 0.1 TMAClO4at –2.5 V under weak current density, with a moderate total quantity of electricity;
(b) Reduction of a platinised layer (0.30 mg cm–2) in 0.1 M tetra-n-octylammonium bromide with a large
over-reduc-tion of the layer. Part of the deposit then collapses; (c) Reducover-reduc-tion of a platinised film of thickness 2 μm in 0.1 M
TBABF4. A large quantity of electricity passed. Evidence for strongly swollen structures; (d) Same conditions as image
times and limited amounts of electricity. This find-ing strongly supports the conclusion that the platinum reactivity is fully reversible, at least in the case of tetra-n-butylammonium salts. Over-reduced ionometallic layers in the presence of TMA+(up to one TMA+ ion per platinum atom) could be reached in certain cases. Over-reduction of platings could cause spectacular swelling and deformation of platinum surfaces.
Returning to the quasi-uniform size of the spheres (as shown in Figures 7 (a)–(d)), it may be proposed that these structures grow under ther-modynamic conditions. (This assumption is supported by the quasi-reversibility of the electro-chemical process). The total conformational energy per sphere, Esp, may be split into two
terms, relating to the bulk (ρV) and surface (ρS)
energies respectively as shown in Equation (vi):
Esp= (4/3)πR3ρV– 4πR2ρS (vi)
where R is the radius of the spheres. At the equilibrium (Equation (vii)):
(dE/dR)eq= 4πR2ρV– 8πRρS (vii)
The equilibrium radius, Req, of the spheres is given
by Equation (viii):
Req= 2ρS/ρV (viii)
Thus the radii of the spheres depend only on energy factors specific to the experimental condi-tions (such as the nature of the salt used, its concentration, its level of dissociation, as well as the number of available platinum atoms). The for-mation of contiguous spheres of very similar volume suggests that the ionometallic layer turns out to be very mobile on the conducting substrate. Therefore the model of growth of swollen struc-tures from randomly distributed activated centres onto the substrate surface is probably incorrect.
A range of experiments were performed to visualise clearly the occurrence of the platinum swelling. The degree of swelling may be approxi-mately estimated by adopting the following formula for the charged phase obtained with TMAX salts (Equation (ix)):
[Pt4–, TMA+, TMAX] (ix)
The example of TMAI is then taken as representa-tive. On the basis of literature values of ionic radii for the phase constituents (43, 46), i.e., Pt = 0.80 Å, I–
= 2.2 Å, and TMA+
= 3.01 Å, it is possible to assess the degree of swelling for closely-adjacent ions. This calculation predicts a volume increase of approximately 30 times – which is huge. Similar swelling factors can be estimated for X = ClO4–
and BF4–. By contrast, the swelling at platinum
without a specific insertion of the salt would be limited to 14 times – again in the case of TMAI, on attaining complete reduction of the platinum layer. Thus the deposition of a few platinum atoms onto a conducting surface would lead to the progressive formation of a sphere-like volume, until attain-ment of the final stoichiometry based on the availability of strongly reactive platinum atoms. Although the mode of swelling is unclear so far, the rate of growth of such spheres would be deter-mined both by the number of starting points (perhaps randomly distributed on the surface) and the diffusion of ions. The mobility of the spheres on the platinum substrate (under the condition that an electric contact promotes their growth) should probably also be taken into account. Since the volume of the spheres is not directly depen-dent on the amount of platinum deposited, one can foresee the existence of zones weakly covered by a small number of spheres (see, for example, Figures 7(c) and 7(d)).
then the interface would be expected to keep roughly its original structure. The oxidation first forms a platinum shell, and continues by the dif-fusion of dioxygen through this shell. The action of dioxygen would tend to preserve the general features of the layer, including cracks and smaller spheres (often not immediately adjacent) owing to shrinkage. Scheme II depicts swelling processes on a much larger scale, which would lead to cauli-flower-like structures in the case of thicker platinum layers and/or over-reduction of deposits.
Conclusions
Under super-dry conditions, platinised plat-inum layers exhibit a high cathodic reactivity towards the electrolyte. In particular, with TMA+ salts, a fully reversible ion insertion has been demonstrated. A stoichiometry for this insertion process has been proposed for the condition that the platinised layer reaches is full reactivity. Coulometric results for a large number of TAAX salts strongly support the conclusion that two or four platinum atoms are associated with the trans-fer of one electron to produce thin modified
Schematic representation of the morphology change of a platinised platinum interface (shown in green) during the charging process by TMAX followed by air oxidation. The ionometallic layer (the fully reduced form of the platinised layer) is shown in red. The oxidation by dioxygen would lead to smaller spongy spheres, of which the shrinking factor illustrated here is arbitrary
Scheme I
Schematic representation for the swelling of the platinised platinum surface. Note the possible transformation of (c) for a moderate swelling into (d), obtained with larger amounts of electricity injected into the platinum layer (termed “cauliflower-like” structures in the text). Arrows indicate the supposed direction of the swelling
1 A. J. Bard and L. R. Faukner, “Electrochemical Methods: Fundamentals and Applications”, J. Wiley and Sons, New York, 1980
2 H. Lund, in “Organic Electrochemistry”, 4th Edn., eds. H. Lund and O. Hammerich, Marcel Dekker, New York, 2001, p. 243
3 R. Albalat, J. Claret, E. Gómez, C. Muller, M. Sarret and J. M. Feliu, J. Chem. Soc., Faraday Trans., 1990, 86, (10), 1845
4 L. D Burke and K. J. O’Dwyer, Electrochim. Acta, 1990, 35, (11–12), 1821
5 M. Shibata and S. Motoo, Hyomen Gijutsu, 1989, 40, (10), 78
6 V. N. Korshunov, V. A. Safonov and L. N. Vykhodtseva, Russ. J. Electrochem., 2005, 41, (9), 911 7 P. Ocon and J. Gonzalez Valesco, J. Appl.
Electrochem., 1988, 18, (1), 43
8 Lj. V. Minevski and R. R Adzic, J. Appl. Electrochem., 1988, 18, (2), 240
9 R. R. Adzic, W. E. O’Grady and S. Srinivasan, J. Electrochem. Soc., 1981, 128, (9), 1913
10 B. F. Gianetti, C. M. V. B. Almeida, S. H. Bonilla, M. O. A. Mengod and R. Raboczkay, Phys. Chem., 2003, 217, (10), 35
11 H. A. Lyazidi, M. Moukhedena, M. Mestre and J. F. Fauvarque, Bull. Soc. Chim. Fr., 1995, 132, (10), 1039 12 J. M. Felui, E. Herrero and M. J. Llorca, Book of Abstracts, 210th ACS National Meeting, Chicago, IL, 20th–24th August, 1995
13 K. B. Kokoh, J.-M. Léger, B. Beden, H. Huser and C. Lamy, Electrochim. Acta, 1992, 37, (11), 1909 14 Y.-Y. Yang and S.-G. Sun, J. Phys. Chem. B., 2002,
106, (48), 12499
15 J. Clavilier, A. Fernandez-Vega, J. M. Feliu and A. Aldaz, J. Electroanal. Chem., 1989, 261, (1), 113 16 H. J. Schäfer, ‘Organic Electrochemistry’, in
“Encyclopedia of Electrochemistry”, Volume 8, eds. A. J. Bard and M. Stratmann, Wiley-VCH, Weinheim, 2004
17 G. W. Morrow, in “Organic Electrochemistry”, 4th Edn., eds. H. Lund and O. Hammerich, Marcel Dekker, New York, 2001, p. 589
18 G. P. Klein, K. J. Vetter and J. W. Schultze, Z. Phys. Chem., 1976, 99, (1–3), 1
19 B. E. Conway and G. Jerkiewicz, Electrochim. Acta, 2000, 45, (25–26), 4075
20 J. Fournier, L. Brossard, J. Y. Tilquin, R. Côté, J.-P. Dodelet, D. Guay and H. J. Ménard, J. Electrochem. Soc., 1996, 143, (3), 919
21 O. Hammerich and V. D. Parker, Electrochim. Acta, 1973, 18, (8), 537
22 J. Heinze, Angew. Chem., 1984, 96, (11), 823 23 J. Simonet, E. Labaume and J. Rault-Berthelot,
Electrochem. Commun., 1999, 1, (6), 252
24 C. Cougnon and J. Tanguy, unpublished observa-tions
25 C. Cougnon and J. Simonet, J. Electroanal. Chem., 2002, 531, (2), 179
(ionometallic) layers (up to 1 μm in thickness). Under appropriate conditions, the salt-infused ionometallic layer shows a huge swelling, which depends on the applied potential, the nature of the interface and the mode of the discharging process. With the aid of SEM techniques, it has been found that charging and discharging of platinum layers are correlated with their swelling and shrinkage. Dramatic structural changes of the platinised plat-inum layer have been analysed and explained in terms of both the existence of a reversible charg-ing-discharging process and the specific nature of the electrolytes employed. Finally, analysis of reduction processes revealed that side reactions may occur, such as the slow decomposition of the ionometallic layer obtained at quite negative poten-tials. For example, methane may be evolved with TMAX salts as shown in Equations (x) and (xi):
[Ptn–, TMA+, TMAX]
→nPt + (CH3)3N + CH3• + TMAX (x)
In the presence of a source of electrons and pro-tons:
CH3•→CH4 (xi)
Thus controlling the roughness of the platinum interface by the various treatments presented here might be used to impart particular proper-ties to the resulting surface – for instance, by achieving a clean platinum surface in the absence of dioxygen, for catalytic and electrocatalytic applications.
Acknowledgments
The author is grateful to the Centre de Microscopie Electronique à Balayage et MicroAnalyse (Dr Le Lannic, Université de Rennes) for its efficient help, and to Professor C. Amatore (ENS, Université de Paris VI) for fruitful technical discussions.
26 C. Cougnon and J. Simonet, Electrochem. Commun., 2001, 3, (5), 209
27 C. Dano, Ph.D. Thesis, Université de Rennes 1, 1998
28 E. Zintl and G. Woltersdaf, Z. Electrochem., 1935, 41, 876
29 E. Zintl and W. Dullenkopf, Z. Phys. Chem., 1932, B16, 183
30 E. Zintl, J. Goubeau and W. Dullenkopf, Z. Phys. Chem., 1931, 154, (Abt. A), 1
31 J. B. Chlistunoff and J. J. Lagowski, J. Phys. Chem. B, 1997, 101, (15), 2867
32 J. B. Chlistunoff and J. J Lagowski, J. Phys. Chem. B, 1998, 102, (30), 5800
33 V. Svetlicic, P. B. Lawin and E. Kariv-Miller, J. Electroanal. Chem., 1990, 284, (1), 185
34 E. Kariv-Miller, P. D. Christian and V. Svetlicic, Langmuir, 1995, 11, (5), 1817
35 E. Kariv-Miller and P. B. Lawin, J. Electroanal. Chem., 1988, 247, (1–2), 345
36 C. Cougnon and J. Simonet, Platinum Metals Rev., 2002, 46, (3), 94
37 C. Cougnon, Ph.D. Thesis, Université de Rennes 1, 2002
38 A. Merz and G. Thumm, Justus Liebigs Ann. Chem., 1978, 1526
39 C. E. Dalm and D. G. Peters, J. Electroanal. Chem., 1996, 402, (1–2), 91
40 J. Simonet, Electrochem. Commun., 2003, 5, (6), 439 41 A. F. Diaz, K. K. Kanazawa and G. P. Gardini, J.
Chem. Soc., Chem. Commun., 1979, (14), 635 42 G. Tourillon and F. Garnier, J. Electroanal. Chem.,
1982, 135, (1), 173
43 “Handbook of Chemistry and Physics”, 46th Edn., CRC Press, Cleveland, OH, 1965–1966, F 117 44 J. Ghilane, M. Delamar, M. Guilloux-Viry, C.
Lagrost, C. Mangeney and P. Hapiot, Langmuir, 2005, 21, (14), 6422
45 J. Ghilane, M. Guilloux-Viry, C. Lagrost, P. Hapiot and J. Simonet, J. Phys. Chem. B, 2005, 109, (31), 14925
46 Y. H. Zhao, M. H. Abraham and A. M. Zissimos, J. Chem. Inf. Comput. Sci., 2003, 43, (6), 1848
The Author